Abstract
Silver nanoparticles (AgNPs) have been widely used in biomedical fields because of their intrinsic therapeutic properties. Here, we introduce methods of synthesizing AgNPs and discuss their physicochemical, localized surface plasmon resonance (LSPR) and toxicity properties. We also review the impact of AgNPs on human health and the environment along with the underlying mechanisms. More importantly, we highlight the newly emerging applications of AgNPs as antiviral agents, photosensitizers and/or radiosensitizers, and anticancer therapeutic agents in the treatment of leukemia, breast cancer, hepatocellular carcinoma, lung cancer, and skin and/or oral carcinoma.
Keywords: silver nanoparticles, physico-chemical properties, localized surface plasmon resonance, cytotoxicity, antiviral effect, photosensitizers/radiosensitizers, anticancer, assessment of the risks to humans and environment
Introduction
AgNPs with unique optical, electronic, and antibacterial properties have been widely used in biosensing [1], photonics [2], electronics [3], and antimicrobial [4] applications, among others. The remarkably strong broad-spectrum antimicrobial activity of AgNPs is a major direction for the development of AgNP products, including textiles, food storage containers, antiseptic sprays, catheters, and bandages. The biocidal activity of AgNPs depends on their size, shape, and surface coatings. Therefore, the development of AgNPs with well-controlled morphological and physicochemical features for physiological application in humans is necessary to expand their biomedical applications. Recently, AgNPs have gained increased attention because of their therapeutic applications, such as their promising role as anticancer agents [5]. Positive outcomes have been achieved when incorporating AgNPs into cancer treatments [6]. Here, we focus mainly on the recently reported therapeutic applications of AgNPs as virucidal agents and anticancer agents, and on the safety issues relating to the use of AgNPs in humans and their effects on the environment. We conclude by discussing the prospects for additional uses of AgNPs in the clinic.
Synthesis methods
Many routes have been introduced for the synthesis of silver nanostructures, which can be categorized as: (i) chemical methods [7–10]; (ii) physical methods [11–14], and (iii) biological methods [15–17]. Chemical methods for the syntheses of silver nanostructures can be subdivided into chemical reduction [7], electrochemical techniques [8], irradiation-assisted chemical methods [9], and pyrolysis [10]. The synthesis of silver nanostructures in solution usually contains three main components: metal precursors, reducing agents, and stabilizing/capping agents. Widely used reducing agents include borohydride, sodium citrate, ascorbic acid, alcohol, and hydrazine compounds. It has been reported that AgNPs supported on nanostructured SiO2 were obtained by flame aerosol technology, which enables close control of Ag content and size [9]. Similarly, Ag/silica nanoparticles with relatively narrow size distribution were made by flame spray pyrolysis (flame aerosol technology) [10]. By contrast, physical methods do not involve toxic chemicals and usually have fast processing times. Such methods include physical vapor condensation [11], Arc-discharge [12], energy ball milling method [13], and direct current (DC) magnetron sputtering [14]. Another advantage of physical methods is that the AgNPs formed have a narrow size distribution [14]; however, a major drawback is their high energy consumption. In the biological synthesis of AgNPs, the toxic reducing agents and stabilizers are replaced by nontoxic molecules (proteins, carbohydrates, antioxidants, etc.) produced by living organisms, including bacteria, fungi, yeasts, and plants. Biological methods based on microorganisms such as bacteria [15], fungi [16], and yeast [17], have been widely reported. The cheaper plant systems, such as lemongrass, Aloe vera, seaweed, alfalfa, tea, neem, mustard, safeda, lotus, and tulsi, have been explored for the synthesis of AgNP. The possible mechanisms of biological synthesis include enzymatic (e.g., NADPH reductase) and nonenzymatic reduction [18]. In general, AgNP synthesis using plant extracts is the most-used environmentally friendly method of production.
Properties of AgNPs
Major physicochemical properties of AgNPs
Some physicochemical properties of AgNPs, including size (surface area), shape, surface charge and coating, agglomeration, and dissolution rate, are particularly important for determining their biological interactions and impacts. Smaller particles have a larger surface area and, therefore, have greater toxic potential [19]. It is well known that the shape of silver nanostructures can dramatically affect their physical and chemical properties. Frequently utilized silver nanostructures in the biomedical field include silver spherical nanoparticles, nanowires, nanorods, nanoplates, and nanocubes [20]. Studies have found that the biological effects of AgNPs depend on the different surface charges of their coatings, which can affect the interaction of AgNPs with living systems [21]. Agglomeration is known to occur with most engineered nanoparticles. It was shown that agglomeration of AgNPs occurs in culture media and within the cytoplasm and nuclei of HepG2 cells [22]. Dissolution of AgNPs as a result of surface oxidation leads to the production of ionic silver. The rate of dissolution depends on the chemical and surface properties of the particle as well as its size, and is further affected by the surrounding media [23].
