Abstract
Unlike chemotherapy drugs, the safety of natural compounds such as curcumin has been well established. However, the potential use of curcumin in cancer has been compromised by its low bioavailability, limited tissue distribution and rapid biotransformation leading to low in vivo efficacy. To circumvent these problems, more potent and bioavailable analogs have been synthesized. In the current study, we investigated the mechanism of anti-tumor effect of one such analog, FLLL12, in lung cancers. IC50 values measured by sulforhodamine B (SRB) assay at 72 h and apoptosis assays (annexin V staining, cleavage of PARP and caspase-3) suggest that FLLL12 is 5–10-fold more potent than curcumin against a panel of premalignant and malignant lung cancer cell lines, depending on the cell line. Moreover, FLLL12 induced the expression of death receptor-5 (DR5). Ablation of the expression of the components of the extrinsic apoptotic pathway (DR5, caspase-8 and Bid) by siRNA significantly protected cells from FLLL12-induced apoptosis (p < 0.05). Analysis of mRNA expression revealed that FLLL-12 had no significant effect on the expression of DR5 mRNA expression. Interestingly, inhibition of global phosphatase activity as well as protein tyrosine phosphatases (PTPs), but not of alkaline phosphatases, strongly inhibited DR5 expression and significantly inhibited apoptosis (p < 0.05), suggesting the involvement of PTPs in the regulation of DR5 expression and apoptosis. We further showed that the apoptosis is independent of p53 and p73. Taken together, our results strongly suggest that FLLL12 induces apoptosis of lung cancer cell lines by post-transcriptional regulation of DR5 through activation of protein tyrosine phosphatase(s).
Keywords: Apoptosis, FLLL12, Lung cancer, Death receptor-5, Protein tyrosine phosphatases (PTPs)
Introduction
Lung cancer represents ~13% of all cancers with approximately 224,000 new cases and 159,000 deaths in the United States in 2014 [1]. Lung cancer remains the top leading cause of cancer related mortality because of low survival rate and high prevalence of advanced stage disease at diagnosis [2]. Extensive worldwide epidemiological data have established cigarette smoking (direct as well as second hand) as the key cause of lung cancers [3,4]. Occupational exposures to environmental pollutants are other causes of lung cancers. Non-small cell lung cancer (NSCLC) is the most prevalent subtype and accounts for 85% of all lung cancer cases, and is relatively resistant to chemotherapy and radiation. The treatment of lung cancer still remains a challenge due to lack of effective screening tools for early detection, the presence of smoking-related comorbid illnesses, and the inherent molecular heterogeneity [5]. Despite advances in conventional surgical procedures, radiotherapy, and chemotherapy, the 5 year survival rate for lung cancer remains almost unchanged at ~15% [6]. Moreover, radiation and chemotherapy are frequently associated with serious side effects including pneumonitis and/or long-term fibrosis, renal, otologic, and bone marrow-suppressive sequelae [7]. Thus, there is an increasing emphasis on strategies to maximize tumor control, prolong overall survival, minimize chemotherapy side effects, and improve quality of life. As a result, there has been continued investigation into potential alternative and less toxic therapies for lung cancer, with the aim of achieving a more favorable clinical outcome while reducing treatment morbidity.
The safety of natural dietary agents present in fruits, vegetables and spices has been established through years of human consumption and these agents have drawn increasing attention when designing anti-cancer drugs [8]. Curcumin, the most abundant component of the spice turmeric and isolated from the rhizome of the Indian medicinal plant Curcuma longa, is one such compound extensively investigated for its potential anti-cancer effects [7,9,10]. Accumulated evidence suggests that curcumin affects a variety of biological pathways involved in apoptosis, tumor proliferation, chemo- and radiotherapy sensitization, tumor invasion, and metastases [7,9,11,12]. However, low bioavailability, limited tissue distribution, rapid metabolism and subsequent excretion from the body limit the potential use of curcumin in cancers [13]. To circumvent these problems, numerous analogs of curcumin have been synthesized. For example, the potential activity of recently synthesized curcumin analogs (2E,6E)-2,6-bis(thiophen-3-methylene) cyclohexanone (AS), (3E,5E)-3,5-bis(thiophen-3-methylene)-tetrahydrothiopyran-4-one (FS), A501, and GO-Y031 has been studied in prostate cancers, NSCLC cells and gastric cancers [14–16].
Successful elimination of cancer cells from the body depends on the induction of cell death. Apoptosis is the major form of cell death and serves as one of the most powerful tools in the development of cancer chemotherapeutics. Defects in apoptosis are implicated in both tumorigenesis and drug resistance, and are the major causes of treatment failures [17]. Apoptosis can be initiated by two major pathways: either through mitochondria (intrinsic pathway) or through death receptors located in the cell surface (extrinsic pathway). In the case of extrinsic apoptosis, activation of death receptors via ligand binding or drugs initiates the formation of the death inducing signaling complex (DISC). DISC formation involves recruitment of the adaptor protein FADD to the receptor via the death domain (DD) and the inactive pro-caspases 8 and 10. This facilitates the activation and self-processing of caspases 8 and 10, leading to their release into the cytoplasm, where they activate effector caspases 3 and 7, leading to the induction of apoptosis [18]. Activated caspase-8 can also cleave the Bcl-2-family member Bid, which links the extrinsic pathway to the intrinsic pathway. Death receptor 5 (DR5) is a tumor necrosis factor related apoptosis inducing ligand (TRAIL) receptor that activates apoptosis via the extrinsic apoptosis pathway [19]. Recent studies have shown that cancer cells resistant to TRAIL can be sensitized by co-treatment with classical chemotherapeutic drugs. This synergism is partially due to the ability of chemotherapeutic drugs to induce DR5 expression [20–22]. Hence, targeting the expression of DR5 and induction of apoptosis by anti-cancer drugs has drawn special attention for cancer therapy.
In the current study, we investigated the mechanism of FLLL12-induced apoptosis in lung cancer cell lines. FLLL12 is a potent synthetic curcumin analog [23]. The anti-cancer efficacy of FLLL12 has been tested against breast, prostate, colorectal and pancreatic cancers [23–25], and shown to be ~10-fold more potent than natural curcumin and to possess selective activity against cancer cells. FLLL12 induces apoptosis of these cancer cells by inhibition of two major survival pathways, AKT and STAT3. However, the detailed mechanism underlying FLLL12-induced apoptosis is not fully understood. Moreover, FLLL12 has never been tested against lung cancer cells. In the present study, for the first time, we investigated the anti-tumor effects of FLLL12 in lung cancers and showed that depending on the cell line, FLLL12 is 5–10-fold more potent than curcumin and induces apoptosis. Moreover, we further explored the fact that FLLL12, but not curcumin, induces DR5-mediated apoptosis in lung cancer cell lines.
