Abstract
Human C-reactive protein (CRP) is a serum soluble pattern recognition receptor (PRR) that serves as a marker of inflammation and directly contributes to innate immunity. Herein we show that human CRP also directly contributes to adaptive immunity, i.e. native CRP binds specifically to human Jurkat T cells and to mouse naïve CD4+ T cells and modulates their T helper (Th) 1 and Th2 responses. In vitro both exogenously added (purified) and endogenously expressed (via transfection) human CRP inhibited Th1 differentiation and augmented Th2 differentiation of naïve CD4+ T cells. In vivo for human CRP transgenic (CRPtg) compared to wild type mice, a lesser proportion of the T cells recovered from the spleens of healthy animals were Th1 cells. Moreover in both CRPtg mice and in wild type mice treated with human CRP, during myelin oligodendrocyte glycoprotein peptide induced experimental autoimmune encephalomyelitis both the Th1 cell response and disease severity were inhibited. These pattern recognition-independent actions of CRP directly on T cells highlights the potential for this soluble PRR to act as a tonic regulator of immunity, shaping global adaptive immune responses during both homeostasis and disease.
Keywords: C-reactive protein, T cell differentiation, inflammation, autoimmune diseases, experimental autoimmune encephalomyelitis
Introduction
C-reactive protein (CRP) is evolutionarily conserved and there is no known natural deficiency in humans (1). CRP is a major human acute phase reactant, thus the plasma concentration of CRP can increase up to 1000-fold upon tissue injury or infection (1). Although a rise in circulating CRP is widely used as a non-specific clinical marker of inflammation and several of its properties have been well defined in vitro, there is still no consensus about the exact function of CRP in vivo (1, 2). The recognized capacity of CRP to bind Fc receptors, to activate the classical pathway of complement, and to opsonize both apoptotic cells and microbes supports the proposition that CRP acts as a soluble pattern recognition receptor (PRR) in vivo and thereby directly contributes to innate host defense (3, 4). Additional studies done using human CRP transgenic mice (CRPtg) indicate that CRP might also regulate autoimmunity (5–8), and our recent identification of highly recurrent promoter mutations in CRP gene in multiple types of cancers suggests CRP might also play a critical role therein (9, 10).
CD4+ effector T cells are key component of adaptive immunity and they play a major role in controlling infections and the development of autoimmunity and cancer (11–16). The propagation of effector CD4+ T cells begins when T cell receptors (TCRs) on naïve CD4+ T cells are engaged by cognate antigens in the context of MHC II and co-stimulation provided by antigen-presenting cells (APCs). Thusly activated and depending on the nature of cytokines produced by cells of the innate immune system, naïve T cells differentiate into multiple kinds of effectors including IFN-γ secreting T helper (Th) 1 cells, IL-4 secreting Th2 cells and IL-17 secreting Th17 cells (17, 18). PRRs were originally thought to regulate T cell differentiation and effector responses indirectly, via their actions on APCs and other kinds of innate immune cells. However, recent evidence indicates that Toll-like receptors (TLRs), the representative membrane PRRs, are themselves expressed by T cells and hence can directly modulate T cell responses following TLR ligation by their cognate ligands (19–21).
In the mid-1970s it was initially reported that CRP could bind T cells and thereby modulate their effector functions (22–24). Subsequently however, that observation could not be reproduced by the same group (25). The paradoxical outcomes were attributed to differences in CRP purity (25). Nevertheless, because T cell heterogeneity was not fully appreciated at the time, its likely contribution to the observed variance in CRP binding and actions was not explored. Importantly, although Fc receptors (FcRs) were identified as major receptors for CRP (26, 27), there is little evidence that T cells express FcRs (28). Thus whether purified CRP is able to directly interact with T cells still remains equivocal.
In the present work we rigorously characterized both the CRP preparations and T cells that we used and revisited the question of CRP binding by T cells. We demonstrate that human CRP in its native pentameric conformation does indeed bind to both primary mouse naïve T cells and to human leukemic Jurkat T cells. This binding is independent of calcium or the classic CRP ligand phosphorylcholine, and require neither FcR nor LOX-1, another recently identified CRP receptor (29). CRP binding to T cells is abrogated by pretreatment of cells with proteases however, indicating a requirement for an as yet unidentified receptor. Importantly, we show for the first time that CRP binds preferentially to the naïve T cell subset and thereby modulates their differentiation, favoring the Th2 effector program while inhibiting the Th1 program both in vitro and in vivo. Although the identity of the CRP-binding receptor on naïve T cells has yet to be determined, these new results suggest that CRP might play a direct role in regulating the adaptive immune response. Given the inducible nature of CRP and its systemic presence, it remains plausible that CRP is an important tonic regulator of adaptive immunity (8).
Materials and Methods
Animals
Wild type mice (strain C57BL/6) were from the animal center of Lanzhou University. Human CRP transgenic mice (CRPtg) have been fully described elsewhere (30). Human CRP is present in the blood of CRPtg at concentrations manifested in humans i.e., low levels under steady-state conditions (<1 to 10 µg/ml) and high levels during the acute phase response (approx. 30 to 500 µg/ml). Mice were housed at constant humidity (60 ± 5%) and temperature (24 ± 1°C) with a 12 hour light cycle (6 AM to 6 PM) and maintained ad libitum on sterile bottled water and regular chow (Harlan Teklad). 8–12 weeks old mice were used unless specifically noted otherwise. All animal use protocols were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham and Lanzhou University and were consistent with the Guide for the Care and Use of Laboratory Animals, 8th Edition (2010).
Reagents
Native human CRP purified (>99 % purity) from ascites was purchased from the BindingSite (Birmingham, UK). To ensure that calcium and ligand binding ability was retained, CRP were re-purified with PC-Agarose beads (Thermo Fisher Scientific, Rockford, IL, USA), dialyzed extensively to remove any residual NaN3. Finally, CRP was passed through Detoxi-Gel columns (Thermo Fisher Scientific) to remove endotoxin. The functional integrity of the CRP molecule was then directly verified by sodium dodecyl sulphate (SDS; 1/20th the normal concentration) polyacrylamide-gel electrophoresis (1/20 SDS-PAGE) (31) (Figure S1A), electron-microscopy (Figure S1B), conformation-specific ELISA (32) (Figure S1C) and calcium-dependent binding to the classic CRP ligand phosphorylcholine (PC) (1, 4) (Figure S1D). As a further safeguard, polymyxin B (20 μg/ml) was included in cell response experiments to exclude possible confounding effects from residual endotoxin in any of the culture media used. Anti-CD3 mAb (2C11; catalog no. 555273), anti-CD28 mAb (37.51; catalog no. 553295), anti-IL4 mAb (catalog no. 554432), anti-IFN-γ mAb (catalog no. 554408), anti-IL-12 mAb (catalog no. 554475), mIL-2 (catalog no. 550069), mIL-12p70 (catalog no. 554592), mIL-4 (catalog no. 550067), ELISA kits for IL-4 (catalog no. 555232) and IFN-γ(catalog no. 555138) were from BD Biosciences (San Jose, CA). Anti-Ly6G mAbs were from BioLegend (San Diego, CA; catalog number 127611) and other reagents were from Sigma-Aldrich (St. Louis, MO). Anti-CRP mAbs 1D6, 2C10 and 3H12 were prepared as described (33).