Localized surface plasmon resonance of AgNPs
The remarkable optical properties of silver nanostructures result from their unique interaction with light, which causes the collective coherent oscillation of their free conduction band electrons, or LSPR. Oscillation of the free electrons results in either radiative decay with a strong visible scattering of light or nonradiative decay, which causes the conversion of photon energy into thermal energy. These two decay mechanisms have been readily utilized in biodiagnostic and imaging (both exploiting radiative SPR decay), and therapeutic (exploiting nonradiative SPR decay) applications [24].
LSPR of AgNPs depends on the size, shape, dielectric environment, and mutual electromagnetic interactions among particles in close proximity [25]. These parameters can be used to tune the plasmon peak of AgNPs in the range of 393–738 nm [26] and 500–1000 nm [27]. Therefore, LSPR of AgNPs results in strong visible and near-infrared (NIR) scattering and absorption, which enables the development of photothermal and thermolytic laser therapies [6,28,29]. Moreover, it was revealed that AgNPs could enhance the effect of cancer cell killing in radiation treatment [30].
Toxicity of AgNPs
Impact on human health
There are several possible ways in which patients can be exposed to AgNPs, such as dermal contact, oral administration, inhalation, and blood circulation. Macrophages are the first cells that AgNPs will encounter in the human body [31]. It is known that the size of the AgNP dictates its mode of cytotoxicity to murine macrophages (Ag+ ion-specific and/or particle-specific). The toxicity of AgNPs (<10 nm) is mostly mediated by the released Ag+ ions, with liver being the major target organ, followed by spleen, lungs, and kidney. One study showed that the effect of both 20 nm and 100 nm AgNPs on Wistar-derived WU rats treated at 6 mg/kg body weight doses was an increase in spleen weight; moreover, the clinical chemistry parameters also indicated liver damage [32]. A separate study on the inhalation toxicity of AgNPs showed that AgNPs had an influence on the neutral mucins in the respiratory mucosa of Sprague–Dawley (SD) rats exposed to AgNPs at concentrations of 0.5–61 μg/m3, yet without toxicological significance [33]. Notably, another study showed that AgNPs had negligible impact on the nasal cavity and lungs [34]. Furthermore, it was reported that levels of silver reported from nanomaterial-manufacturing workers exposed to silver concentrations of 0.35–1.35 g/m3 were only 0.0135–0.034 mg/m3 for blood and 0.043 mg/m3 for urine, and there were no significant findings on their health status [35].
Although many toxicological studies using AgNPs have been published, it is still difficult to draw a definite conclusion about their toxicity. We can conclude that AgNPs might have different toxicological properties owing to the different synthesis methods, their various sizes, the presence or absence of capping agents, different organisms, and/or culture cells. Hence, their risks should be assessed on a case-by-case basis.
Impact on the environment
The toxicity of AgNPs to the environment depends on their chemical form and the availability of free silver ions. Once released into the environment, AgNPs are dispersed in different ways, which modifies their properties and alters their transport, fate, and toxicity. According to a study by Blaser et al. [36], silver can release into solid waste, which is disposed of in solid waste landfills or incinerated in thermal waste treatment (TWT), and wastewater, which is either treated in a sewage treatment plant (STP) or directly discharged into natural waters. Sewage sludge is applied to agricultural soils, disposed of in solid waste landfills, or incinerated in TWT. Solid waste landfilling might allow silver to leach into subsoil and groundwater. Additionally, immediate release to the environment originates from STP effluents, untreated wastewater, and silver contained in sewage sludge that is spread out on agricultural fields. Furthermore, the release of silver incorporated into textiles and plastics used in all 25 European Union countries was found to be in the range 110–230 t/yr in the three different emission scenarios [36].
It is most likely that AgNPs would react with sulfide, chloride, or other natural substances, altering the original properties of the nanoparticles. Levard et al. found that even a very low degree of sulfidation of AgNPs can result in a significant decrease in their toxicity because of the lower solubility of silver sulfide [37]. Meanwhile, Tiede et al. [38] also revealed that >90% of AgNPs were removed during wastewater treatment. Although the dissolution of AgNPs in the presence of chloride in aqueous solution has not been thoroughly investigated, toxicity of Ag+ in the presence of Cl− among various species of fish has been studied [39].
Overall, little is known about the specific effects of AgNPs on the environment. Therefore, it is currently impossible to assess reliably the environmental risks associated with the production and use of AgNPs.