Materials and methods
Cell lines
Human lung cancer cell lines used in this study were obtained from Dr. Sun's laboratory (Emory University). All cell lines were authenticated through genotyping and maintained in RPMI medium supplemented with 5% heat-inactivated fetal bovine serum in a 37 °C and 5% CO2 humidified incubator. Generation and maintenance of the premalignant cell lines 1799 and 1198 were described elsewhere [26].
Reagents
Curcumin was purchased from Sigma Chemical (St. Louis, MO, USA) and FLLL12 was obtained from Dr. James R. Fuchs laboratory (Ohio State University, Columbus, USA). FLLL12 and curcumin were dissolved in DMSO and preserved as stock solutions for in vitro studies. During experiments, the reagents were further diluted directly in a cell culture dish with RPMI medium. The final concentration of DMSO was <0.1%.
Measurement of IC50
Appropriate numbers of cells were seeded with 100 μL medium in 96 well culture plates and incubated overnight before treatment with FLLL12 or curcumin. Then, cells were treated with various concentrations of FLLL12 or curcumin and incubated for 72 h. Inhibition of cell growth was determined by SRB assay as described elsewhere [27]. IC50 value was calculated by using CalcuSyn software (Biosoft, UK).
Western blot analysis
Whole cell lysates were extracted from cells using lysis buffer. The protein concentration of each sample was determined by protein assay kit (Bio-Rad, CA, USA). Equal amounts of protein (20 μg) from each sample were separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, USA) and incubated with appropriately diluted specific primary antibodies. Mouse anti-β-actin (Sigma) or rabbit anti-GAPDH (Trevigen, MD, USA) antibody was used as a sample loading control. Immunostained protein bands were detected with an enhanced chemiluminescence kit (Pierce, IL, USA).
Annexin V-phycoerythrin staining for apoptosis
Lung cancer cells were treated with different concentrations of FLLL12 and curcumin for the indicated time, then trypsinized and washed in cold 1× PBS. The cells were then resuspended in 1× Annexin V binding buffer (BD PharMingen), and stained with Annexin V-phycoerythrin (Annexin V-PE; BD PharMingen) and 7-AAD (BD PharMingen) for 15 min at room temperature. The stained samples were measured using a fluorescence-activated cell sorting caliber bench-top flow cytometer (Becton Dickinson). FlowJo software (Tree Star) was used for apoptosis analysis. Total apoptosis was considered the sum of early and late stage apoptoses.
Caspase-8 activity assay
Caspase-8 activity was determined by Caspase-Glo 8 Assay kit (Promega, WI, USA) according to the manufacturer's protocol. Briefly, 5 × 103 cells were seeded in a 96 well culture plate and incubated for 24 h before FLLL12 treatment. After treatment of cells with FLLL12 for 24 h, cells were normalized at room temperature for ~10 min and then incubated with 100 μL of caspase-Glo 8 reagent for 2 h. Luminescence of each sample was detected by SynergyMx multi-mode plate reader (BioTech, VT, USA). Luminescence reading of each sample is considered as proportional of caspase-8 activity. Triplicate wells were used for untreated (NT) and FLLL12 treated group.
Reverse transcription (RT) PCR analysis
Total RNA was extracted from cells using RNeasy mini kit (Qiagen, Valencia, CA, USA). A total of 2 μg of RNA was reverse transcribed to cDNA using a cDNA synthesis kit (Bio-Rad, CA, USA) according to the manufacturer's protocol. Synthesized single stand cDNA was amplified by RT-PCR with the following primer pairs: DR5 forward, 5′-AAGACCCTTGTGCTCGTTGT-3′ and reverse, 5′-GACACATTCGATGTCACTCCA-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-TGCACCACCAACTGCTTA-3′ and reverse, 5′-GGATGCAGGGATGATGTTC-3′. All samples were denatured at 94 °C for 5 min and the PCR conditions were optimized for each primer set: DR5, 35 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 35 cycles of 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min. After optimized PCR conditions, the final extensions were performed at 72 °C for 5 min. PCR products were analyzed by electrophoresis on 1% agarose gel and expression levels of each molecule were normalized to GAPDH levels within the same sample.
siRNA transfection
DR5, caspase-8, and Bid specific siRNA were purchased from Qiagen (Valencia, CA, USA) and non-targeting control siRNA was obtained from Dharmacon (Chicago, IL, USA). Cells were seeded in 6-cm plates 24 h before transfection in medium containing 5% FBS, so that they reached 30%–50% confluency. siRNA was complexed with Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer's instructions and applied to each plate. Transfection media was removed and replaced with fresh media after 6 h of transfection. Knockdown efficiency of each target gene was evaluated by Western blotting after 48 h of transfection (Supplementary Fig. S1).
Plasmid transfection
Dominant negative p73 (amino acids 327–636) plasmid was obtained from David Boothman (University of Texas Southwestern, Dallas, TX). Generation and validation of the plasmid was described elsewhere [28]. Lung cancer cells were transfected with plasmid using the Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's instructions. Individual clones were selected by using G418 and validated elsewhere [29]. Stable knockdown of p53 in the H292 cell line was described elsewhere [30].
Statistical analysis
Experimental values are represented as mean ± standard deviation in triplicate. The significance of differences was determined by the Student's t-test. A value of p < 0.05 was considered statistically significant.