T cell differentiation
CD4+ and CD62L+ naïve T cells were purified from the spleens of male C57BL/6 mice using MACS kits (catalog no. 130-093-227; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. 2 × 105 T cells were cultured in 500 μl medium for 3 days with plate-bound anti-CD3 (2 μg/ml, immobilized overnight at 4 °C) and fluid-phase anti-CD28 mAbs (2 μg/ml), in the presence or absence of CRP under Th1 polarizing conditions (10 ng/ml mIL-2, 20ng/ml mIL-12p70, 10 μg/ml anti-IL-4 mAb) or Th2 polarizing conditions (10 ng/ml mIL-2, 20 ng/ml mIL-4, 10 μg/ml anti-IL12 mAb, 10 μg/ml anti-IFN-γ mAb). In some experiments, cells were transfected with control or CRP-expressing pcDNA 3.1 plasmids via electroporation with a Multiporator apparatus (Eppendorf AG, Hamburg, Germany) or by using the X-tremeGENE HP DNA Transfection Reagent (catalog no. 550067; Roche, Basel, Switzerland). The cells were cultured under resting condition for an additional 2 days followed by treatment with PMA (20 ng/ml), calcium ionophore (1 μg/ml) and monensin (catalog no. 550069; BD Biosciences) for 5 h. Cells were washed with PBS and FcRs blocked with anti-mouse CD16/32 mAb (catalog no. AF1460; Abcam, Cambridge, UK) for 10 min on ice. After staining with FITC-rat anti-mouse CD4 (catalog no. 553046) or rat IgG2a κ isotype control (catalog no. 553929; BD Biosciences) for 30 min at 4 °C, cells were washed twice and treated with cytofix/cytoperm fixation/permeabilization buffer (catalog no. 554714; BD Biosciences). Intracellular IL-4 and IFN-γ were stained with PE-rat anti-mouse IL-4 (catalog no. 554435) and PerCP-Cy5.5-rat anti-mouse IFN-γ (catalog no. 560660) or corresponding isotype controls (catalog no. 554685 and 560537; BD Biosciences) for 30 min at 4 °C followed by washing and flow cytometry detection. Negligible cell staining was observed with all isotype control antibodies.
The mRNA levels of cytokines and transcription factors were measured by q-PCR using the SYBR Premix DimerEraser kit (catalog number: DRR091C; Takara, Shiga, Japan) and a CFX96 real-time thermal cycler (Bio-Rad, Hercules, CA). Cytokine and transcription factor mRNA levels were normalized to the β-actin (ACTB) or GAPDH. The primer sequences used are: human IFN-γ forward: 5’-GAATGTCCAACGCAAAGCAAT-3’; reverse: 5’-GACCTCGAAACAGCATCTGACTCCT-3’; human IL-4 forward: 5’-CTGTGCACCGAGTTGACCGTA-3’; reverse: 5’-GTCCTTCTCATGGTGGCTGTAGAAC-3’; human ACTB forward: 5’-GCAAAGACCTGTACGCCAACA-3’; reverse: 5’-ACACGGAGTACTTGCGCTCAG-3’; mouse IFN-γ forward: 5’-CCATCAGCAACAACATAAGCGTC-3’; reverse: 5’-TTGACCTCAAACTTGGCAATACTCA-3’; mouse IL-4 forward: 5’-TTCCAAGGTGCTTCGCATA-3’; reverse: 5’-TGCAGCTTATCGATGAATCCA-3’; mouse IL-12Rβ2 forward: 5’-TCTGCGAAATTCAGTACCGAC-3’; reverse: 5’-GCCCACCGTGATGATAGC-3’; mouse IL-4Ra forward: 5’-GGGCATGGAGGCTACAAG-3’; reverse: 5’-CTCCGTGTCTAGTCCGAAAGT-3’; mouse T-bet forward: 5’-CCATTCCTGTCCTTCACCG-3’; reverse: 5’-CTGCCTTCTGCCTTTCCAC-3’; mouse GATA-3 forward: 5’-AGTCCTCATCTCTTCACC-3’; reverse: 5’-CACTCTTTCTCATCTTGC-3’; mouse GAPDH forward: 5’-GGAGAAACCTGCCAAGTATGA-3’; reverse: 5’-GTGGGTGCAGCGAACTTTA-3’.
CRP binding
CRP was incubated with 5 × 105 Jurkat or mouse T cells for 1 h at 4 °C in PAB buffer (PBS, 0.1 % BSA, 0.05 % NaN3, 1 mM Ca). After washing twice with PAB, cells were incubated sequentially with anti-CRP mAbs 2C10, 1D6 or 3H12 (33) and DyLight 650-labelled anti-mouse antibody (catalog no. ab96874; Abcam). Cells were then incubated with propidium iodide (PI) for 15 min at room temperature to allow identification of live/dead cells by flow cytometry. In some binding experiments, APC-labelled streptavidin (catalog no. 405207; Biolegend, San Diego, CA) was used to detect the binding of biotin-labelled CRP.
Immunoblotting
Mouse naïve T cells stimulated in vitro as indicated above were lysed in the presence of protease/phosphatase inhibitor cocktail (catalog no. 5872; Cell Signaling Technology, Danvers, MA) for 30 min on ice. The lysates were centrifuged at 12000 × g for 30 min, 4 °C and the supernatants were subjected to standard SDS-PAGE followed by transferring to PVDF membranes for detection of STAT, T-bet or GATA-3 proteins. Rabbit anti-STAT1 (catalog no. 9172S), anti-phospho-(Tyr701) STAT 1 (catalog no. 9167S), anti-STAT4 (catalog no. 2653S), and anti-phospho-(Tyr693) STAT 4 (catalog no. 4134S) were from Cell Signaling Technology. Rabbit anti-STAT6 (catalog no. ab32520) and anti-phospho-(Tyr641) STAT 6 (catalog no. ab54461) were from Abcam. Anti-T-bet mAb 4B10 (catalog no. sc-21749) and anti-GATA-3 HG3-31 (catalog no. sc-268) were from Santa Cruz (Dallas, TX, USA).
Experimental autoimmune encephalomyelitis (EAE)
Female C57BL/6 mice housed in conventional conditions were immunized s.c. with 200 μg myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (>99 % purity; Shanghai Science Peptide Biological Technology, China) in complete Freund’s adjuvant containing 1 mg Mycobacterium tuberculosis strain H37Ra (catalog no. 7027; Chondrex, Redmond, WA). On days 0 and 2, immunized mice were injected i.p. with 200 ng pertussis toxin (catalog no. BML-G101; Enzo Life Sciences, Farmingdale, NY). On day 2, immunized mice received s.c. a single injection of 200 μg human CRP or buffer control. The clinical severity of EAE was scored using the standard grading scale: 0, asymptomatic; 1, limp tail; 2, limp tail and weakness of hind limb; 3, limp tail and partial hind limb paralysis; 4, limp tail, complete hind limb and partial foreleg paralysis; 5, moribund. The splenocytes were isolated on day 7 or at the peak of EAE symptoms and re-stimulated ex vivo with 100 μg/ml MOG peptide 35–55. Intracellular and secreted cytokines were then determined by flow cytometry and ELISA, respectively. In experiments comparing wild type and CRPtg splenocytes, flow cytometry was done without prior MOG restimulation in vitro.
Assessing spinal cord cell infiltration
To examine spinal cord infiltrating cells, spinal cords were removed from mice at the peak of EAE. After perfusion with PBS, the spinal cords were ground through a cell strainer, washed in buffer, resuspended in 30% Percoll and layered onto 70% Percoll. After centrifugation at 2500 rpm (RT, 30 min), cells at the interface were carefully removed and washed in PBS. Cells were plated in complete medium using round bottom 96-well plates and incubated at 37°C for four hours in the presence of Golgi Stop (BD Biosciences). After treatment with fixing/permeabilization buffer the cells were washed and stained with cell marker specific mAbs and processed for flow cytometry.
Statistical Analysis
Data are presented as mean ± SEM. Statistical analysis was performed using two-tailed Student’s t-tests or one-way ANOVA with Tukey post hoc comparisons. Values of p<0.05 were considered significant.