Mechanism of AgNP-induced toxicity
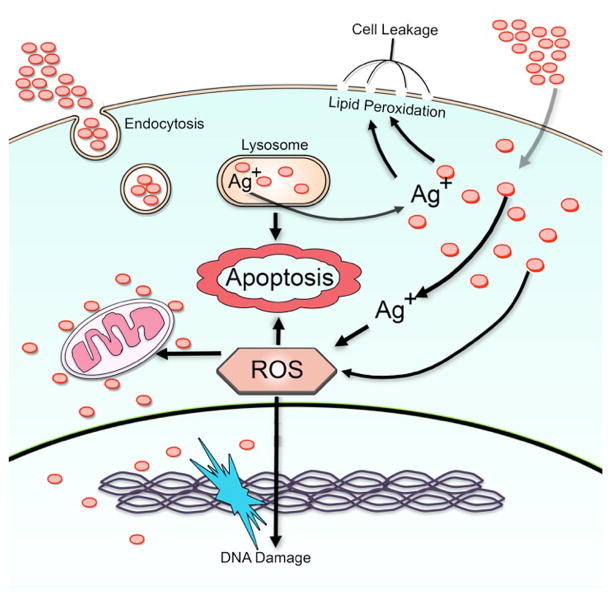
The interactions between nanomaterials and cells, the cellular uptake, and subsequent toxic response of the cell are among the most crucial issues relating to AgNP-induced toxicity. For most cells, uptake of AgNPs mainly through endocytosis depends on time, dose, and energy, and the major target organelles are endosomes and lysosomes [40,41]. Nanoparticles can induce reactive oxygen species (ROS) production directly once they are exposed to the acidic environment of lysosomes [42]. Additionally, Singh et al. also demonstrated the accumulation of Ag+ in lysosomes [43]. ROS contain superoxide anions (O2−), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2). Reactions between H2O2 and AgNPs are thought to be one of the factors leading to Ag+ release in vivo. The possible chemical reaction involves: 2Ag + H2O2 + 2H+ → 2Ag+ + 2H2O E0 = 0.17 V [41]. The reaction can occur upon contact with cell culture medium or proteins in the cytoplasm. Furthermore, ROS are highly reactive and result in oxidative damage to proteins and DNA, and induce mitochondrial dysfunction. AgNPs and Ag+ ions can also escape from lysosomes, further inducing the increase of intracellular ROS. AgNPs and released Ag+ ions prefer to interact with the thiol groups of molecules present in the cytoplasm, cell membrane, and inner membrane of mitochondria, which might release lipid peroxide and increase permeation of the cell membrane and mitochondrial systems [40,44]. Damage to the cell membrane results in leakage of cytoplasmic contents and eventual necrosis, whereas rupture of lysosomal membranes activates lysosome-mediated apoptosis. Furthermore, damage to mitochondria impairs electron transfer, thereby activating mitochondrion-dependent apoptosis [45]. In addition, it has been reported that AgNPs could readily diffuse into, and translocate to, the nucleus through nuclear pore complexes, thereby leading to the formation of ROS, which directly trigger DNA damage and chromosomal abnormalities [41]. Recent studies also indicated that Ag+ can directly lead to DNA damage in addition to damaging mitochondria and inducing ROS production [46].
Few studies have investigated gene expression induced via AgNPs. RT-PCR analysis [47] indicated that the upregulation of metabolism and oxidative stress genes by AgNPs might have increased the production of ROS as a byproduct of oxidation. AgNPs also significantly upregulate the gene encoding Thioredoxin-interacting protein (Txnip), inducing the intrinsic mitochondrial pathway of apoptosis. It was also found that the Atr gene, which senses DNA damage, was significantly upregulated in the caudate nucleus of mice exposed to AgNPs.
In brief, exposure to AgNPs causes cytotoxicity by elevating ROS levels and increasing lipid peroxidation; it also leads to genotoxicity by inducing DNA and chromosomal damage (Figure 1).
Figure 1.
Schematic representation of possible mechanisms for silver nanoparticle (AgNP)-induced cytotoxicity. The red circles denote AgNPs. Abbreviation: ROS, reactive oxygen species.
Key factors mediating toxicity of AgNPs
Toxicological investigations of AgNPs imply that, in addition to time, dose, and temperature, other factors, including particle size, shape, surface coatings, and cell type, could also influence the toxicity of AgNPs. In general, the toxicity of nanomaterials is related to their reactivity, which in turn depends on the nanoparticle size [48]. As reported elsewhere, the effect of AgNP size on macrophages was seen to affect cell viability, induction of oxidative stress, and release of cytokines [49]. To achieve stable AgNPs and reduce the potential risks of AgNPs on human cells and the environment, chemical modification of the AgNP surface with biological ligands has been investigated. It was shown that three organocoated AgNPs have different toxicity against two model organisms, Escherichia coli and Daphnia magna, that is dependent on the particle size, surface charge, and concentration [50]. Interestingly, Sotiriou et al. reported that coating AgNPs with a thin SiO2 layer preserves their plasmonic performance and minimizes their toxicity by blocking ion release and bacteria and/or cell contact [51]. The shape of AgNPs also has an important role in their toxic and immunological effects. Recently, George et al. [52] reported that plate-shaped AgNPs are comparatively more toxic against a fish gill epithelial cell line (RT-W1) and zebrafish embryos compared with sphere- or wire-shaped AgNPs because of the presence of surface defects. In addition, it was demonstrated that the human sperm cells exhibit a lower cytotoxic response to 8–10 nm AgNPs compared with human lymphocytes [53].