Results
FLLL12 is 5–10-fold more potent than curcumin and induces apoptosis
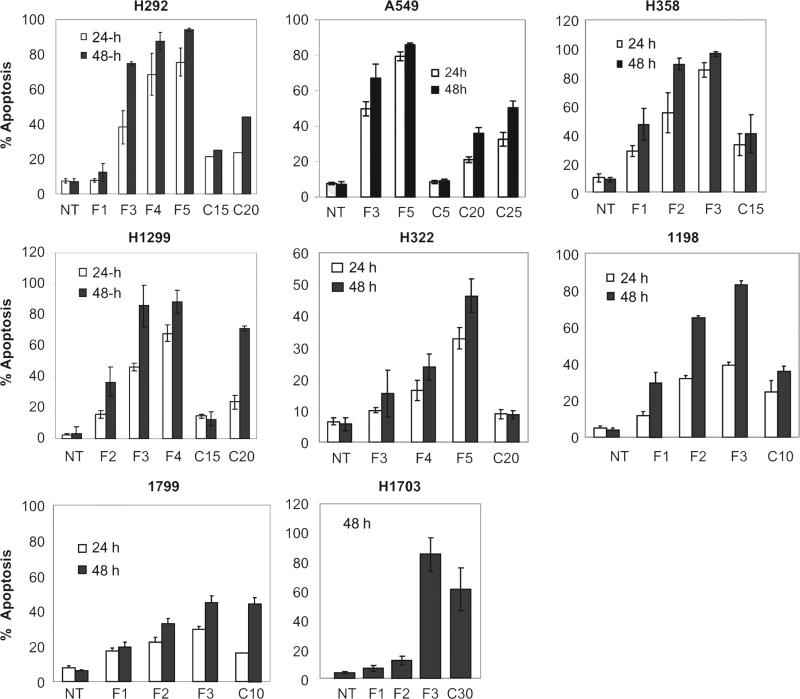
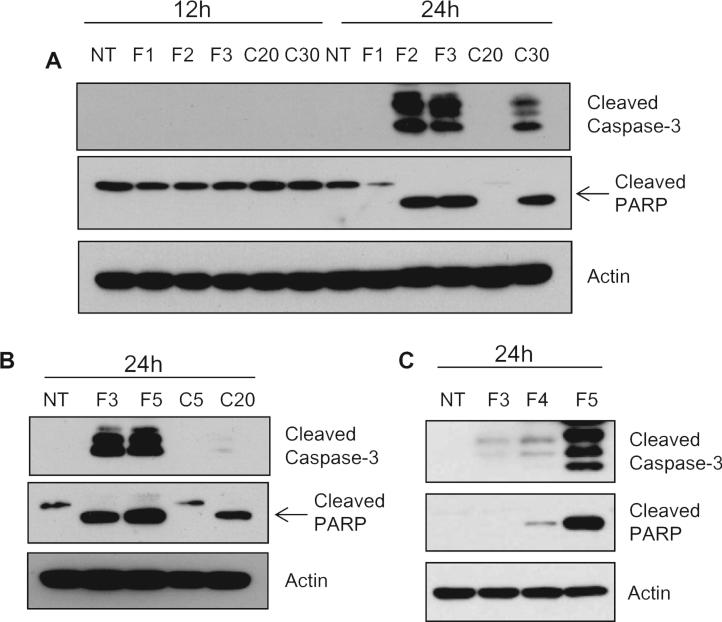
Although the antitumor effects of FLLL12 have been investigated in prostate, breast, pancreatic and colon cancer cell lines and compared with those of curcumin [23–25], the agent has never been evaluated in lung cancer cell lines. In order to explore the mechanism of anti-tumor effect of FLLL12 in lung cancers, we first assessed the sensitivity of different premalignant and malignant lung cancer cell lines to FLLL12 versus curcumin by comparing IC50 values measured using SRB assays at 72 h. As shown in Table 1, depending on the cell line, the IC50 values of FLLL12 ranged from 0.63 to 1.87 μM, compared with 6.07–12.4 μM for curcumin. These results suggest that FLLL12 is 5–10-fold more potent than curcumin, depending on the cell line. Since induction of apoptosis is critical for effective tumor regression, we next measured apoptosis by annexin V staining. As shown in Fig. 1, FLLL12 dose- and time-dependently induced apoptosis in lung cancer cell lines. While 3–5 μM of FLLL12 was sufficient to induce ~80% apoptosis by 48 h, 15–30 μM of curcumin was required to induce comparable apoptosis. These results further confirm that FLLL12 is 5–10-fold more potent than curcumin against lung cancer cell lines. Finally, to confirm apoptosis induction by FLLL12, we assessed the cleavage of caspase-3 and PARP, which is considered a marker of apoptosis in response to apoptotic signal. Treatment of cells with FLLL12 markedly induced the cleavage of caspase-3 and PARP in different lung cancer cell lines (Fig. 2).
Table 1.
IC50 of FLLL12 and curcumin. All cell lines were seeded in 96-well culture plates and treated with different concentrations of FLLL12 and curcumin for 72 h. Determination of cell growth inhibition and calculation of IC50 values are described in experimental procedures.
| Cell lines | IC50 (μM) |
|
|---|---|---|
| FLLL12 | Curcumin | |
| H1299 | 0.78 | 6.07 |
| A549 | 1.61 | 9.2 |
| H358 | 0.80 | 6.21 |
| H292 | 1.67 | 12.4 |
| H322 | 1.55 | 11.7 |
| H1703 | 1.87 | 10.5 |
| 1198 | 0.75 | 7.25 |
| 1799 | 0.63 | 6.07 |
Fig. 1.
FLLL12 dose- and time-dependently induces apoptosis in lung cancer cells. Human premalignant and malignant lung cancer cell lines were treated with different concentrations of FLLL12 and curcumin for the indicated time points. Apoptosis was quantified by Annexin V-PE staining. NT is no treatment, F denotes FLLL12 and C denotes curcumin throughout the manuscript. Numbers beside F and C indicate the concentration (μM) of FLLL12 and curcumin, respectively.
Fig. 2.
Cleavage of caspase-3 and PARP by FLLL12 and curcumin. (A) H1299, (B) H292 and (C) A549 lung cancer cells were treated with the indicated doses of FLLL12 and curcumin and the cleavage of caspase-3 and PARP were determined by Western blotting.
FLLL12-induced apoptosis is independent of p53 and p73 activation
The p53 family consists of three structurally and functionally closely related proteins, p53, p63 and p73, functionally classified as transcription factors and tumor suppressors, which play critical roles in apoptosis [31]. Curcumin is known to induce p53-dependent apoptosis in various cancer cell lines [32–34]. Our unpublished data have also shown that curcumin induces p73-dependent apoptosis in the lung cancer cell line H1299. In this study, we also found that FLLL12 increased the expression of p73 in the H1299 lung cancer cell line (Supplementary Fig. S2A). To investigate the role of p53 and p73 in FLLL12-induced apoptosis, we ablated expression of p53 in H292 cells expressing wild type p53 and inactivated p73 in H1299 cells by overexpressing a dominant negative p73 and measured apoptosis after FLLL12 treatment. As shown in Supplementary Fig. S2B and C, FLLL12 markedly inhibited cell survival and induced apoptosis, and abrogation of p53 or inactivation of p73 could not protect cells from FLLL12-induced growth inhibition and apoptosis suggesting that apoptosis of lung cancer cells by FLLL12 is independent of p53 and p73.
