Abstract
Drug resistance remains a major impediment in the development of durable cancer therapies. Studies in acute myeloid leukemia (AML) patients revealed a new form of multi-drug resistance. Here, increased Glioma associated protein GLI1 leads to elevation of the UDP-glucuronosyl transferase (UGT) enzymes. UGTs add glucuronic acid to xenobiotics and metabolites. Traditionally, loss of these enzymes is thought to contribute to cancer as a result of impaired clearance of environmental carcinogens. However, we demonstrate that overexpression of UGTs can contribute to oncogenesis by promoting drug resistance. Indeed, UGT levels in AML patients treated with ribavirin and/or cytarabine were elevated at relapse relative to diagnosis. This was reversed by GLI1 inhibition suggesting a clinically relevant strategy to overcome drug resistance. Further, overexpression of UGTs can also lead to drug resistance in other cancers such as certain Hsp90 inhibitors and vorinostat in colorectal and chronic lymphoblastic leukemia, respectively. Not all drugs are targets of glucuronidation suggesting that UGT status could be relevant to treatment choice. Here, we describe several facets of UGT biology and how these could be exploited clinically. These studies demonstrate how drugs in cancer cells can be metabolised differentially than their normal counterparts. In summary, we describe a new form of drug resistance relevant to a variety of cancer contexts.
Background
The development of durable cancer therapeutics continues to be impaired by the development of drug resistance. Resistance arises to both traditional chemotherapies as well as newly developed targeted treatments. Drug resistance can arise for many different reasons including modulation of drug transporters and/or metabolism, mutation of protein targets of drugs and re-wiring of targeted pathways, to name a few (1, 2). Further complicating matters, development of resistance to one drug can lead to resistance to drugs that the patient has never received. This is referred to as cross-resistance (1). For instance, increased drug efflux through the MDR transporters affects many drugs. Genetic re-wiring can lead to oncogenic bypass where the drug still hits its cellular target, but no longer has a physiological effect (2). Below, a new form of multi-drug resistance is described, inducible drug glucuronidation (Fig. 1), which was discovered while targeting the eukaryotic translation initiation factor 4E (eIF4E) with a small molecule inhibitor ribavirin in acute myelogenous leukemia (AML) patients (3). Initially, these results were quite promising with some refractory and relapsed patients achieving complete and partial remissions with ribavirin monotherapy and also ribavirin in combination with low dose Ara-C (4, 5). However, all patients eventually relapsed and investigations into the molecular reasons for this led to the discovery that ribavirin and Ara-C became glucuronidated specifically in resistant cells.
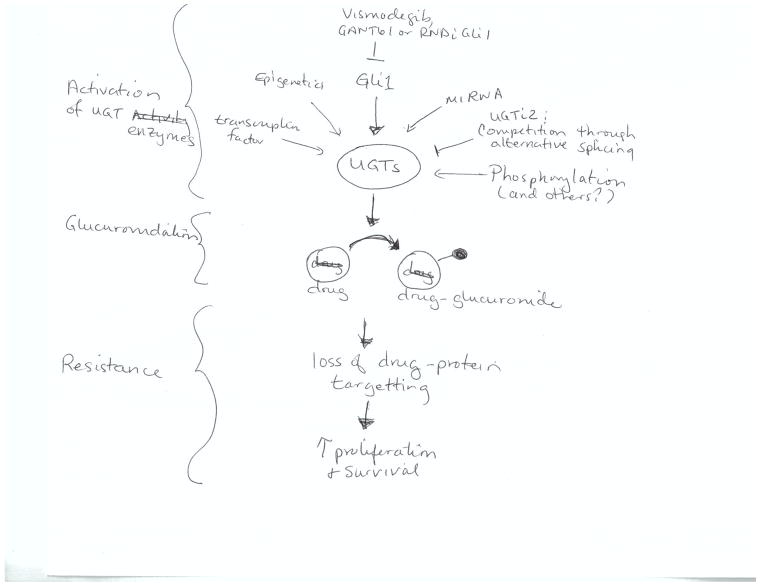
Figure 1.
Summary of means to regulate glucuronidation enzymes. For simplicity, only a subset of pathways are depicted.
Glucuronidation is an important step in Phase II drug metabolism (6). There are several glucuronidation enzymes, UDP-glucuronosyl transferases, which are divided into two major families: UGT1As with nine members and UGT2B with seven members (7). UGT1As catalyze the addition of glucuronic acid to the nucleophilic part of the drug rendering it more hydrophilic and either enhancing its efflux or modulating its affinity for a given cellular target. Family members differ in their preference to target specific chemical moieties such as nitrogens, sulphurs and oxgen (8). This activity, once thought limited to the liver, can occur throughout the body (8). Although glucuronidation is usually considered a steady state process, it can have somewhat unpredictable effects. For instance, glucuronidation of morphine increases its potency (6) while glucuronidation of testosterone modulates its repertoire of protein partners without altering efflux (6).