Results
Specific binding of CRP to naïve CD4+ T cells
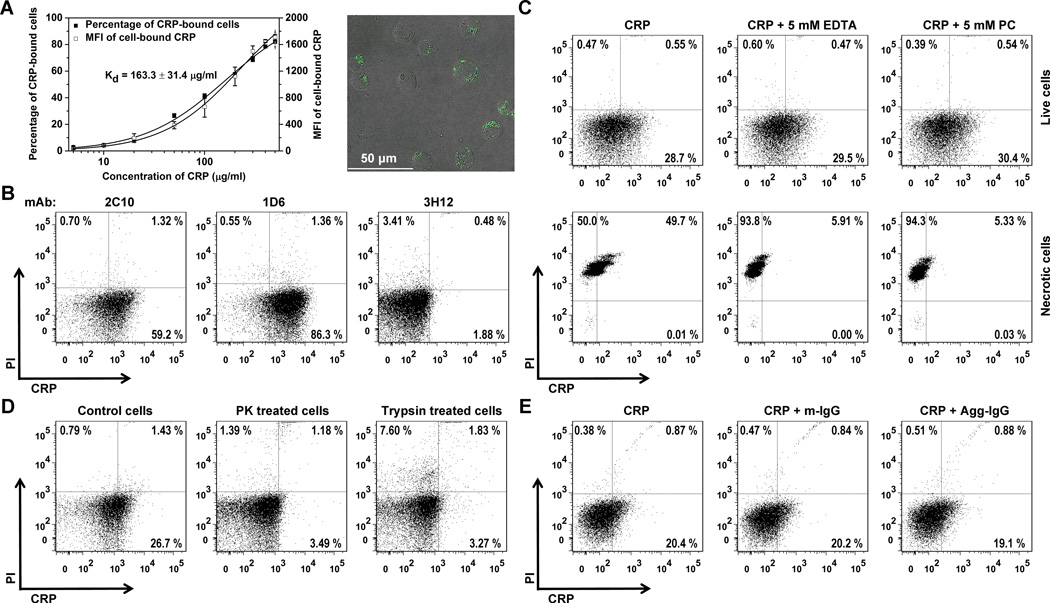
Human Jurkat clone E6-1 is a widely used human T cell line. We found that highly purified human CRP bound to live E6-1 Jurkat T cells in a concentration-dependent manner with over 70 % of cells staining positive for CRP at saturation (Figure 1A). The calculated KD of this interaction was 163.3 μg/ml, but substantial CRP binding could still be observed at lower concentrations. This binding was not attributable to CRP with an altered conformation, as the native structure of our CRP preparations were thoroughly validated by documenting the integrity of CRP’s pentameric assembly (Figure S1A and S1B), the exclusive expression of its native antigenicity (Figure S1C), and the calcium-dependence of its interaction with PC (Figure S1D). Moreover, CRP bound to E6-1 cells could be detected with a mAb specific to native CRP (1D6) but not with a mAb (3H12) that recognizes a CRP epitope exposed in the altered CRP conformation (Figure 1B).
Figure 1. Binding of native human CRP to human Jurkat T cells.
5×105 Jurkat T cells were incubated with purified endotoxin free CRP in its native pentameric conformation (see Figure S1) at the indicated concentrations at 4 °C for 1 h, followed by washing with PAB buffer as described in the Materials and Methods. CRP binding was probed with anti-CRP mAbs in conjunction with DyLight 650-labelled secondary antibody. Cells were counterstained with PI. (A) The percentage of Jurkat T cells to which CRP bound and the mean DyLight 650 fluorescence intensity (MFI) is CRP concentration-dependent (average of triplicate assays shown in graph). The micrograph shows interaction of CRP (green) with Jurkat T cells as visualized by confocal microscopy. (B) Native CRP binds to live Jurkat T cells. Results of a representative experiment (1 of 3) show that binding of CRP (200 μg/ml) to PI-negative (live) cells could be detected using mAb 2C10 (59.2% of cells) and mAb 1D6 (86.3% of cells), two mAbs that recognize native CRP (33), but not using mAb 3H12 that does not (33). On average 2C10, 1D6, and 3H12 detected 61.0 ± 1.5 %, 89.1 ± 3.6 %, and 1.35 ± 0.31 % of live Jurkat T cells, respectively. Using the same antibodies but without addition of CRP only 1.24 ± 0.02 %, 0.98 ± 0.22 %, and 1.57 ± 0.27 % of live Jurkat T cells were scored positive. (C) CRP (100 μg/ml) binding to live Jurkat T cells (top panels) was calcium-independent and was not PC-inhibitable. In stark contrast, both EDTA and PC nearly abrogated the binding of CRP to necrotic cells generated by boiling of live cells at 95 °C for 15 min (bottom panels). (D) CRP (100 μg/ml) binding to live Jurkat T cells is surface protein (receptor)-dependent as evidenced by near complete abrogation of CRP binding to cells pretreated with proteinase K (PK, 0.5 mg/ml) or trypsin (1 mg/ml). (E) CRP (100 μg/ml ) binding to live Jurkat T cells is FcγR-independent, as neither monomeric IgG (m-IgG, a ligand for the high affinity receptor FcγRI) nor heat-aggregated IgG (Agg-IgG, a ligand for the low affinity receptors FcγRII and FcγRIII) at 100 μg/ml was able to compete for CRP binding (n=3). In (C)-(E), one representative of 3 experiments is shown.
Since CRP binds avidly to necrotic or apoptotic cells (34), this might also account for the observed binding of CRP to E6-1 cells. However, counterstaining cells with PI or Annexin V revealed only negligible proportions of dead or dying cells in our cultures (Figure1, B–E and Figure S2). Moreover, the binding of CRP to live E6-1 Jurkat T cells was both calcium- and PC-independent (i.e., not inhibited with 5 mM EDTA and 5 mM PC, respectively) (Figure 1C, top panels).In stark contrast, interaction of CRP with necrotic cells (generated by exposure to boiling buffer) was both EDTA- and PC-inhibitable (Figure 1C, bottom panels). Surface receptors were likely required for the association of CRP with live E6-1 cells, as pre-treating the cells with proteinase K or trypsin abrogated CRP binding (Figure 1D). Nonetheless, CRP binding to live E6-1 cells was not FcγR-dependent because these cells did not express FcγRs (Figure S3A) and because neither monomeric nor aggregated IgG was able to prevent CRP binding (Figure 1E). LOX-1 has recently been identified as a potential receptor for CRP on endothelial cells (29). However the presence of polyinosinic acid, an inhibitor of LOX-1 (35), also failed to reduce the binding of CRP to live Jurkat cells (Figure S3B).
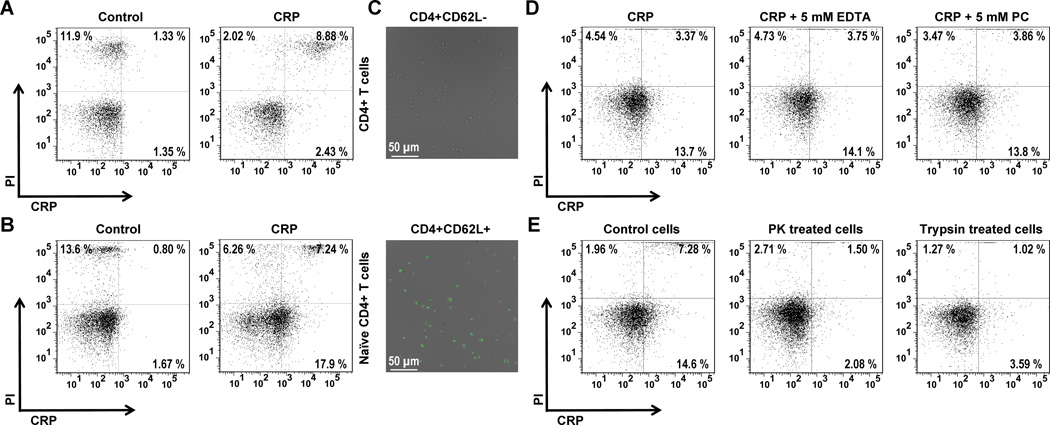
To test if CRP binding to T cells extended beyond the special case of E6-1 Jurkat cells, we also examined the interaction of CRP with primary mouse CD4+ T cells. Unexpectedly, only a small percentage of live CD4+ T cells (isolated from the spleens of healthy adult mice) bound CRP under the conditions we tested (Figure 2A). As Jurkat T cells more closely resemble the naïve phenotype (36), we purified primary mouse naïve T cells based on their high expression of the homing receptor CD62L (L-selectin) (37). On this CD4+CD62L+ T cell subpopulation we detected appreciable binding of CRP (Figure 2B). Confocal microscopy was used to confirm prominent binding of CRP to CD4+CD62L+ T cells but not to CD4+CD62L− T cells (Figure 2C). Like binding to human Jurkat cells, CRP binding to mouse primary CD4+CD62L+ naïve T cells did not require calcium nor PC, but did require surface (proteinaceous) receptors (Figure 2, D and E) other than FcγR and LOX-1 (Figure S3C). Taken together, these results establish that human CRP binds preferentially to the naïve CD4+ T cell subset and that this action requires proteinaceous surface receptors.