Generally, the smaller the size of AgNPs, the stronger cytotoxic effects they could have. Moreover, different surface coatings of AgNPs can trigger different events depending on the cell type.
Therapeutic applications of AgNPs
The function of AgNPs as antibacterial and antifungal agents has been well documented [54,55] and is not discussed here. Moreover, applications of AgNPs have been expanded to emerging fields, such as drug delivery and diagnosis. Here, we focus on their therapeutic applications as antiviral, photosensitizer and/or radiosensitizer, and anticancer agents.
AgNPs as virucidal agents
AgNPs have been shown to inhibit HIV-1, Tacaribe virus (TCRV), hepatitis B virus (HBV), recombinant respiratory syncytial virus (RSV), monkey pox virus, murine norovirus (MNV)-1, and influenza A/H1N1 virus. Table 1 summarizes the antiviral effects of AgNPs reported in recent publications. Park et al. [56] recently developed and evaluated a novel micrometer-sized magnetic hybrid colloid (MHC) decorated with variously sized AgNPs that could be used to inactivate viral pathogens ΦX174 and MNV with minimum chance of potential release into the environment. In addition, Xiang et al. [57] showed that AgNPs have beneficial effects in preventing A/Human/Hubei/3/2005 (H3N2) influenza virus infection both in vitro and in vivo. Another study [58] observed that AgNPs had better antiviral activity (80–90% inhibition) against Herpes simplex virus (HSV)-1 and human parainfluenza virus (hPIV)-3 and were less cytotoxic to Vero cells. In addition, it was found that AgNPs can inhibit the replication of Vaccinia virus (VACV) [59].
Table 1.
Antiviral effect of AgNPs
| Type | Size (nm) | Surface stability | Microbial strains | Function | Refs |
|---|---|---|---|---|---|
| AgNPs | 1–10 | Carbon, PVP and BSA | HIV-1 | Size-dependent interaction with HIV-1; inhibits virus from binding to host cells | [55] |
| 10 and 25 | None and polysaccharide | TCRV | Inhibits viral replication | [55] | |
| 10, 50, and 800 | None | HBV | Only 10-nm AgNPs inhibit replication of HBV | [55] | |
| N/A | PVP- AgNPs, recombinant RSV fusion (F) protein and BSA | RSV | 44% inhibition of syncytial virus | [54] | |
| 10–80 | None or polysaccharide-coated | (MPV) | AgNPs of approximately 10 nm inhibit MPV infection in vitro | [55] | |
| 11.2 | biogenic Ag0 | MNV-1 | Addition of 31.25 mg biogenic Ag0 m−2 on the filter caused 3.8-log decline of the virus compared with 1.5-log decrease by original filter | [55] | |
| 10 | None | H1N1 influenza A virus | Rapidly inhibits H1N1 influenza A virus hemagglutination of chicken RBCs | [55] | |
| AgNP@MHCs | 7, 15, and 30 | None | ΦX174, MNV, and AdV2 | Inactivates viral pathogens with minimum chance of potential release into environment | [56] |
| AgNPs | 9.5 nm | None | H3N2 influenza virus | Prevents H3N2 influenza virus infection both in vitro and in vivo | [57] |
| 4–13, 5–23, and 7–20 | None | HSV-1, HSV-2, and HPIV-3 | AgNPs with a size of 4–13 nm and 5–23 nm, had better antiviral activity against HSV-1 and HPIV-3 viruses | [58] | |
| 25 | None | VACV | Inhibits vaccinia virus replication by preventing viral entry | [59] |
Abbreviations: BSA: bovine serum albumin; N/A: not available..
AgNPs as photosensitizers and/or radiosensitizers
LSPR of nanoparticles enables the use of AgNPs in nonionizing radiation and ionizing radiation. In a report by the Cai group, it was revealed that aptamer–Ag–Au shell–core nanostructures have a high ability to absorb NIR irradiation and are able to perform photothermal therapy of the A549 cells at a low irradiation power density (0.20 W cm−2) without destroying the healthy cells and the surrounding normal tissues [28]. Moreover, it was reported that grapheneoxide@Ag-doxorubicin-DSPE-PEG2000-NGR (GO@Ag-DOX-NGR) showed excellent chemophotothermal therapeutic efficacy, tumor-targeting properties, NIR laser-controlled drug-releasing functions, and X-ray imaging ability in an in vivo murine tumor model [29]. Furthermore, it was revealed that hollow Au–Ag nanoshells (HGNS) showed potential for photothermal therapy because of their stability when PEGylated under laser illumination [6]. In addition, Zhao et al. [30] reported that Fe3O4/Ag/C225 (epidermal growth factor receptor) combined with X-ray treatment could increase the sensitivity of human nasopharyngeal carcinoma cell lines (CNEs) to irradiation.