FLLL12, but not curcumin, induces DR5-mediated apoptosis
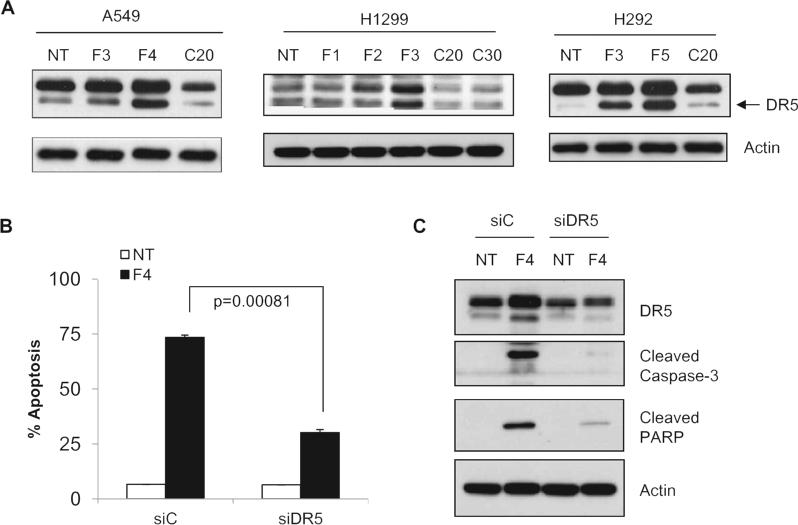
Cancer cells can undergo apoptosis by activating extrinsic pathways mediated through death receptors or intrinsic pathways mediated through mitochondrial depolarization, respectively. The extrinsic pathway can be linked to intrinsic pathways through truncation of Bid by activated caspase-8. To understand the molecular mechanisms underlying FLLL12-induced apoptosis, we examined the expression of DR5 after FLLL12 and curcumin treatment in three different lung cancer cell lines. As shown in Fig. 3A, treatment of cells with FLLL12 markedly enhanced the expression of DR5. On the other hand, treatment of cells with the concentration of curcumin that induced comparable apoptosis had no effect on the expression of DR5. To confirm the role of DR5 in FLLL12-induced apoptosis, the expression of DR5 was knocked down by siRNA. Ablation of DR5 significantly protected cells from apoptosis induced by FLLL12 (Fig. 3B) and almost completely abrogated the cleavage of caspase-3 and PARP (Fig. 3C), suggesting that the expression of DR5 is required for FLLL12-induced apoptosis.
Fig. 3.
FLLL12 induces DR5-mediated apoptosis. (A) A549, H1299 and H292 cells were treated with FLLL12 and curcumin for 24 h and the expression of DR5 protein was determined by Western blotting. (B) A549 cells were transfected with control (siC) and DR5 (siDR5) siRNA as described in the Materials and methods and apoptosis was quantified after 24 h of FLLL12 treatment. (C) A549 cells were transfected and treated with FLLL12 as in (B). Expression of DR5, cleaved caspase-3 and PARP were analyzed by Western blotting.
Caspase-8 is required for FLLL12 induced apoptosis
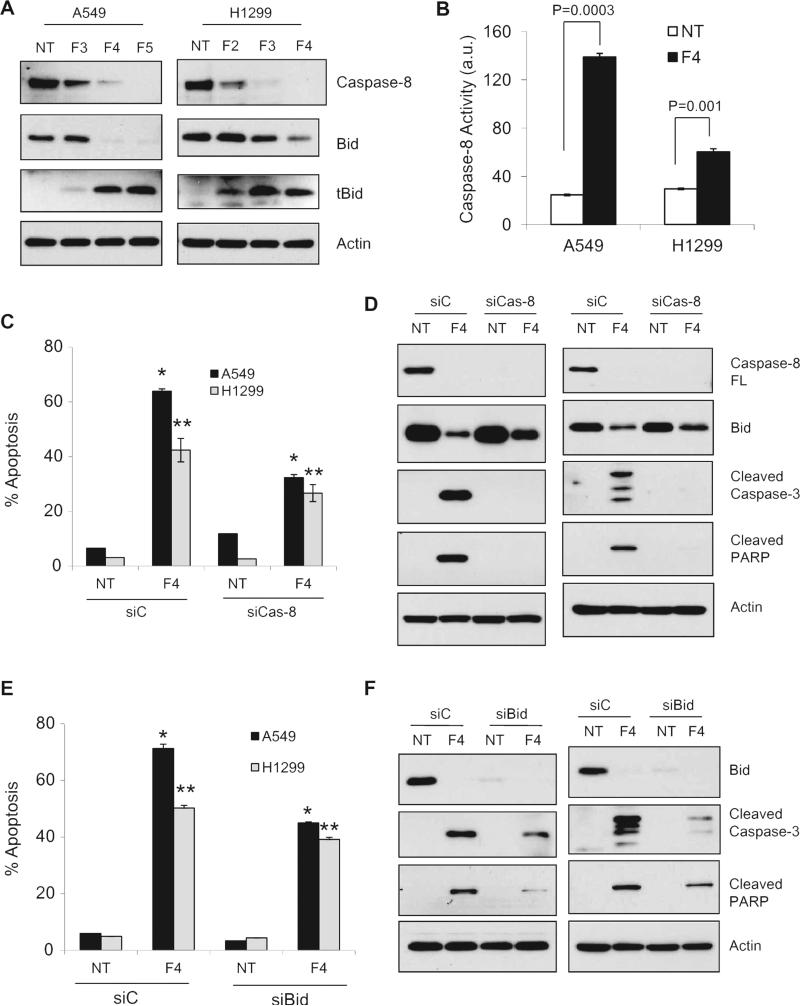
DR5-mediated apoptosis initiates with the formation of DISC containing FADD and caspase-8 followed by activation of caspase-8. To further explore DR5-mediated apoptosis by FLLL12, we examined caspase-8 activation and cleavage of Bid. FLLL12 dose dependently induced cleavage/activation of caspase-8 and truncation of Bid to tBid as evidenced by the reduction of full length caspase-8 and Bid (Fig. 4A), and significantly increased the caspase-8 activity and expression of tBid (Fig. 4A and B). These results suggest that FLLL12 activated caspase-8 and caused truncation of Bid to tBid. The expression of caspase-8 was knocked down by siRNA and apoptosis as well as apoptotic markers were examined. As shown in Fig. 4C and D, knockdown of caspase-8 significantly inhibited FLLL12 induced apoptosis (Fig. 4C) and strongly reduced FLLL12-induced cleavage of caspase-3 and PARP (Fig. 4D). These results demonstrate that FLLL2-induced expression of DR5 and activation of caspase-8 are important for apoptosis. During death receptor-mediated apoptosis, activated caspase-8 can directly activate effector caspase-3 and -7 or cleave Bid to generate a truncated form, tBid. tBid migrates to mitochondria where it induces permeabilization of the outer mitochondrial membrane and links extrinsic apoptosis to intrinsic apoptosis [35,36]. FLLL-12 treatment also caused truncation of Bid to tBid and knockdown of caspase-8 markedly rescued the expression of Bid (Fig. 4A). To investigate the role of Bid in FLLL12-induced apoptosis downstream of DR5 and caspase-8, we analyzed apoptosis and cleavage of caspase-3 and PARP after knocking down Bid. Although knockdown of Bid significantly protected cells from FLLL12-induced apoptosis (Fig. 4E) and reduced the cleavage of caspase-3 and PARP (Fig. 4F), the protection was not as dramatic as after knockdown of DR5 and caspase-8. Together, the results presented in Figs. 3 and 4 suggest that FLLL12 induces apoptosis in lung cancer cells through activation of DR5-mediated caspase-8 pathways involving truncation of Bid and activation of caspase-3.
Fig. 4.
Caspase-8 is required for FLLL12-induced apoptosis. (A) A549 and H1299 cells were treated with FLLL12 for 24 h and expression of caspase-8, Bid and tBid were analyzed. (B) A549 and H1299 cells were treated with FLLL12 for 24 h and caspase-8 activity was determined by Caspase Glo 8 kit. (C) Expression of caspase-8 was ablated in A549 and H1299 cells by siRNA and apoptosis was quantified after treatment with FLLL12 for 24 h. (D) A549 and H1299 cells with ablated caspase-8 were treated as in (C) and expression of caspase-8, cleaved caspase-3, cleaved PARP and Bid were analyzed in whole cell lysates. (E) Expression of Bid was ablated in A549 and H1299 cells and apoptosis was quantified as in (C). (F) A549 and H1299 cells with ablated Bid were treated as in (E) and expression of Bid, cleaved caspase-3 and cleaved PARP were analyzed in whole cell lysates.