Figure 2. Binding of native human CRP to mouse primary naïve T cells.
Binding of biotinylated CRP was probed using APC-labelled streptavidin. Biotinylation of CRP was carefully performed and quality control experiments confirmed CRP maintained its native conformation after biotinylation (see Figure S1). (A) Biotinylated CRP (100 μg/ml) bound to 2.2 ± 0.3 % (n=3) of PI-negative (live) primary mouse CD4+ T cells. This binding was negligible as 1.7 ± 0.3 % of cells not incubated with biotinylated CRP (controls) were detected as false-positives.(B) Binding of biotinylated CRP (100 μg/ml) to enriched live mouse CD4+CD62L+ (naïve) T cells was more frequent (16.1 ± 1.1 % of cells incubated with CRP vs. 2.7 ± 0.1 % of control cells, p < 0.001) (n=3). (C) Confocal microscopy showing scant CRP (100 μg/ml) binding to mouse CD4+CD62L− (non-naïve) T cells (upper panel) versus obvious CRP binding to CD4+CD62L+ (naïve) T cells (lower panel). The two cell populations were purified from the same pool of mixed CD4+ cells. Note the more intense and frequent labelling of CD4+CD62L+ cells. (D) Biotinylated CRP (100 μg/ml) binding to live mouse CD4+CD62L+ naïve T cells is calcium-independent (14.2 ± 0.3 % of cells remain CRP-decorated after EDTA washing) and not PC-inhibitable (15.0 ± 1.2 % of cells remain CRP-decorated after PC washing) (n=3). In the absence of either inhibitor, 15.3 ± 1.2 % of cells bound biotinylated CRP. (E) Biotinylated CRP (100 μg/ml) binding to live mouse CD4+CD62L+ naïve T cells is surface protein (receptor)-dependent, as the proportion of cells that bound biotinylated CRP was reduced to 2.5 ± 0.2 % and 4.3 ± 0.3 % when cells were pre-treated with PK and trypsin, respectively. Without enzyme treatment, 16.2 ± 1.5 % of control cells bound biotinylated CRP (p < 0.001; n=3).
Binding of CRP to naïve CD4+ T cells alters their Th1/Th2 responses
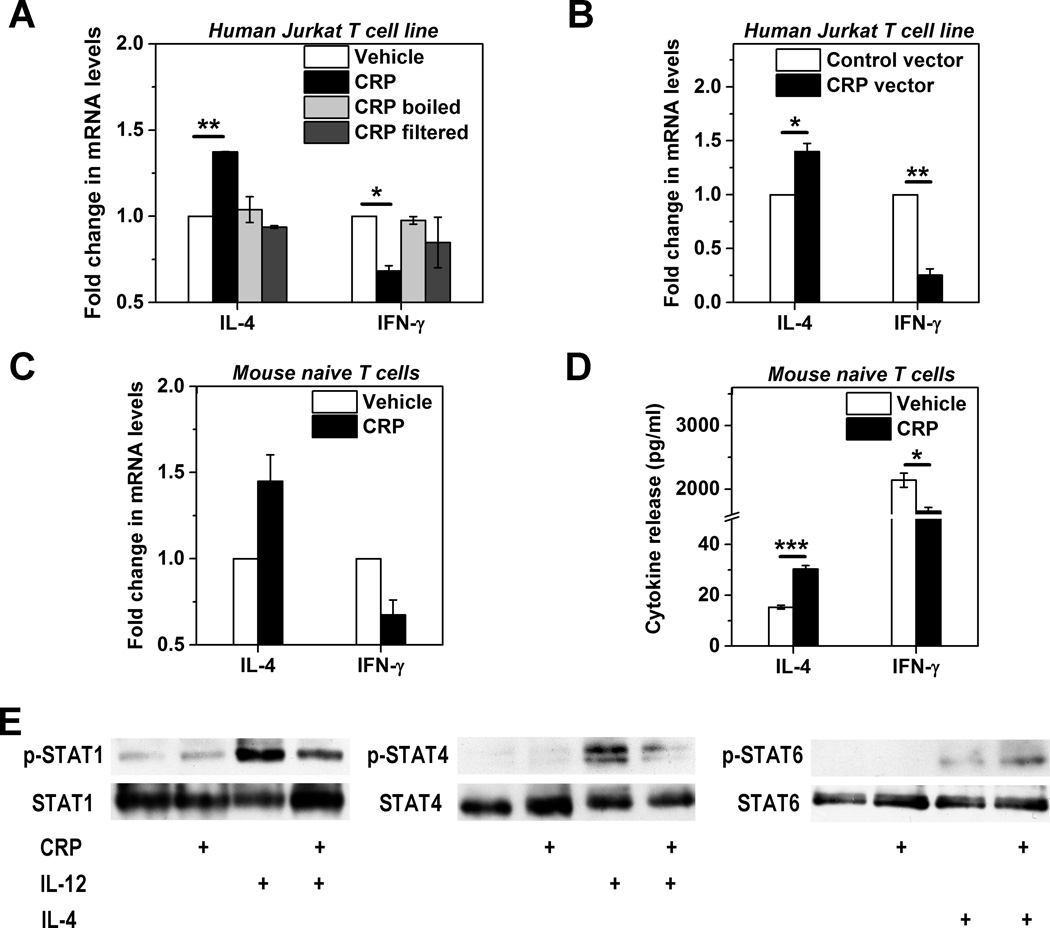
Having established that CRP binds to human Jurkat and mouse naïve CD4+ T cells, we asked if this was sufficient to drive functional consequences. The effector functions of T cells are predicated on their activation and differentiation consequent to TCR recognition of cognate antigen and co-stimulation by APCs (17, 18). To investigate the impact of CRP binding on this process we first measured expression of IL-4 and IFN-γ, two key effector T cell cytokines, produced by Jurkat T cells activated with immobilized anti-CD3 and soluble anti-CD28 mAbs – a combination that drives polyclonal T cell activation. To ensure that any effect we observed was not due to NaN3 or endotoxin contamination, we removed these from our CRP preparations by extensive dialysis, passage through Detoxi-Gel columns, and inclusion of polymyxin B to neutralize any residual endotoxin. Even after these exhaustive measures, CRP significantly enhanced IL-4 expression and inhibited IFN-γ expression by CD3/CD28-activated Jurkat cells (Figure 3A). These effects were attributable to native CRP as both boiling the protein and eliminating it by filtration through a 10 kDa cut-off membrane abrogated these actions (Figure 3A). Importantly, the IL-4 augmenting and IFN-γ suppressing effects were also recapitulated in E6-1 cells transfected with a human CRP-expression vector (Figure 3B).
Figure 3. Direct modulation of human Jurkat T cell and mouse T cell responses by human CRP.
Human Jurkat T cells (A and B) and mouse primary naïve T cells (C–E) were activated with immobilized anti-CD3 plus soluble anti-CD28 antibodies. (A) Jurkat cells co-incubated with human CRP for 3 days (100 μg/ml, black bars) expressed significantly more IL-4 mRNA and significantly less IFN-γ mRNA than cells not treated with CRP (vehicle, white bars; n=3). This effect of CRP was completely abrogated if CRP was denatured by boiling or if the CRP stock solution was filtered through a 10 kDa cut-off membrane. Polymyxin B (20 μg/ml) was included in all experiments to ensure there was no contribution made by residual endotoxin. (B) Jurkat cells transfected with a human CRP expressing vector (black bars) made significantly more IL-4 mRNA and significantly less IFN-γ mRNA than cells transfected with an empty vector (white bars; n=3). (C and D) Mouse naïve CD4+CD62L+ T cells co-incubated with human CRP for 3 days (100 μg/ml, black bars) expressed more IL-4 and less IFN-γ than cells not treated with CRP (white bars) at both mRNA (C) and protein levels (D) (n=3). (E) Mouse naïve CD4+CD62L+ T cells were activated with anti-CD3/anti-CD28 and treated with CRP, IL-4, and IL-12 alone or in combination. CRP (100 μg/ml) inhibited IL-12 induced phosphorylation of STAT1 (left blot) and STAT4 (middle blot) but enhanced IL-4 induced phosphorylation of STAT6 (right blot). * p < 0.05, ** p < 0.01, *** p < 0.001.