Potential therapeutic applications of AgNPs in cancer
AgNPs have proven promising antitumor effects. It was reported that a low concentration of AgNPs can cause DNA damage and chromosomal aberrations (genotoxicity), although no significant cytotoxicity was recorded [40]. However, Lima et al. showed that no genotoxicity effects were observed for different human culture cells treated with up to 10 mg/mL of capped AgNPs (diameter 6–80 nm) [60]. The generation of many toxicological data concerning nanoparticles sometimes creates a negative perception of their use. However, toxicity itself can be useful for cancer therapies because it is highly sought. Positive outcomes have been achieved when incorporating AgNPs into cancer treatments. They can not only passively interact with cells, but also actively mediate molecular processes to regulate cell functions. Table 2 summarizes the potential therapeutic applications of AgNPs in cancer reported in recent publications.
Table 2.
Anticancer effect of AgNPsa
| Application | Cells and/or organisms | Size (nm) | Surface stability | Dose | Exposure time | Function | Refs |
|---|---|---|---|---|---|---|---|
| Antiangiogenesis | BREC/female C57BL/6 mice | 40–50 | None | IC50: 500 nM; injection: AgNPs (500 nM) | 24 h for cell assay; injection for 7 days | Inhibits formation of new blood vessels | [61] |
| DLA/female Swiss albino mice | 50 | N/A | IC50: 500 nM; intraperitoneal injection: AgNPs (500 nM) | 24 h for MTT assay; injection of AgNPs for 15 days | Increased survival time, decreased volume of ascitic fluid in tumor-bearing mice by 65%, returning body weight to normal | [62] | |
| Antileukemia | AML cells | 3,11, and 30 | PVP-AgNPs | 0–10 μg/mL | 24 h | 11-nm AgNPs have significant inhibition effect with low IC50 (0.90 – 3.43 μg/mL) on six AML cells | [46] |
| K562 cells | --- | PVP-AgNPs | ----- | --- | Enters K562 cells in a dose-dependent manner and locates in endosomes | [63] | |
| Antibreast cancer | MCF-7 | NA | Grenetine-stabilized colloidal silver | LD50: 3.5 ng/mL | 5 h | Dose-dependent cytotoxic effect in MCF-7 cells | [64] |
| MDAMB-231 | 5 | None | IC50: 6.0 μg/mL | 24h | Inhibits cell viability and induces membrane leakage | [65] | |
| MDAMB-231 | 2–10 | N/A | IC50: 8.7 μg/mL | 24h | Inhibit the growth of cells in a dose-dependent manner | [66] | |
| Antihepatoma | HepG2 | 5–10 | None | IC50: 3.38 μg/mL | 28 h | AgNPs exhibited cytotoxicity with a potency comparable with that of Ag+ ions | [22] |
| HepG2 | 20 | None | 1–20 μg/mL | 24 h | Oxidative stress did not have major role in observed cytotoxicity of AgNPs in HepG2 | [67] | |
| HepG2 and primary liver cells of mice | 20–40 | None | IC50 2.764 μg/mL for HepG2 cell line and IC50 121.7 μg/mL for primary liver cells of mice | 24 h | 44 times stronger inhibitory effect on growth of HepG2 cell line compared with normal cells | [68] | |
| Antilung caner | A549 | 30–50 | PVP-AgNPs | 0–20 μg/mL | 24 h | Reduction in mitochondrial function | [69] |
| H157 | 10–20 | AgNPs capped with table sugar | IC50: 3.6 μM | 48 h | Remarkable inhibition on H157 | [70] | |
| Antiskin/oral cancer | HT144 | 10–20 | AgNPs capped with table sugar | IC50: 0.36 μM | 48 h | Remarkable inhibition on HT144 | [70] |
| HSC-3 and HaCat | 35 | Peptide–AgNP | 0.1 nM | 24 h | DNA ds breaks and a subsequent increase in the sub-G1 (apoptotic) population | [71] |
Abbbreviations: IC50, the drug concentration that inhibited cell survival by 50%; LD50, the concentration of 50% cell growth inhibition; N/A: not available.
AgNPs as antiangiogenic agents
It is well established that angiogenesis has a central role in several diseases including cancer. Eom’s group [61] reported AgNPs and a natural antiangiogenic molecule, PEDF, almost contributed equally to the inhibitory effects of vascular endothelial growth factor (VEGF)-induced angiogenesis by blocking PI3K/Akt phosphorylation at Ser-473 in bovine retinal epithelial cells (BREC) in vitro. In addition, it was revealed that the formation of new blood vessels is inhibited by AgNPs in vivo. Another study [62] by the same group also revealed that AgNPs showed cytotoxicity against Dalton’s lymphoma ascites (DLA) cells in vitro and in vivo and significantly increased the survival time in the tumor mouse model by approximately 50% compared with tumor controls.