FLLL12 regulates the expression of DR5 through tyrosine phosphatase activation
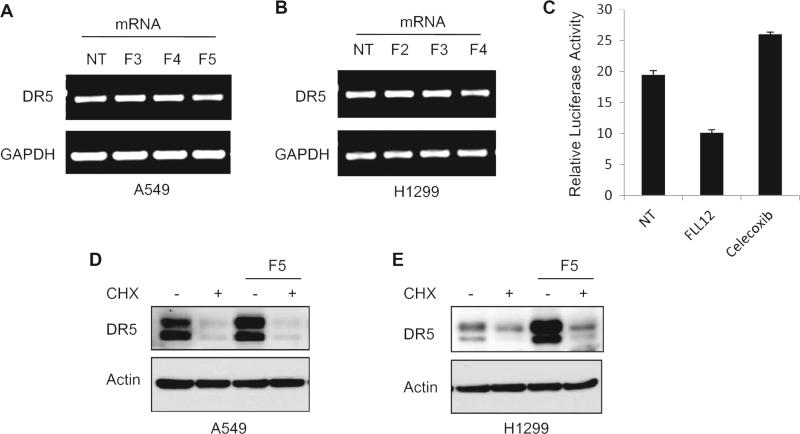
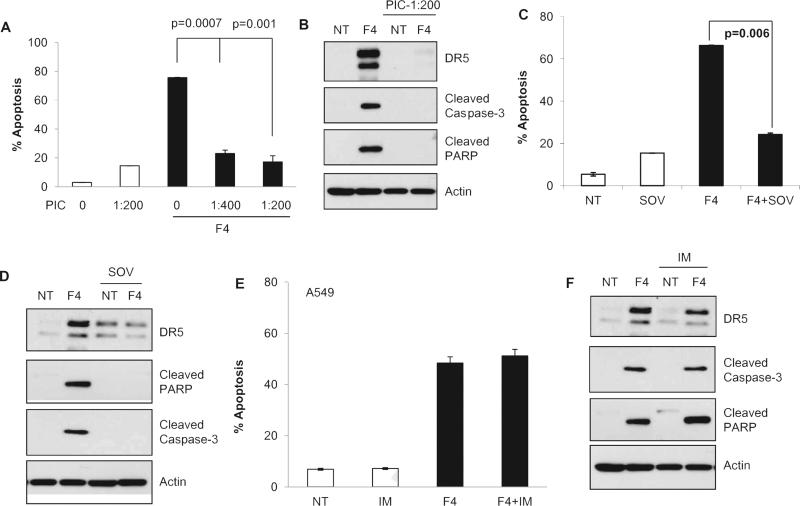
Accumulated evidence has demonstrated that a number of pharmacological endoplasmic reticulum (ER) stressors induce apoptosis by activating DR5 at the mRNA level through the transcription factor CHOP [37–39]. To determine the mechanism of regulation of DR5 by FLLL12, we assessed the expression of DR5 mRNA by RT-PCR after FLLL12 treatment. As shown in Fig. 5A and B, no significant differences in the mRNA expression of DR5 were observed in cells treated with different concentrations of FLLL12. We also carried out a luciferase reporter assay containing up to −552 of the 3′ UTR of the DR5 promoter as described [39]. Celecoxib was used as a positive control. Although celecoxib increased luciferase activity, it was inhibited by FLLL12, suggesting that FLLL12 post-transcriptionally regulates the expression of DR5 (Fig. 5C). We also analyzed the expression of DR5 after blocking global protein translation by cyclohexamide pretreatment followed by treatment with FLLL12. As shown in Fig. 5D and E, FLLL12 failed to increase the expression of DR5 after inhibition of protein translation. Supplementary Fig. S3 shows the time-dependent degradation of DR5 after inhibition of global protein synthesis by cyclohexamide. These results rule out the possibility of posttranslational stabilization of DR5 by FLLL12. Together, the data presented in Fig. 5 suggest that FLLL12 regulates DR5 at the posttranscriptional level. Co-activation of ERK/RSK and JNK signaling pathways and subsequent cooperative effects among the transcriptional factors CHOP, Elk1, and c-Jun enhance DR5 gene transcription [39,40]. We also found that FLLL12 increased the phosphorylation of ERK, p38 and c-Jun (Supplementary Fig. S4). Therefore, we explored the effects of pharmacological inhibitors of p38 (SB203580), JNK (SP600125), ERK (U0126), and AKT (LY294002) and of a phosphatase inhibitor cocktail (PIC) on FLLL12-induced expression of DR5. No notable changes in FLLL12-induced DR5 as well as apoptotic marker expression were found after inhibition of either p38, JNK, ERK or AKT (Supplementary Fig. S5). However, inhibition of global phosphatase activity by PIC, which inhibits protein tyrosine phosphatases (PTPs), serine threonine phosphatases, acid phosphatases and alkaline phosphatases, completely abolished FLLL12-induced DR5 expression (Supplementary Fig. S5). We next examined whether inhibition of phosphatases protected cells from FLLL12-induced apoptosis and found that inhibition of phosphatases by PIC significantly protected cells from apoptosis and inhibited the cleavage of apoptosis markers caspase-3 and PARP (Fig. 6A and B). Treatment with PIC drastically increased the basal level of p-AKT and p-ERK, and FLLL12 treatment had no further effect on p-AKT and p-ERK after PIC pretreatment. To rule out that the possibility that the protective effect of PIC is due to high p-AKT after PIC treatment, we pretreated cells with PIC and LY294002 together and measured FLLL12-induced apoptosis. PIC was still able to protect cells from FLLL12-induced apoptosis (Supplementary Fig. S6). Since PIC affects all phosphatase types, we next investigated the effect of sodium orthovanadate (SOV), which specifically inhibited PTPs and alkaline phosphatases, on the expression of DR5 and induction of apoptosis. As shown in Fig. 6C and D, pretreatment of cells with sodium orthovanadate clearly reduced FLLL12-induced apoptosis, expression of DR5, and completely abolished cleavage of caspase-3 and PARP. To further narrow down the involvement of specific phosphatase type, we pretreated cells with the alkaline phosphatase inhibitor imidazole and examined the expression of DR5 and induction of apoptosis after FLLL12 treatment. Interestingly, pre-treatment of cells with imidazole neither inhibited FLLL12-induced DR5 expression nor suppressed apoptosis and caspase-3 and PARP cleavage (Fig. 6E and F), suggesting the regulation of DR5 by FLLL12 through the activation of PTPs.