Next, we examined the effects of CRP on primary mouse naïve CD4+CD62L+ T cells and obtained comparable results. Thus CD3/CD28-activated primary mouse naïve T cells treated with purified human CRP expressed increased IL-4 and decreased IFN-γ at both the mRNA (Figure 3C) and protein levels (Figure 3D). In addition, IL-5 was found to be also significantly upregulated by CRP treatment (data not shown). Without CD3/CD28 activation however, naïve T cells barely secreted IL-4 and IFN-γ, regardless of the presence or absence of CRP (not shown), indicating that CRP alone is incapable of evoking effector programs in naïve T cells, which instead require antigen and co-stimulatory signals. Further experiments revealed little impact of CRP on the proliferation of CD3/CD28-activated naïve T cells (data not shown). These findings thus demonstrate that CRP is able to directly regulate the cytokine responses of activated naïve mouse CD4+ T cells.
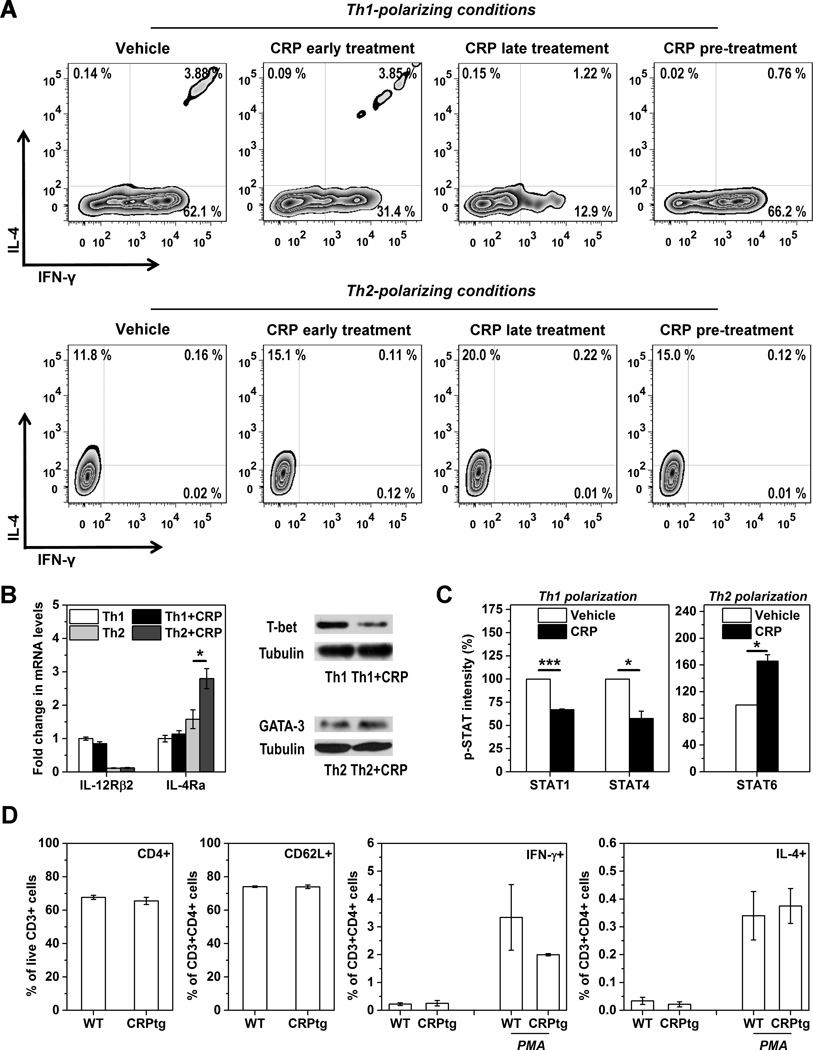
Because IFN-γ and IL-4 production is influenced by CRP and since these are Th1 and Th2 cell signature cytokines (36, 37), respectively, we reasoned that CRP binding may also influence Th1/Th2 differentiation of naïve CD4+ T cells. The fate of antigen-activated naïve T cells is dictated primarily by specific master cytokines, such as Th1-inducing IL-12 (38) and Th2-inducing IL-4 (39). Accordingly, we found that CRP treatment suppressed phosphorylation of STAT4 and STAT1 downstream of IL-12 signaling (38) whereas it augmented phosphorylation of STAT6 evoked by IL-4 (39) (Figure 3E). Moreover, the inclusion of CRP nearly halved the number of IFN-γ+IL-4− cells generated under Th1-polarizing conditions (41.2 ± 6.6 % decrease for CRP early treatment compared to vehicle controls, p<0.001; n=7) whereas it increased the number of IFN-γ−IL-4+ cells by ~40 % under Th2-polarizing conditions (40.8 ± 6.0 % increase for CRP early treatment compared to vehicle controls, p<0.001; n=7) (Figure 4A). Parallel and consistent changes were also observed in the expression of lineage-specific transcription factors (Figure 4B) and STAT phosphorylation (Figure 4C).
Figure 4. Direct modulation of Th1/Th2 differentiation of mouse primary naïve CD4+CD62L+ T cells by human CRP.
(A–C) 2 × 105 CD4+CD62L+ naïve T cells were cultured for 3 days with plate-bound anti-CD3 and fluid-phase anti-CD28 under Th1 polarizing conditions (10 ng/ml mIL-2, 20ng/ml mIL-12p70, 10 μg/ml anti-IL-4 mAb) or Th2 polarizing conditions (10 ng/ml mIL-2, 20 ng/ml mIL-4, 10 μg/ml anti-IL12 mAb, 10 μg/ml anti-IFN-γ mAb). Human CRP (100 μg/ml) was present continuously in the cell cultures starting from the beginning of polarization (early treatment) or starting 24 h after the beginning of polarization (late treatment). For pre-treatment, CRP was added to cells 24 h before the beginning of polarization but was absent thereafter. Intracellular staining for IL-4 and IFN-γ was assessed by flow cytometry. (A) CRP late treatment was most effective at inhibiting Th1 differentiation (upper panels) and promoting Th2 differentiation (lower panels) of mouse naïve T cells. (B and C) Th1 and Th2 signature cytokine receptors and transcription factor expression (B) and STAT phosphorylation levels (C) for mouse naïve T cells polarized towards Th1 or Th2 phenotypes with or without early CRP treatment. Following immunoblotting of GATA-3 or T-bet using the cell lysates, the blots were then stripped and re-probed for β-tubulin as the loading controls. Under Th1 polarizing conditions CRP significantly inhibited STAT1 and STAT4 phosphorylation, whereas under Th2 polarizing conditions CRP significantly increased IL-4Ra expression and STAT6 phosphorylation (n=3). * p < 0.05, ** p < 0.01, *** p < 0.001. (D) Mixed splenocytes were isolated from CRPtg (black bars) and wild type mice (white bars; n=4–6 mice per genotype) and live T cells identified using cell viability stain (EBiosciences) plus anti-CD45 and anti-CD3 mAbs. Amongst these cells there was no difference in the proportion of CD4+ T cells (leftmost panel) nor CD4+ CD62L+ naïve T cells (middle-left panel) in wild type versus human CRPtg mice. Four hours after activation with PMA/ionomycin (PMA) the proportion of CD3+CD4+ T cells producing IFN-γ was lower for CRPtg than wild type mice (middle-right panel), whereas the proportion producing IL-4 was not different (rightmost panel).