Analysis of model systems and material from AML patients who had received either ribavirin or standard of care therapies revealed that levels of the sonic hedgehog transcription factor GLI1 became substantially elevated upon drug resistance and this correlated with an increase in levels of the UGT1As enzymes (3, 5). Here, it was shown that this family of enzymes mediated glucuronidation of ribavirin resulting in the loss of ribavirin-eIF4E interaction and ultimately the loss of response to the drug. It was noted that in this case, glucuronidation did not modulate the efflux of ribavirin. Furthermore, inducible glucuronidation was also observed for cytarabine, the cornerstone in the treatment for AML. Preliminary studies suggest other drugs will also be targets for this mode of inactivation (H.A. Zahreddine and K.L.B. Borden; unpublished results). Excitingly, this mechanism is reversed by genetic or pharmacological inhibition of GLI1, including with Vismodegib a drug approved for metastatic basal cell carcinoma. Thus, our studies reveal a potential strategy to overcome drug resistance which will be tested in AML patients in the coming months (NCT02073838).
Clinical-Translational Advances
Relevance of UGTs to drug resistance
Most studies on glucuronidation in malignancy have focused on the effects of loss of UGTs. Such losses can lead to reduced clearance of xenobiotics such as the carcinogens in cigarette smoke (9). In the absence of clearance of these metabolites, increased DNA damage can occur leading to cancer (9). In support of this idea, individuals that lack activity of UGT1A7, have a higher risk of lung cancer (9).
Recent studies demonstrate that glucuronidation can become elevated in malignancy (3, 5, 10, 11). This suggests that there is some sort of “Goldilocks zone” for UGT expression, where levels should not be too high or too low, but rather “just right”. Our studies show that inducible drug glucuronidation can be part of an adaptive response of cancer cells to evade the effects of certain drugs. Furthermore, these new findings demonstrate that cancer cells metabolize drugs differently than normal counterparts. For instance, ribavirin- and cytarabine-glucuronides are not observed in normal tissues. Indeed, this metabolism even differs amongst cancer cell populations (resistant versus responsive). Two other recent studies support this hypothesis and suggest that these findings go beyond AML. First, in Chronic Lymphocytic Leukemia (CLL), elevation of UGT2B17 may also underpin drug resistance (10). In these studies, elevated UGT2B17 mRNA expression in conjunction with other prognostic markers for CLL predicted significantly shorter survival. Indeed, glucuronides are observed for the HDAC-inhibitor vorinostat upon UGT2B17 overexpression; suggesting that UGT2B17 may have an impact on drug response at least in some patients. In a separate study, resistance to some Hsp90 inhibitors in a subset of colorectal cancer cell lines directly correlated with increased expression and enzymatic activity of UGT1As (11). Glucuronidation abrogated the Hsp90 interaction of a subset of chemically related inhibitors (11). Chemically unrelated Hsp90 inhibitors were not modified and still targeted Hsp90 in these cells demonstrating the specificity of targeting in these cells. For the glucuronidation target drugs, knockdown of UGT led to renewed drug sensitivity. Given that not all Hsp90 inhibitors were targets for glucuronidation, specific UGTs likely play a role with these favoring specific chemical moieties for glucuronidation. In summary, evidence for inducible drug glucuronidation is observed in several contexts including AML, CLL and colorectal cancers.
Regulation and targeting of UGTs
In order to develop means to target UGTs, it is critical to understand how these enzymes are regulated (Fig. 1). UGT levels can be regulated both post-transcriptionally and transcriptionally. For the AML case, there is a clear correlation between elevated GLI1 and elevated UGT protein levels. Further, reduction of GLI1 by knockdown or pharmacological targeting with Vismodegib or GANT61 leads to reduced UGT levels and reverts drug resistance. However, UGT is not a transcriptional target of GLI1, as UGT1A mRNA levels are not elevated. However, GLI1 increases UGT1A protein stability. We postulate that GLI1 decreases UGT1A protein turnover through downregulation of specific protein ligases or it induces some posttranslational modifications of these enzymes rendering them less susceptible to degradation. These possibilities will be examined in future. Hence, in addition to using GLI1 inhibitors as a means to revert resistance, identifying key factors mediating the link between GLI1 and UGT1A could also serve as potential therapeutic modalities.