Fig. 5.
Posttranscriptional regulation of DR5 expression by FLLL12. (A) A549 and (B) H1299 cells were treated with the indicated concentrations of FLLL12 for 24 h and the expression of DR5 mRNA was determined by RT PCR. (C) HEK293 T cells were transfected with luciferase plasmid containing the 3′UTR of DR5 and luciferase activities were determined after treating with FLLL12 and celecoxib for 2 h. (D) A549 and (E) H1299 cells were pretreated with 100 μg/mL of cycloheximide (CHX) for 2 h, followed by treatment with FLLL12 for 8 h. The expression of DR5 was determined by Western blotting.
Fig. 6.
Activation of PTP is required for FLLL12-induced expression of DR5 and apoptosis. (A–E) A549 cells were pretreated with phosphatase inhibitor cocktail (PIC, supplied solution was diluted with culture media as shown in the figure) (A, B), sodium orthovanadate (SOV) (C, D) and alkaline phosphatase inhibitor imidazole (IM) (E, F) for 2 h followed by treatment with FLLL12 for 24 h. Apoptosis was quantified by Annexin V-PE staining and expression of DR5, cleaved caspase-3 and cleaved PARP were analyzed by Western blotting.
Discussion
Treatment outcomes in advanced or metastatic NSCLC remain frustrating, with low long-term survival rates. Overall, five-year survival for lung cancer patients is ~15%, and patients with advanced stage disease often die within 12 months [6]. Although recent advances in molecularly targeted treatments have made lung cancer therapy more personalized, the benefits are typically lost within months to a few years [41]. Long-term disease control requires activation of the apoptotic machinery that selectively kills cancer cells while sparing normal cells [35,36]. Several approaches are actively being explored to induce apoptosis, including switching on pathways that are controlled by death receptors, switching off survival pathways, inhibition of anti-apoptotic Bcl-2 proteins and apoptosis inhibitory proteins (IAPs) and induction of pro-apoptotic Bcl-2 proteins [42]. In the current study, we investigated the mechanism of anti-tumor effect, particularly induction of apoptosis, of a potent curcumin analog, FLLL12, in lung cancers and for the first time we explored FLLL12-induced extrinsic apoptosis triggered by the activation of DR5.
Our findings clearly demonstrate that FLLL12 induces apoptosis and is 5–10-fold more potent than the parental compound curcumin, depending on the cell lines. Apoptosis can be intrinsic or extrinsic depending on the involvement of mitochondria. DR5 is a pro-apoptotic cell-surface receptor that plays a central role in extrinsic apoptosis upon binding with its ligand TRAIL [43,44]. Activation of DR5 without ligand stimulation and subsequent induction of apoptosis have also been reported [37,43,44]. DR5-dependent apoptosis involves a cascade of events, such as formation of DISC comprising death receptor, FADD and caspase-8, processing of procaspase-8 to active caspase-8, truncation of Bid to tBid, activation of effector caspases and finally cleavage of PARP [18]. We investigated the mechanism of FLLL12-induced apoptosis in lung cancer cells, and found that FLLL-12 increased the expression of DR5 and that knock-down of DR5 and the downstream members of the extrinsic apoptotic machinery, caspase-8 and Bid, significantly protected cells from FLLL12-induced apoptosis. These results clearly demonstrate the importance of DR5 expression in FLLL12-induced apoptosis. Interestingly, the expression of DR5 is selective for the analog; the parent compound did not increase DR5 expression. We used three different cell lines and high doses of curcumin that induced comparable apoptosis. In all three cell lines, FLLL12 markedly increased DR5 expression, but curcumin failed to do so in any of the cell lines tested. After formation of DISC, activated caspase-8 can directly activate effector caspases or involve mitochondria through the truncation of Bid. Our results demonstrated that, although statistically significant, protection of cells from FLLL12-induced apoptosis was less pronounced in the case of Bid knockdown than caspase-8 and DR5 knockdowns. These results suggest that activated caspase-8 might activate effector caspases directly and via truncation of Bid.