The results shown above demonstrate that in vitro, CRP is able to directly bind primary mouse naïve T cells and thereby regulates their effector responses. To determine if human CRP alters the Th1/Th2 balance in vivo during homeostasis, we analyzed splenocytes harvested from healthy adult wild type versus CRPtg mice (30) housed under conventional conditions. Splenocytes recovered from CRPtg versus wild type mice contained nearly equal proportions of CD3+CD4+ double-positive cells and CD3+CD4+CD62L+ triple-positive cells (Figure 4D). Importantly however, following PMA/ionomycin activation fewer T cells from CRPtg mice expressed the Th1 signature cytokine IFN-γ whereas there was no effect of genotype on the number of cells producing the Th2 signature cytokine IL-4 (Figure 4D). Although the observed difference between genotypes did not achieve statistical significance, in light of the in vitro data described above the results do support a role for CRP in the direct modulation of Th1/Th2 differentiation in vivo.
CRP-mediated suppression of the Th1 response in MOG-induced EAE
In the aforementioned in vitro studies CRP was present in cell cultures throughout the Th1/Th2 polarization process. This “early treatment” should mimic the homeostatic state. In the context of disease however, because any rise in CRP serum levels necessarily lags behind the onset of inflammation, prominent actions of CRP on T cell differentiation might be manifest at a later stage. We modeled this scenario in vitro by adding CRP to naïve T cells 24 h after the initiation of Th1 or Th2 polarization, an approach we term “late treatment”. Interestingly, we found that the effect of late treatment was more pronounced than that of early treatment, with the former leading to a maximum of ~67 % inhibition of Th1 differentiation (p<0.01; n=4) and ~71 % enhancement of Th2 differentiation (p<0.05; n=5) at the doses we tested (Figure 4A, top and bottom panels respectively, and Figure S4A). Importantly, with the late treatment regimen the effect of CRP on both Th1 and Th2 cell differentiation were dose-dependent, and an effect on Th1 polarization was obvious even with as little as 1 μg/ml CRP (Figure S4A). In stark contrast to the early and late treatment scenarios, “pre-treatment” - wherein CRP was incubated with naïve T cells for 24 h under resting conditions but was absent during the following Th1 or Th2 polarization (a non-physiological scenario) – had little effect (Figure 4A).
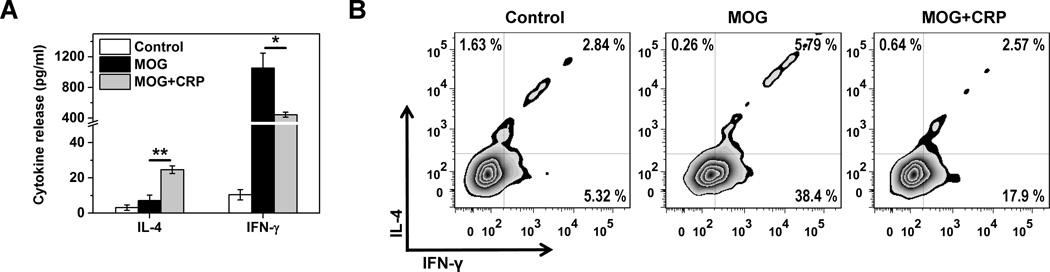
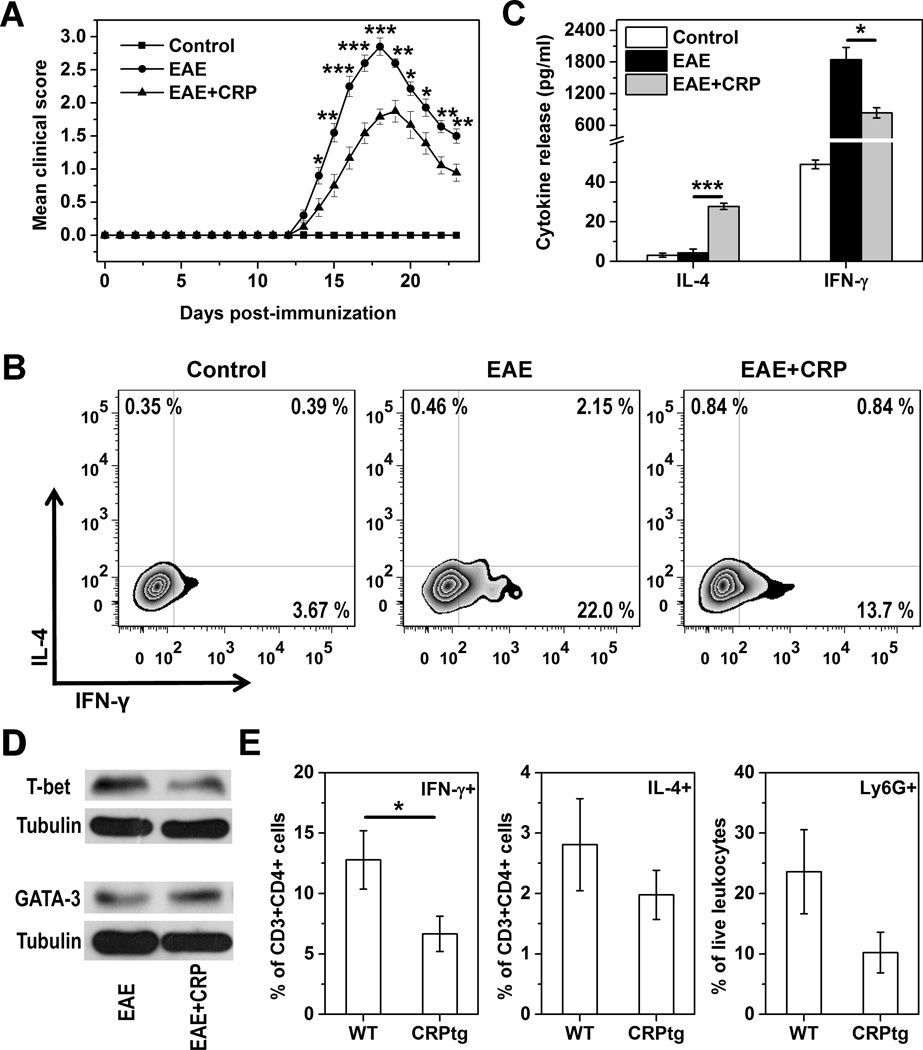
To extend our findings to an in vivo setting wherein T cells are known to be integral and wherein CRP has been shown to play a beneficial role, we immunized C57BL/6 mice with MOG peptide to induce EAE, followed 2 days later by a single s.c. injection of CRP (6, 7). Splenocytes were collected 7 days later to assess Th1/Th2 balance. We found that splenocytes from MOG immunized and CRP treated mice secreted 40 % less IFN-γ and ~5-fold more IL-4 than splenocytes from MOG immunized/vehicle treated mice (Figure 5A). Moreover, the proportion of MOG-reactive CD4+IFN-γ+ T cells recovered from the spleens was reduced by 60% for MOG immunized and CRP treated mice (p<0.001; n=3) (Figure 5B). Only a few MOG-reactive CD4+IL-4+ T cells were recovered, likely because of the strong Th1 polarizing milieu created by MOG immunization (40). Importantly, the weaker Th1 response seen early after s.c. treatment with purified human CRP was associated with a significant reduction in severity of ensuing EAE (Figure 6A). At the peak of the disease, a reduction in the proportion of MOG-reactive splenic Th1 cells (Figure 6B) and their impaired ability to secret IFN-γ upon activation ex vivo (Figure 6C) were also noted. Corroborating these results, we further found that CRP treatment decreased T-bet whereas increased GATA-3 levels in splenocytes of mice with EAE (Figure 6D). We did not directly assess Th17 cells in these experiments but since the encephalitogenicity of Th17 cells has been shown to be dependent on T-bet (41), weakening of the Th17 response might also contribute to the protection of CRP against EAE. Finally, the number of cells infiltrating the spinal cord and the extent of spinal cord demyelination were dramatically less in CRP treated mice compared to vehicle treated mice (not shown). In CRPtg mice with EAE, wherein disease is less severe compared to wild type mice (not shown and ref. 6, 7), significantly fewer CD3+CD4+IFN-γ+ T cells (p<0.05) were recovered from the spinal cord (Figure 6E). Collectively, these data suggest that the observed capacity of CRP to suppress Th1 responses in vitro is recapitulated in vivo, and that CRP’s ability to alleviate EAE likely involves inhibition of the pathological Th1 response.