It seems likely that there will be many pathways leading to UGT dysregulation upon cancer resistance, in addition to GLI1 dysregulation. Numerous recent studies revealed a variety of molecular mechanisms governing expression and activity of UGT enzymes (11–15). Due to space restraints; only a few of these are described here. Aside from effects on protein stability described above, these include transcriptional regulation, phosphorylation, DNA methylation, histone modification, microRNA regulation, as well as alternative splicing (11–15). For instance, DNA hypermethylation of UGT1A1 promoter correlated with reduced transcription and increased sensitivity of colorectal cancer cells to SN-38 (the active metabolite of Irinotecan) (12). Also, a recent study identified miRNAs that negatively regulate the expression of UGT2B17 in human liver samples, but the identity of these miRNAs remains to be experimentally validated (14). Another interesting discovery revealed the presence of a novel class of human UGT proteins named UGT isoforms 2 (or i2s) (16). Like isoforms 1, i2s are encoded by the same genetic locus, but instead of incorporating the usual common C-terminus exon 5a, these utilize a shorter exon 5b which causes a premature arrest of translation and subsequent loss of the transmembrane domain. Consequently, UGT i2s are located in the lumen and cytoplasm rather than the membrane of the endoplasmic reticulum. Functionally, the i2 enzymes lack glucuronidation activity but act in a dominant-negative capacity, possibly by forming inactive heteromeric complexes with i1 enzymes thus reducing enzymatic activity. Recent reports showed differential i1/i2 expression ratio in normal versus cancer tissues, with increased i2 in liver tumors and decreased levels of i1 and i2 in colon cancer samples, compared to normal specimens (17). Given the close tie between protein levels and activity, it seems likely that these tumours will have different glucuronidation activity relative to normal controls. As such, understanding the mechanisms of regulation of i2s might provide a means for increasing their expression in resistance context allowing for inhibition of UGT i1s, as one of many possible targeting strategies.
Inducible modifications beyond glucuronidation?
Phase II drug metabolism involves conjugation of drugs via multiple pathways, including sulfation, glutathione addition, acetylation, methylation as well as glucuronidation. Accordingly, similar to glucuronidation, one can speculate that these modifications may also be induced in some forms of drug resistance. As such a better understanding of the mechanisms that control these enzymes in both normal and cancer contexts could help improve the efficacy of cancer therapy. In addition, our findings suggest that when developing drugs, mechanisms that could drive drug modifications should be considered in patients particularly at relapse and not only in the initial stages of drug development in normal cells.
Conclusions and Future Directions
UGTs could serve not only as a biomarker for drug resistance but also guide choice of treatment. For instance, knowledge of which Hsp90 inhibitors become glucuronidated in cell lines would be important for guiding which inhibitors to administer when UGT levels become elevated in patients. In this way, the most effective Hsp90 inhibitors could be selected based on patient UGT status. Once there is a more global understanding of the drugs that become glucuronidated in different cancer contexts, UGT status could be used more globally for guiding the best options for other treatments. In AML, we will examine the efficacy of targeting glucuronidation by combining the GLI1 inhibitor Vismodegib to target glucuronidation with ribavirin to target elevated eIF4E (NCT02073838). In this way, we will examine the efficacy of targeting UGT as a means to overcome drug resistance in patients.
One of the great challenges in studying drug metabolism is developing appropriate model systems. Human cell lines have been used for such experiments but are limited in terms of monitoring in vivo disease and further, recapitulating drug compartmentalization and associated features. However, the substantial differences in drug modifications and even drug transporter distribution, between humans and rodents (18–20), means that this is a difficult solution as well. For instance, rodents have no N-linked glucuronidation which is a major pathway in humans. Mice with human UGT1As expressed in their liver have more similar, but still not identical, glucuronidation activity as humans (19). Further issues arise as there are glucuronidation enzymes that are mainly in the gastro-intestinal track and thus would not be accounted for by the humanized liver model (19). Other species-specific aspects of drug metabolism need to be accounted for as well such as differences in drug transporter distribution (20). Thus, only with great effort and care can a model system be developed to truly parallel the human system.
In summary, inducible drug glucuronidation as a novel form of multi-drug resistance is described. A better understanding of the modalities that cause UGT dysregulation will likely provide therapeutic strategies beyond the GLI1 example used here. Such studies should include monitoring UGTs at the protein level in various types and stages of cancer to assess the scope of this dysregulation. Also, determining which of the UGTs is upregulated in a given type of cancer first will help predict which class(es) of drugs will be subject to glucuronidation and potentially guide treatment choice. Finally, our studies strongly suggest that drug metabolism can differ between cancer and normal cells. These differences need to be better defined and could be launching point for novel therapeutic strategies.