Previous studies suggest that DR5 is mostly regulated at the transcriptional level via activation of multiple transcription factors, such as CHOP, NF-κB and p53 [38,45,46]. Recent studies have demonstrated that the activation of CHOP is critical for the transcriptional regulation of DR5 expression and that the transcriptional efficiency of CHOP is positively modulated by co-activation of the ERK, JNK and p38 MAP kinase pathway [40,47–49]. We investigated the regulation of DR5 expression by FLLL12 and also found that FLLL12 increased the phosphorylation of ERK p38 and c-jun. Interestingly, inhibition of these pathways had no effect on the expression of DR5. We observed no significant changes in the expression of DR5 mRNA after FLLL12 treatment, which suggests that FLLL12 regulates DR5 at a posttranscriptional level. We further excluded the possibility of posttranslational stabilization of DR5 since FLLL12 failed to stabilize DR5 after inhibition of global protein translation by cyclohexamide treatment, suggesting that FLLL12 regulates DR5 at the posttranscriptional processing of mRNA stage or at the translational level.
Like many physiological processes, apoptosis is posttranslationally regulated by reversible phosphorylation of apoptotic signaling proteins, which is controlled by a balance in protein kinase and protein phosphatase activities [50]. Although protein kinases implicated in the prevention or generation of apoptotic signals are already identified, the counter-regulatory protein phosphatases are less well defined [50]. In our current study, we found that activation of phosphatases plays a role in the expression of DR5 and induction of apoptosis, since inhibition of phosphatase activities with chemical inhibitors strongly inhibited the expression of DR5 and protected cells from FLLL12-induced apoptosis. We further identified that the activation of PTPs is important for the expression of DR5 and apoptosis. PTPs constitute a large family of enzymes consisting of receptor type PTPs, non-receptor type PTPs, dual specific PTPs and others [51,52]. Currently, it is not clear whether a specific PTP can act as an oncogene or as a tumor suppressor. Although our study clearly demonstrates a link between PTP and DR5 expression and a tumor suppressive function of PTP, it is not clear whether PTP regulates DR5 directly or via dephosphorylating a tyrosine kinase. So far, no report is available to support that DR5 is regulated by direct phosphorylation. Moreover, we have established that FLLL12 regulates DR5 at the translational level. The protein translational machinery is complex and most members of this machinery are regulated by phosphorylation/dephosphorylation events. A recent study reported the regulation of DR5 by miRNA, which also regulates protein translation [53]. Most probably, FLLL12 treatment activates PTPs that dephosphorylate a component of the protein translational machinery or regulate miRNA to increase DR5 translation. Finally, the specific tumor suppressor PTPs activated by FLLL12 that can exert a negative effect on tumor cell survival remain to be elucidated.
Selective activation of the apoptotic pathway provides tremendous therapeutic potential for cancer treatment [54]. Death receptor agonists and Bcl-2 inhibitors are currently under active clinical investigation [55]. Among the death receptor targeted agents, the therapeutic use of TNF-α and agonistic CD95-specific antibodies has been hampered by their toxic side effects, particularly a severe inflammatory response through NF-κB activation [56,57]. Death receptor agonist antibodies or recombinant TRAIL may be promising as a safe anticancer therapy; however, the clinical use of TRAIL and antibodies is limited by the fast turnover rate of TRAIL in the blood and the dimeric nature of the antibody, whereas death receptors are activated as a trimer [58]. In the current study, we have shown that FLLL12 induces apoptosis in lung cancer cell lines by expression of DR5 and activation of a DR5-dependent pathway. This is the first study to report that DR5-mediated extrinsic pathways play a major role in FLLL12-induced apoptosis and this signaling is selective for the analog. Linkage of PTP to FLLL12-induced DR5 expression and apoptosis is also novel. Further in vivo efficacy and safety as well as mechanistic studies are warranted to develop this promising compound for clinical application.
Supplementary Material
Acknowledgments
We are thankful to Dr. Anthea Hammond and Shi-Yong Sun for carefully editing the manuscript and providing the luciferase construct, respectively.
Funding
This study was supported by R03CA171663 and Robbins Scholar Award of Winship Cancer Institute of Emory University.
Footnotes
Authors’ contributions
Conception and design: Haque and Amin.
Development of methodology: Haque, Amin, and Rahman.
Acquisition of data: Haque and Amin.
Analysis and interpretation of data: Haque, Amin, Chen, and Shin.
Writing, review and/or revision of the manuscript: Haque, Amin, Fuchs, Saba, Khuri and Shin.
Study supervision: Amin.