Figure 5. CRP-mediated suppression of the MOG immunization induced Th1 response in mice.
Wild type mice were immunized with MOG peptide and 48 h later injected s.c. with human CRP (MOG+CRP group) or vehicle (MOG group) (n=3 mice per treatment). (A) 7 days after immunization, splenocytes were isolated and stimulated ex vivo with MOG peptide and the amount of IL-4 and IFN-γ produced measured by ELISA. The control group represent MOG-stimulated splenocytes from non-immunized mice. * p<0.05, ** p<0.01. (B) Intracellular staining for IL-4 and IFN-γ produced by CD4+ T cells isolated as in panel A.
Figure 6. CRP-mediated suppression of MOG immunization induced EAE in wild type and CRPtg mice.
EAE was induced in wild type and CRPtg mice as described in the legend for Figure 5. (A) EAE severity (mean clinical score) for wild type mice that received a single s.c. injection of either purified human CRP (EAE+CRP group) or vehicle (EAE group) 48 h after immunization with MOG peptide. Controls are non-immunized and non-treated mice. (B–D) Splenocytes were isolated from mice at the peak of EAE and stimulated ex vivo with MOG peptide. Intracellular (B) and secreted (C) cytokines produced by CD4+ T cells was then determined. (D) T-bet and GATA-3 expression in cell lysates were determined by immunoblotting using specific antibodies. The blots were then stripped and re-probed for β-tubulin as the loading controls. (E) IFN-γ+ (left panel) and IL-4+ T cells (middle panel) and Ly6G+ neutrophils (right panel) isolated from spinal cord infiltrates of wild type and CRPtg mice at the peak of EAE (n=6 mice per genotype). * p<0.05, ** p<0.01, *** p<0.001.
Discussion
CRP is an ancient soluble PRR whose emergence predates that of the adaptive immune system. Therefore, it should not be surprising if this protein is found to have an impact on adaptive immunity. Unlike the cell type-restricted expression of membrane PRRs like TLRs, the secretion of CRP by hepatocytes into the blood and lymph should, in principle, allow it direct access to a variety of leukocytes in both the circulation and in inflamed tissues. Although the direct interaction of CRP with T cells was investigated previously, these studies yielded conflicting results (22–25). Indeed, little evidence supports the expression by T cells of established CRP receptors, including FcRs (28) and LOX-1 (42). Consequently, it seems more likely that CRP modulation of T cell responsiveness is indirect and mediated by innate immune cells which do express one or more of the receptors for CRP (4). However, in the present study we provide compelling evidence that CRP in its native conformation binds directly to human Jurkat and primary mouse naïve T cells, via an as yet unidentified receptor, and thereby alters T cell differentiation. The failure to detect CRP binding to Jurkat T cells in previous work (34) might have been due to the use of an inappropriate antibody for CRP detection, the epitope of which might have been masked when CRP was bound to the cell. Indeed, we found that mAb 1D6 was much more efficient than mAb 2C10 at revealing T cell-bound CRP (see Figure 1B). Moreover, apparent binding of CRP to live Jurkat T cells has previously been noted, though it was interpreted as non-specific (43). Based on our new finding that binding of human CRP to CD4+ T cells is largely restricted to the naïve CD62L+ subset (see Figure 2B and C), we propose that the inconsistent findings reported about binding of human CRP to T cells (22–25) is most likely due to phenotypic heterogeneity of the T cells used.
Most importantly, we demonstrate herein for the first time that CRP is able to directly modulate Th1/Th2 balance, favoring the Th2 effector program while inhibiting Th1 differentiation of TCR-activated naïve T cells in vitro (see Figures 3 and 4). In contrast to the rather conditional and localized actions of membrane-bound TLR on T cells, which requires the concurrent presence of its cognate ligands (19–21), the effects of CRP are likely to be tonic and global in vivo because CRP circulates in the blood and lymph and because its interaction with naïve T cells does not require additional ligand. Of course CRP undoubtedly can also regulate T cell responses indirectly, via its actions on FcR expressing APCs for example. Indeed, CRP has been shown to promote DC maturation (44), to enhance the uptake of antigens by DCs (45), and to induce cytokine release from monocytes or macrophages (46, 47). Nevertheless, our results show that accessory cells are not an essential requirement. As such, both direct and indirect actions of CRP on T cells could ultimately lead to the suppression of Th1 responses in health and disease. Consistent with this notion, we provide preliminary evidence that the proportion of Th1 cells in healthy CRPtg mice is lower than that in wild type mice (see Figure 4D) and that a reduction of pathological Th1 responses in mice with EAE can be achieved by CRP injection or by its transgenic expression (see Figures 5 and 6). Notably, transgenic expression of CRP has also been shown to be protective in mouse models of other autoimmune diseases wherein Th1 cells play a detrimental role (12–14), including spontaneous lupus (5) and collagen-induced arthritis (CIA) (8). In addition, the prolonged effect of a single dose of administered CRP observed in this study and previous ones (6, 48) strongly suggests that CRP is able to prominently and permanently remodel the adaptive immune response. A direct action of CRP on naïve T cells may be the explanation that ties these observations together, since CRP treatment 2 or 9 days following disease induction is still very effective (see Figures 5 and 6 and refs. 6 and 44). Notably, in preliminary experiments we also observed a moderate influence of CRP on the response of already differentiated Th1 and Th2 cells (data not shown). Given the recognized importance of Th17 cells in EAE as well as in other autoimmune diseases, future studies will be needed to determine if CRP influences the generation of Th17 cells.
CRP is not the panacea for all T cell mediated diseases. In fact, under certain circumstances CRP’s ability to modulate T effector cell balance might prove to be detrimental. For example, in asthma and cancer, CRP-mediated augmentation of Th2 responses would be predicted to promote disease (15, 49). Notwithstanding these caveats, there is growing evidence that CRP is a tonic regulator of the adaptive immune response and this will need to be considered if either purified CRP, or drugs designed to modulate the level of CRP (50, 51), are to be used in patients. In conclusion, CRP can directly affect both innate and adaptive immunity and these capacities are fine-tuned by the protein’s conformation (2, 32, 52, 53), location (54), and concentration (1, 4). This diversity of traits though no doubt accounting for much of the seemingly contradictory effects of CRP reported in the literature, underlies the role of CRP as a central node in the regulatory network of inflammation and immunity (9, 10).
Supplementary Material
Acknowledgements
We thank Ms. Liang Peng, Ms. Li Xie and Mr. Jing Zhao for their excellent technical assistance.
This work was supported by grants from the Ministry of Science and Technology of China [grant number 2011CB910500]; the National Natural Science Foundation of China [grant numbers, 31470718, 31222015, 31270813, 31170696, 30930024]; the Ministry of Education of China [grant numbers PCSIRT: IRT1137]; and the National Institutes of Health [grant numbers F31NS081903 and R01DK099092].
Footnotes
Disclosures. The authors have no conflicts of interest to declare.