Application in leukemia
Leukemia is a group of cancers that usually begins in bone marrow and results in high numbers of abnormal white blood cells. Several investigators have reported that AgNPs induced a cytotoxic effect against leukemic cells, such as THP-1, Jurkat, and K562 cells. Recently, Guo et al. [46] found that poly(N-vinyl-2-pyrrolidone (PVP)-coated AgNPs could inhibit the viability of acute myeloid leukemia (AML) cells, including isolates from patients with AML at low concentrations, suggesting a novel approach for the treatment of AML in the future. Another study by the same group [63] demonstrated that AgNPs were able to enter K562 cells in a dose-dependent manner and localize within the endosomes.
Application in breast cancer
It has also been observed that AgNPs have dose-dependent cytotoxic effects in MCF-7 breast cancer cells through induction of apoptosis, with a concentration of 50% cell growth inhibition (LD50) of 3.5 ng/mL and LD100 of 14 ng/mL [64]. More recently, Gurunathan et al. [65] found that AgNPs induced MDA-MB-231 cell death through ROS generation, activation of caspase 3, and DNA fragmentation. Further work by this group also indicated that single-crystalline AgNPs have cytotoxic effects with apoptotic features [66].
Application in hepatocellular carcinoma
An in vitro cytotoxic study conducted by Kim et al. [22] demonstrated that the cytotoxicity of AgNPs against human liver HepG2 cells is primarily the result of oxidative stress. Recently, Sahu et al. [67] revealed a significant concentration-dependent cytotoxicity of AgNPs in HepG2 cells and that a different mechanism of AgNPs-induced mitochondrial injury leads to the cytotoxicity. Notably, Faedmaleki et al. [68] showed that AgNPs had a 44-times stronger inhibitory effect on HepG2 cells compared with normal cells (primary liver cells of mice).
Application in lung cancer
AgNPs have also been shown to display cytotoxicity to lung cancer cells. Foldbjerg et al. [69] observed a dose-dependent reduction in mitochondrial function of human alveolar cell line A549 cells. It was shown that AgNPs are taken up by the cells, leading to increased production of ROS and ultimately apoptotic and necrotic cell death. Moreover, Nazir et al. [70] showed that AgNPs have effective anticancer properties against the H157 (squamous cell lung carcinoma) cell line with an IC50 of 3.6μM.
Application in skin and/or oral carcinoma
Work by the Nazir group showed that AgNPs have effective anticancer properties with an IC50 of 0.36μM against HT144 melanoma cell lines [70]. Recently, Austin et al. [71] demonstrated that the nuclear-targeting peptide-conjugated AgNPs cause DNA double-strand (ds) breaks and a subsequent increase in the sub-G1 (apoptotic) population in the HSC-3 cancer cell model at lower concentrations compared with nuclear-targeting gold nanoparticles (AuNPs).
Concluding remarks and future perspectives
In summary, silver nanoparticles exhibit particularly unique physical, chemical, optical, and biological properties that different from other biomedical nanomaterials and, thus, can serve as therapeutic platforms in many biomedical applications, including but not limited to: (i) antiviral agents; (ii) photosensitizers and/or radiosensitizers; and (iii) anticancer therapeutic agents in leukemia, breast cancer, hepatocellular carcinoma, lung carcinoma, and skin and/or oral carcinoma. However, despite their promising potential in medical applications, the impact of AgNPs on human health (both positive and negative) needs to be fully understood before their wider use. The successful translation of silver nanotechnology to the clinic requires the development of simple, safe, cost-effective, and eco-friendly preparations of AgNPs, and a fuller understanding of the safety control mechanisms as well as the biodistribution and pharmacokinetics of AgNPs in clinical applications.
Highlights.
AgNPs possess intrinsic therapeutic properties for biomedical applications.
AgNPs are employed in newly emerging applications as photosensitizers/radiosensitizers, antiviral and anticancer agents.
Treatment of a variety of cancers with AgNPs have been documented. with AgNPs.
The underlying anticancer mechanisms of AgNPs include (1) disruption of cell membranes, and (2) production of reactive oxygen species and Ag+ to damage protein or DNA.
The photosensitizing mechanism of AgNPs is based on nonradiative decay converting photo energy to thermal energy.
Acknowledgments
This work was supported by funds from St John’s University Research Seed Grant (No. 579-1110-7002) and the Department of Pharmaceutical Sciences to Z-S.C.