Conflict of interest
The authors declare no potential conflict of Interest.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2015.04.017.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Roeland E, Loprinzi C, Moynihan TJ, Smith TJ, Temel J. In chemotherapy for lung cancer, sometimes less is more. J. Natl Compr. Canc. Netw. 2013;11:232–235. doi: 10.6004/jnccn.2013.0034. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 4.Ancuceanu RV, Istudor V. Pharmacologically active natural compounds for lung cancer. Altern. Med. Rev. 2004;9:402–419. [PubMed] [Google Scholar]
- 5.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13(Suppl. 1):5–13. doi: 10.1634/theoncologist.13-S1-5. [DOI] [PubMed] [Google Scholar]
- 6.Khuri FR, Cohen V. Molecularly targeted approaches to the chemoprevention of lung cancer. Clin. Cancer Res. 2004;10:4249s–4253s. doi: 10.1158/1078-0432.CCR-040019. [DOI] [PubMed] [Google Scholar]
- 7.Mehta HJ, Patel V, Sadikot RT. Curcumin and lung cancer-a review. Target. Oncol. 2014;9:295–310. doi: 10.1007/s11523-014-0321-1. [DOI] [PubMed] [Google Scholar]
- 8.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 10.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman MA, Amin AR, Shin DM. Chemopreventive potential of natural compounds in head and neck cancer. Nutr. Cancer. 2010;62:973–987. doi: 10.1080/01635581.2010.509538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 13.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 14.Zhou DY, Ding N, Van Doren J, Wei XC, Du ZY, Conney AH, et al. Effects of curcumin analogues for inhibiting human prostate cancer cells and the growth of human PC-3 prostate xenografts in immunodeficient mice. Biol. Pharm. Bull. 2014;37:1029–1034. doi: 10.1248/bpb.b14-00044. [DOI] [PubMed] [Google Scholar]
- 15.Xia YQ, Wei XY, Li WL, Kanchana K, Xu CC, Chen DH, et al. Curcumin analogue A501 induces G2/M arrest and apoptosis in non-small cell lung cancer cells. Asian Pac. J. Cancer Prev. 2014;15:6893–6898. doi: 10.7314/apjcp.2014.15.16.6893. [DOI] [PubMed] [Google Scholar]
- 16.Uehara Y, Inoue M, Fukuda K, Yamakoshi H, Hosoi Y, Kanda H, et al. Inhibition of beta-catenin and STAT3 with a curcumin analog suppresses gastric carcinogenesis in vivo. Gastric Cancer. 2014 doi: 10.1007/s10120-014-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: a review. J. Cell. Biochem. 2003;88:885–898. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
- 18.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 20.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 21.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 22.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- 23.Cen L, Hutzen B, Ball S, DeAngelis S, Chen CL, Fuchs JR, et al. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer. 2009;9:99. doi: 10.1186/1471-2407-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman L, Lin L, Ball S, Bekaii-Saab T, Fuchs J, Li PK, et al. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs. 2009;20:444–449. doi: 10.1097/CAD.0b013e32832afc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Hutzen B, Ball S, Foust E, Sobo M, Deangelis S, et al. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009;100:1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein-Szanto AJ, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, et al. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6693–6697. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–387. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Amin AR, Thakur VS, Paul RK, Feng GS, Qu CK, Mukhtar H, et al. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5419–5424. doi: 10.1073/pnas.0700642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruhul Amin AR, Thakur VS, Gupta K, Agarwal MK, Wald DN, Shin DM, et al. N-(phosphonacetyl)-L-aspartate induces TAp73-dependent apoptosis by modulating multiple Bcl-2 proteins: potential for cancer therapy. Oncogene. 2013;32:920–929. doi: 10.1038/onc.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, et al. Enhanced anti-tumor activity by the combination of the natural compounds (–)-epigallocatechin-3-gallate and luteolin: potential role of p53. J. Biol. Chem. 2010;285:34557–34565. doi: 10.1074/jbc.M110.141135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botchkarev VA, Flores ER. p53/p63/p73 in the epidermis in health and disease. Cold Spring Harb. Perspect. Med. 2014;4 doi: 10.1101/cshperspect.a015248. pii: a015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 33.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 35.Korytowski W, Basova LV, Pilat A, Kernstock RM, Girotti AW. Permeabilization of the mitochondrial outer membrane by Bax/truncated Bid (tBid) proteins as sensitized by cardiolipin hydroperoxide translocation: mechanistic implications for the intrinsic pathway of oxidative apoptosis. J. Biol. Chem. 2011;286:26334–26343. doi: 10.1074/jbc.M110.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Perez C, Roy SS, Naghdi S, Lin X, Davies E, Hajnoczky G. Bid-induced mitochondrial membrane permeabilization waves propagated by local reactive oxygen species (ROS) signaling. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4497–4502. doi: 10.1073/pnas.1118244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Yu D, Liu C, Yang X, Zhang N, Ma C, et al. Gadolinium-conjugated PLA-PEG nanoparticles as liver targeted molecular MRI contrast agent. J. Drug Target. 2011;19:657–665. doi: 10.3109/1061186X.2010.531727. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 39.Oh YT, Liu X, Yue P, Kang S, Chen J, Taunton J, et al. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J. Biol. Chem. 2010;285:41310–41319. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh YT, Yue P, Zhou W, Balko JM, Black EP, Owonikoko TK, et al. Oncogenic Ras and B-Raf proteins positively regulate death receptor 5 expression through co-activation of ERK and JNK signaling. J. Biol. Chem. 2012;287:257–267. doi: 10.1074/jbc.M111.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer–is it becoming a reality? Nat. Rev. Clin. Oncol. 2010;7:401–414. doi: 10.1038/nrclinonc.2010.64. [DOI] [PubMed] [Google Scholar]
- 42.Pore MM, Hiltermann TJ, Kruyt FA. Targeting apoptosis pathways in lung cancer. Cancer Lett. 2013;332:359–368. doi: 10.1016/j.canlet.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Wang X, Yu H, Wei S, Williams N, Holmes DL, et al. Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat. Chem. Biol. 2013;9:84–89. doi: 10.1038/nchembio.1153. [DOI] [PubMed] [Google Scholar]
- 44.Ren YG, Wagner KW, Knee DA, Aza-Blanc P, Nasoff M, Deveraux QL. Differential regulation of the TRAIL death receptors DR4 and DR5 by the signal recognition particle. Mol. Biol. Cell. 2004;15:5064–5074. doi: 10.1091/mbc.E04-03-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G, et al. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol. Cell. Biol. 2005;25:5404–5416. doi: 10.1128/MCB.25.13.5404-5416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, et al. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 47.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, et al. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X, et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 50.Van Hoof C, Goris J. Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochim. Biophys. Acta. 2003;1640:97–104. doi: 10.1016/s0167-4889(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 51.Wang WQ, Sun JP, Zhang ZY. An overview of the protein tyrosine phosphatase superfamily. Curr. Top. Med. Chem. 2003;3:739–748. doi: 10.2174/1568026033452302. [DOI] [PubMed] [Google Scholar]
- 52.Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Francis H, McDaniel K, Han Y, Liu X, Kennedy L, Yang F, et al. Regulation of the extrinsic apoptotic pathway by microRNA-21 in alcoholic liver injury. J. Biol. Chem. 2014;289:27526–27539. doi: 10.1074/jbc.M114.602383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 55.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 56.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 57.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 58.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin. Cancer Res. 2010;16:1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.