References
- 1.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma X, Ji SR, Wu Y. Regulated conformation changes in C-reactive protein orchestrate its role in atherogenesis. Chinese Sci Bull. 2013;58:1642–1649. [Google Scholar]
- 3.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 4.Du Clos TW. Pentraxins: structure, function, and role in inflammation. ISRN Inflamm. 2013;2013:379040. doi: 10.1155/2013/379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE. Delayed lupus onset in (NZB × NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 6.Hu XZ, Wright TT, Jones NR, Ramos TN, Skibinski GA, McCrory MA, Barnum SR, Szalai AJ. Inhibition of Experimental Autoimmune Encephalomyelitis in Human C-Reactive Protein Transgenic Mice Is FcgammaRIIB Dependent. Autoimmune Dis. 2011;2011:484936. doi: 10.4061/2011/484936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 8.Jones NR, Pegues MA, McCrory MA, Kerr SW, Jiang H, Sellati R, Berger V, Villalona J, Parikh R, McFarland M, Pantages L, Madwed JB, Szalai AJ. Collagen-induced arthritis is exacerbated in C-reactive protein-deficient mice. Arthritis Rheum. 2011;63:2641–2650. doi: 10.1002/art.30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang MY, Zhou HH, Zhang SC, Hui F, Zhu W, Su HX, Guo HY, Li XW, Ji SR, Wu Y. Recurrent mutations at C-reactive protein gene promoter SNP position-286 in human cancers. Cell Res. 2014;24:505–508. doi: 10.1038/cr.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su HX, Zhou HH, Wang MY, Cheng J, Zhang SC, Hui F, Chen XZ, Liu SH, Liu QJ, Zhu ZJ, Hu QR, Wu Y, Ji SR. Mutations of C-reactive protein (CRP)-286 SNP, APC and p53 in colorectal cancer: implication for a CRP-Wnt crosstalk. PLoS One. 2014;9:e102418. doi: 10.1371/journal.pone.0102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stohl W. Future prospects in biologic therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:705–720. doi: 10.1038/nrrheum.2013.136. [DOI] [PubMed] [Google Scholar]
- 14.Alzabin S, Williams RO. Effector T cells in rheumatoid arthritis: lessons from animal models. FEBS Lett. 2011;585:3649–3659. doi: 10.1016/j.febslet.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 20.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croft SM, Mortensen RF, Gewurz H. Binding of C-reactive protein to antigen-induced but not mitogen-induced T lymphoblasts. Science. 1976;193:685–687. doi: 10.1126/science.1085034. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen RF, Gewurz H. Effects of C-reactive protein on the lymphoid system. II. Inhibition of mixed lymphocyte reactivity and generation of cytotoxic lymphocytes. J Immunol. 1976;116:1244–1250. [PubMed] [Google Scholar]
- 24.Mortensen RF, Osmand AP, Gewurz H. Effects on C-reactive protein on the lymphoid system. I. Binding to thymus-dependent lymphocytes and alteration of their functions. J Exp Med. 1975;141:821–839. [PMC free article] [PubMed] [Google Scholar]
- 25.James K, Hansen B, Gewurz H. Binding of C-reactive protein to human lymphocytes. I. Requirement for a binding specificity. J Immunol. 1981;127:2539–2544. [PubMed] [Google Scholar]
- 26.Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW. C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol. 1995;155:2185–2193. [PubMed] [Google Scholar]
- 27.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 29.Fujita Y, Kakino A, Nishimichi N, Yamaguchi S, Sato Y, Machida S, Cominacini L, Delneste Y, Matsuda H, Sawamura T. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin Chem. 2009;55:285–294. doi: 10.1373/clinchem.2008.119750. [DOI] [PubMed] [Google Scholar]
- 30.Ciliberto G, Arcone R, Wagner EF, Ruther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J. 1987;6:4017–4022. doi: 10.1002/j.1460-2075.1987.tb02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor KE, van den Berg CW. Structural and functional comparison of native pentameric, denatured monomeric and biotinylated C-reactive protein. Immunology. 2007;120:404–411. doi: 10.1111/j.1365-2567.2006.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL, Lu W, Zhao J. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP(m) FASEB J. 2007;21:284–294. doi: 10.1096/fj.06-6722com. [DOI] [PubMed] [Google Scholar]
- 33.Ying SC, Gewurz H, Kinoshita CM, Potempa LA, Siegel JN. Identification and partial characterization of multiple native and neoantigenic epitopes of human C-reactive protein by using monoclonal antibodies. J Immunol. 1989;143:221–228. [PubMed] [Google Scholar]
- 34.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriwaki H, Kume N, Sawamura T, Aoyama T, Hoshikawa H, Ochi H, Nishi E, Masaki T, Kita T. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler Thromb Vasc Biol. 1998;18:1541–1547. doi: 10.1161/01.atv.18.10.1541. [DOI] [PubMed] [Google Scholar]
- 36.Smeets RL, Fleuren WW, He X, Vink PM, Wijnands F, Gorecka M, Klop H, Bauerschmidt S, Garritsen A, Koenen HJ, Joosten I, Boots AM, Alkema W. Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28-mediated signaling. BMC Immunol. 2012;13:12. doi: 10.1186/1471-2172-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawashima H, Fukuda M. Sulfated glycans control lymphocyte homing. Ann N Y Acad Sci. 2012;1253:112–121. doi: 10.1111/j.1749-6632.2011.06356.x. [DOI] [PubMed] [Google Scholar]
- 38.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 39.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Chen B, Song JH, Zhen T, Wang BY, Li X, Liu P, Yang X, Zhang QL, Xi XD, Chen SD, Zuo JP, Chen Z, Chen SJ. Eriocalyxin B ameliorates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells. Proc Natl Acad Sci U S A. 2013;110:2258–2263. doi: 10.1073/pnas.1222426110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, O'Garra A, Zurawski G, Banchereau J, Oh S. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart SP, Alexander KM, MacCall SM, Dransfield I. C-reactive protein does not opsonize early apoptotic human neutrophils, but binds only membrane-permeable late apoptotic cells and has no effect on their phagocytosis by macrophages. J Inflamm (Lond) 2005;2:5. doi: 10.1186/1476-9255-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Vre EA, Bult H, Hoymans VY, Van Tendeloo VF, Vrints CJ, Bosmans JM. Human C-reactive protein activates monocyte-derived dendritic cells and induces dendritic cell-mediated T-cell activation. Arterioscler Thromb Vasc Biol. 2008;28:511–518. doi: 10.1161/ATVBAHA.107.157016. [DOI] [PubMed] [Google Scholar]
- 45.Thomas-Rudolph D, Du Clos TW, Snapper CM, Mold C. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc gamma R on dendritic cells. J Immunol. 2007;178:7283–7291. doi: 10.4049/jimmunol.178.11.7283. [DOI] [PubMed] [Google Scholar]
- 46.Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc gamma R. J Immunol. 2002;169:7019–7025. doi: 10.4049/jimmunol.169.12.7019. [DOI] [PubMed] [Google Scholar]
- 47.Mold C, Du Clos TW. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J Immunol. 2006;176:7598–7604. doi: 10.4049/jimmunol.176.12.7598. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez W, Mold C, Kataranovski M, Hutt J, Marnell LL, Du Clos TW. Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum. 2005;52:642–650. doi: 10.1002/art.20846. [DOI] [PubMed] [Google Scholar]
- 49.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones NR, Pegues MA, McCrory MA, Singleton W, Bethune C, Baker BF, Norris DA, Crooke RM, Graham MJ, Szalai AJ. A Selective Inhibitor of Human C-reactive Protein Translation Is Efficacious In Vitro and in C-reactive Protein Transgenic Mice and Humans. Mol Ther Nucleic Acids. 2012;1:e52. doi: 10.1038/mtna.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, Hawkins PN, Pepys MB. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res. 2014;114:672–676. doi: 10.1161/CIRCRESAHA.114.302770. [DOI] [PubMed] [Google Scholar]
- 52.Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 2009;8:3885–3892. doi: 10.4161/cc.8.23.10068. [DOI] [PubMed] [Google Scholar]
- 53.Wang MY, Ji SR, Bai CJ, El Kebir D, Li HY, Shi JM, Zhu W, Costantino S, Zhou HH, Potempa LA, Zhao J, Filep JG, Wu Y. A redox switch in C-reactive protein modulates activation of endothelial cells. FASEB J. 2011;25:3186–3196. doi: 10.1096/fj.11-182741. [DOI] [PubMed] [Google Scholar]
- 54.Li HY, Wang J, Wu YX, Zhang L, Liu ZP, Filep JG, Potempa LA, Wu Y, Ji SR. Topological localization of monomeric C-reactive protein determines pro-inflammatory endothelial cell responses. J Biol Chem. 2014;289:14283–14290. doi: 10.1074/jbc.M114.555318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.