Footnotes
Teaser: By interacting with cells and mediating molecular processes to regulate cell functions, silver nanoparticles exhibit emerging biomedical applications as antiviral agents, photosensitizers and/or radiosensitizers, and anticancer therapeutic agents.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Habouti S, et al. Synthesis of silver nano-fir-twigs and application to single molecules detection. J Mater Chem. 2010;20:5215–5219. [Google Scholar]
- 2.Hu XH, Chan CT. Photonic crystals with silver nanowires as a near-infrared superlens. Appl Phys Lett. 2004;85:1520–1522. [Google Scholar]
- 3.Alshehri AH, et al. Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl Mater Interfaces. 2012;4:7007–7010. doi: 10.1021/am3022569. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, et al. A novel green synthesis approach for polymer nanocomposites decorated with silver nanoparticles and their antibacterial activity. Analyst. 2014;139:5793–5799. doi: 10.1039/c4an01301h. [DOI] [PubMed] [Google Scholar]
- 5.Braun GB, et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat Mater. 2014;13:904–911. doi: 10.1038/nmat3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman AM, et al. The surprising in vivo instability of near-IR-absorbing hollow Au–Ag nanoshells. ACS Nano. 2014;8:3222–3231. doi: 10.1021/nn405663h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, et al. A systematic study of the synthesis of silver nanoplates: is aitrate a ‘magic’ reagent. J Am Chem Soc. 2011;133:18931–18939. doi: 10.1021/ja2080345. [DOI] [PubMed] [Google Scholar]
- 8.Roldán MV, et al. Electrochemical method for Ag-PEG nanoparticles synthesis. J Nanopart. 2013;2013:7. [Google Scholar]
- 9.Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol. 2010;44:5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- 10.Sotiriou GA, et al. Nanosilver on nanostructured silica: antibacterial activity and Ag surface area. Chem Eng J. 2011;170:547–554. doi: 10.1016/j.cej.2011.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kholoud MM, et al. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010;3:135–140. [Google Scholar]
- 12.Tien D, et al. Discovery of ionic silver in silver nanoparticle suspension fabricated by arc discharge method. J Alloys Compounds. 2008;463:408–411. [Google Scholar]
- 13.Kosmala A, et al. Synthesis of silver nano particles and fabrication of aqueous Ag inks for inkjet printing. Mater Chem Phys. 2011;129:1075–1080. [Google Scholar]
- 14.Asanithi P, et al. Growth of silver nanoparticles by DC magnetron sputtering. J Nanomater. 2012;2012:8. [Google Scholar]
- 15.Shivaji S, et al. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011;46:1800–1807. [Google Scholar]
- 16.Li G, et al. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci. 2012;13:466–476. doi: 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourato A, et al. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl. 2011;2011:8. doi: 10.1155/2011/546074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge L, et al. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int J Nanomed. 2014;9:2399–2407. doi: 10.2147/IJN.S55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston HJ, et al. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 20.Rycenga M, et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem Rev. 2011;111:3669–3712. doi: 10.1021/cr100275d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers CM, et al. Critical contributions of silver ion, particle size, coating, and composition. Environ Health Persp. 2011;119:37–44. doi: 10.1289/ehp.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Misra SK, et al. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ. 2012;438:225–232. doi: 10.1016/j.scitotenv.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 24.Austin LA, et al. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch Toxicol. 2014;88:1391–1417. doi: 10.1007/s00204-014-1245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren J, Tilley RD. Preparation, self-assembly, and mechanistic study of highly monodispersed nanocubes. J Am Chem Soc. 2007;129:3287–3291. doi: 10.1021/ja067636w. [DOI] [PubMed] [Google Scholar]
- 26.Huang T, Nancy X. Synthesis and characterization of tunable rainbow colored colloidal silver nanoparticles using single-nanoparticle plasmonic microscopy and spectroscopy. J Mater Chem. 2010;20:9867–9876. doi: 10.1039/C0JM01990A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud MA, El-Sayed MA. Different plasmon sensing behavior of silver and gold Nanorods. J Phys Chem Lett. 2013;4:1541–1545. doi: 10.1021/jz4005015. [DOI] [PubMed] [Google Scholar]
- 28.Wu P, et al. High specific detection and near-infrared photothermal therapy of lung cancer cells with high SERS active aptamer–silver–gold shell–core nanostructures. Analyst. 2013;138:6501–6510. doi: 10.1039/c3an01375h. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, et al. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials. 2014;35:5847–5861. doi: 10.1016/j.biomaterials.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Zhao D, et al. A novel multifunctional nanocomposite C225-conjugated Fe3O4/Ag enhances the sensitivity of nasopharyngeal carcinoma cells to radiotherapy. Acta Biochim Biophys Sin. 2012;44:678–684. doi: 10.1093/abbs/gms051. [DOI] [PubMed] [Google Scholar]
- 31.Pratsinis A, et al. Toxicity of silver nanoparticles in macrophages. Small. 2013;9:2576–2584. doi: 10.1002/smll.201202120. [DOI] [PubMed] [Google Scholar]
- 32.De Jong WH, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34:8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Hyun JS, et al. Effects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in rats. Toxicol Lett. 2008;182:24–28. doi: 10.1016/j.toxlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Lee HY, et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. J Nanopart Res. 2009;12:1567–1578. [Google Scholar]