Acknowledgments
Grant Support
K.L.B. Borden is supported by grants from the NIH (ROI 80728, 98571), the Leukemia & Lymphoma Society Translation Research Program, and the Canadian Cancer Society Research Institute and holds a Canada Research Chair. H.A. Zahreddine is supported by a Cole Foundation Fellowship.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014;511:90–3. doi: 10.1038/nature13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–60. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 5.Assouline S, Culjkovic-Kraljacic B, Bergeron J, Caplan S, Cocolakis E, Lambert C, et al. A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica. 2015;100:e7–9. doi: 10.3324/haematol.2014.111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutton GJ. Glucuronidation of drugs and other compounds. Boca Raton (FL): CRC Press; 1980. [Google Scholar]
- 7.Guillemette C, Levesque E, Rouleau M. Pharmacogenomics of human uridine diphospho-glucuronosyltransferases and clinical implications. Clin Pharmacol Ther. 2014;96:324–39. doi: 10.1038/clpt.2014.126. [DOI] [PubMed] [Google Scholar]
- 8.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 9.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics. 2003;3:37–52. doi: 10.2165/00129785-200303010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Gruber M, Bellemare J, Hoermann G, Gleiss A, Porpaczy E, Bilban M, et al. Overexpression of uridine diphospho glucuronosyltransferase 2B17 in high-risk chronic lymphocytic leukemia. Blood. 2013;121:1175–83. doi: 10.1182/blood-2012-08-447359. [DOI] [PubMed] [Google Scholar]
- 11.Landmann H, Proia DA, He S, Ogawa LS, Kramer F, Beißbarth T, et al. UDP glucuronosyltransferase 1A expression levels determine the response of colorectal cancer cells to the heat shock protein 90 inhibitor ganetespib. Cell Death Dis. 2014;5:e1411. doi: 10.1038/cddis.2014.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belange AS, Tojcic J, Harvey M, Guillemette C. Regulation of UGT1A1 and HNF1 transcription factor gene expression by DNA methylation in colon cancer cells. BMC Mol Biol. 2010;11:9. doi: 10.1186/1471-2199-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda S, Fukami T, Yokoi T, Nakajima M. Epigenetic regulation of the tissue-specific expression of human UDP-glucuronosyltransferase (UGT) 1A10. Biochem Pharmacol. 2014;87:660–7. doi: 10.1016/j.bcp.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Rieger JK, Klein K, Winter S, Zanger UM. Expression variability of absorption, distribution, metabolism, excretion-related microRNAs in human liver: influence of nongenetic factors and association with gene expression. Drug Metab Dispos. 2013;41:1752–62. doi: 10.1124/dmd.113.052126. [DOI] [PubMed] [Google Scholar]
- 15.Yokoi T, Nakajima M. microRNAs as mediators of drug toxicity. Annu Rev Pharmacol Toxicol. 2013;53:377–400. doi: 10.1146/annurev-pharmtox-011112-140250. [DOI] [PubMed] [Google Scholar]
- 16.Girard H, Lévesque E, Bellemare J, Journault K, Caillier B, Guillemette C. Genetic diversity at the UGT1 locus is amplified by a novel 3′ alternative splicing mechanism leading to nine additional UGT1A proteins that act as regulators of glucuronidation activity. Pharmacogenet Genomics. 2007;17:1077–89. doi: 10.1097/FPC.0b013e3282f1f118. [DOI] [PubMed] [Google Scholar]
- 17.Bellemare J, Rouleau M, Harvey M, Popa I, Pelletier G, Têtu B, Guillemette C. Immunohistochemical expression of conjugating UGT1A-derived isoforms in normal and tumoral drug-metabolizing tissues in humans. J Pathol. 2011;223:425–35. doi: 10.1002/path.2805. [DOI] [PubMed] [Google Scholar]
- 18.Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45:1121–32. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Kutsuno Y, Sumida K, Itoh T, Tukey RH, Fujiwara R. Glucuronidation of drugs in humanized UDP-glucuronosyltransferase 1 mice: Similarity with glucuronidation in human liver microsomes. Pharmacol Res Perspect. 2013;1:e00002. doi: 10.1002/prp2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Nishimura T, Nishimura S, Zhang H1, Zheng M, Guo YY, et al. Fialuridine induces acute liver failure in chimeric TK-NOG mice: a model for detecting hepatic drug toxicity prior to human testing. PLoS Med. 2014;11:e1001628. doi: 10.1371/journal.pmed.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]