- 35.Lee JH, et al. A health surveillance case study on workers who manufacture silver nanomaterials. Nanotoxicology. 2012;6:667–669. doi: 10.3109/17435390.2011.600840. [DOI] [PubMed] [Google Scholar]
- 36.Blaser SA, et al. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ. 2008;390:396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Levard C, et al. Sulfidation of silver nanoparticles: natural antidote to their toxicity. Environ Sci Technol. 2013;47:13440–13448. doi: 10.1021/es403527n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiede K, et al. Application of hydrodynamic chromatography–ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J Anal At Spectrom. 2010;25:1149–1154. [Google Scholar]
- 39.Nichols J, et al. Influence of salinity and organic matter on silver accumulation in Gulf toadfish (Opsanus beta) Aquat Toxicol. 2006;78:253–261. doi: 10.1016/j.aquatox.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, et al. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55:283–291. doi: 10.3349/ymj.2014.55.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AshaRani PV, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 42.Chang YN, et al. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials. 2012;5:2850–2871. [Google Scholar]
- 43.Singh RP, Ramarao P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol Lett. 2012;213:249–259. doi: 10.1016/j.toxlet.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Almofti MR, et al. Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003;134:43–49. doi: 10.1093/jb/mvg111. [DOI] [PubMed] [Google Scholar]
- 45.Arora S, et al. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Guo D, et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials. 2013;34:7884–7894. doi: 10.1016/j.biomaterials.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Rahmana MF, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Buzea C, et al. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR172. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 49.Park J, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun. 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 50.Silva T, et al. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ. 2014;468–469:968–976. doi: 10.1016/j.scitotenv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Sotiriou GA, et al. Non-toxic dry-coated nanosilver for plasmonic biosensors. Adv Functional Mater. 2010;20:4250–4257. doi: 10.1002/adfm.201000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.George S, et al. Surface defects on plate-shaped silver nanoparticles contribute to its hazard potential in a fish gill cell line and zebrafish embryos. ACS Nano. 2012;6:3745–3759. doi: 10.1021/nn204671v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikramullah A, et al. In vitro cytotoxicity testing of silver nano-particles in lymphocyte and sperm cells. Ind J Fund Appl Life Sci. 2013;3:44–47. [Google Scholar]
- 54.Tran QH, et al. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv Nat Sci Nanosci Nanotechnol. 2013;4:20. [Google Scholar]
- 55.dos Santos CA, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103:1931–1944. doi: 10.1002/jps.24001. [DOI] [PubMed] [Google Scholar]
- 56.Park S, et al. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl Environ Microbiol. 2014;80:2343–2350. doi: 10.1128/AEM.03427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang D, et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int J Nanomed. 2013;8:4103–4114. doi: 10.2147/IJN.S53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaikwad S, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed. 2013;8:4303–4314. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trefry JC, Wooley DP. Silver nanoparticles inhibit vaccinia virus infection by preventing viral entry through a macropinocytosis-dependent mechanism. J Biomed Nanotechnol. 2013;9:1624–1635. doi: 10.1166/jbn.2013.1659. [DOI] [PubMed] [Google Scholar]
- 60.Lima RD, et al. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 2012;32:867–879. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- 61.Gurunathan S, et al. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30:6341–6350. doi: 10.1016/j.biomaterials.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Sriram MI, et al. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomed. 2010;5:753–762. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo D, et al. The cellular uptake and cytotoxic effect of silver nanoparticles on chronic myeloid leukemia cells. J Biomed Nanotechnol. 2014;10:669–678. doi: 10.1166/jbn.2014.1625. [DOI] [PubMed] [Google Scholar]
- 64.Franco-Molina MA, et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Cancer Res. 2010;29:148–154. doi: 10.1186/1756-9966-29-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurunathan S, et al. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res Int. 2013:535796–535805. doi: 10.1155/2013/535796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurunathan S, et al. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int J Nanomed. 2013;8:4399–4413. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahu SC, et al. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J Appl Toxicol. 2014;34:1155–1166. doi: 10.1002/jat.2994. [DOI] [PubMed] [Google Scholar]
- 68.Faedmaleki F, et al. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran J Pharm Res. 2014;13:235–242. [PMC free article] [PubMed] [Google Scholar]
- 69.Foldbjerg R, et al. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85:743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 70.Nazir S, et al. Novel and cost-effective green synthesis of silver nano particles and their in vivo antitumor properties against human cancer cell lines. J Biosci Tech. 2011;2:425–430. [Google Scholar]
- 71.Austin LA, et al. Nuclear targeted silver nanospheres perturb the cancer cell cycle differently than those of nanogold. Bioconjugate Chem. 2011;22:2324–2331. doi: 10.1021/bc200386m. [DOI] [PMC free article] [PubMed] [Google Scholar]