Abstract
Objective
Supraventricular tachycardia (SVT) is the most common arrhythmia in infants. Infants are typically treated with antiarrhythmic medications, but there is a lack of evidence guiding management, thus exposing infants to risks of both inadequate therapy and medication adverse events. We used data from a large clinical database to better understand current practices in SVT management, safety of commonly used medications, and outcomes of hospitalized infants treated for SVT.
Methods
This retrospective data analysis included all infants discharged from Pediatrix Medical Group neonatal intensive care units between 1998 and 2012 with a diagnosis of SVT who were treated with antiarrhythmic medications. We categorized infants by presence of congenital heart disease other than patent ductus arteriosus. Medications were categorized as abortive, acute, or secondary prevention therapies. We used descriptive statistics to describe medication use, adverse events, and outcomes including SVT recurrence and mortality.
Results
A total of 2848 infants with SVT were identified, of whom 367 (13%) had congenital heart disease. Overall, SVT in-hospital recurrence was high (13%), and almost one fifth of our cohort (18%) experienced an adverse event. Mortality was 2% in the overall cohort and 6% in the congenital heart disease group (p<0.001). Adenosine was the most commonly used abortive therapy, but there was significant practice variation in therapies used for acute treatment and secondary prevention of SVT.
Conclusion and Practice Implication
Significant variation in SVT treatment and suboptimal outcomes warrant future clinical trials to determine best practices in treating SVT in infants.
Keywords: supraventricular tachycardia, infants, anti-arrhythmic
1. Introduction
Supraventricular tachycardia (SVT) is the most common arrhythmia in infants, with an estimated incidence of 1/250 to 1/1000 among all infants and 1/10 among infants with congenital heart disease (CHD) [1–6]. Medications used to treat SVT typically fall into one of three categories: 1) abortive therapies; 2) acute management therapies used to achieve rate control or improve the likelihood of arrhythmia abortion; and 3) secondary prevention or “prophylactic” therapies used to prevent SVT recurrence [7,8]. Across this therapeutic spectrum, over a dozen different therapies are used to treat SVT.
Although a broad armamentarium of therapies is available, there is limited evidence to guide management. Current practices are based on survey data, small clinical trials, and retrospective studies involving few (<300) infants [7,9–16]. There are no Food and Drug Administration (FDA)-labeled medications for SVT in pediatric populations, and the safety profile of commonly used medications has not been well described in infants. This is of particular importance in infants with CHD who are at high risk of adverse events and poor outcomes.
To better understand current practices in SVT management, safety of commonly used medications, and outcomes of hospitalized infants treated for SVT, we conducted a retrospective cohort study using a large database. Results of our study are useful for guiding management and identifying priorities for future clinical trials required for FDA medication labeling.
2. Methods
2.1. Database and study cohort
We performed a retrospective cohort study using data generated from electronic medical records (EMR) of infants cared for by clinicians in one of 348 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 1998–2012. The data are de-identified and stored in the Pediatrix Clinical Data Warehouse [17]. We included all infants discharged with a diagnosis of SVT who received SVT therapy during their first 120 days of life. SVT diagnosis was based on the clinical documentation of the bedside providers. The study was approved by the Duke University Institutional Review Board with waiver of informed consent.
2.2. Definitions
We categorized infants by presence of any congenital heart disease other than patent ductus arteriosus (Appendix). We defined need for inotropic support and mechanical ventilation as exposure to any inotrope (dopamine, dobutamine, epinephrine, milrinone, norepinephrine, or phenylephrine) and any invasive mechanical ventilation on days of exposure to antiarrhythmic medications. Arrhythmias were categorized as atrial flutter if the infant ever had a diagnosis of atrial flutter, Wolff Parkinson White (WPW) syndrome if the infant ever had a diagnosis of WPW syndrome, or unspecified SVT if the infant only had a diagnosis of SVT. Note that there were no infants with a diagnosis of both atrial flutter and WPW syndrome.
We defined abortive therapy as adenosine or cardioversion used at any time. We defined acute therapy as amiodarone, esmolol, or procainamide if started on day one of diagnosis. We defined secondary prevention therapy as amiodarone or esmolol if started after day one of diagnosis, or any other beta-blocker, digoxin, flecainide, or sotalol started on day one of or any time after diagnosis. Based on this definition, in case of recurrence of arrhythmia defined as any arrhythmia observed after the first day of diagnosis, adenosine or cardioversion would be classified as abortive therapy, while the initiation of another drug would be classified as secondary prevention. We defined arrhythmia recurrence as the administration of adenosine or cardioversion (abortive therapy) after day one of diagnosis. We defined multidrug therapy as exposure to more than one secondary prevention medication concomitantly. We defined mortality as death prior to hospital discharge.
To evaluate the safety of secondary prevention medications, we assessed incidence of adverse events (AEs). The list of potential AEs was compiled by reviewing the FDA labels for the medications of interest. AEs evaluated included hypotension requiring inotropes, bradycardia, hyperkalemia, hypoglycemia, and elevated liver enzymes. We defined bradycardia as a new diagnosis of bradycardia made by the treating providers while infants were receiving secondary prevention therapies. We defined elevated liver enzymes as any elevation of aspartate transaminase (AST) >600 IU/L, alanine transaminase (ALT) >225 IU/L, or gamma glutamyl transferase (GGT) >90 IU/L. We defined hyperkalemia as a serum potassium >6 mEq/L and hypoglycemia as serum glucose <40 mg/dL. We included only AEs that occurred while the infant was exposed to secondary prevention therapies.
2.3. Statistical methods
We used standard summary statistics to describe baseline characteristics. We reported the proportion of infants on antiarrhythmic medications and used chi-square tests of association or Fisher’s exact tests to compare antiarrhythmic therapy use across diagnosis groups. We described changes in secondary prevention medications over time by calculating the proportion of infants exposed to an antiarrhythmic medication in a given year. We reported hospital length of stay as median (interquartile range) and compared its distribution across diagnosis groups using Mann Whitney U tests. We described the incidence of AEs as the number of infant-days with AE occurrence/1000 infant-days on secondary prevention therapies, and used chi-square tests of association to compare incidence of AEs between medications.
3. Results
3.1. Patient demographics
Of the 887,910 infants present in the database, 2848 (0.3%) infants met our inclusion criteria and were included in this analysis. The median (interquartile range) gestational age and birth weight of the included infants was 37 weeks (34, 38) and 2950 g (2210, 3520). Median postnatal age at diagnosis was 2 days (0, 8), and 1869/2848 (66%) were diagnosed with SVT in the first week of life. Underlying arrhythmia diagnoses included unspecified SVT (2200/2848, 77%), atrial flutter (448/2848, 16%), and WPW syndrome (200/2848, 7%). Overall, 367/2848 (13%) had CHD (Table 1).
Table 1.
Patient characteristics, n (%)
| No CHD N = 2481 | CHD N= 367 | Overall N= 2848 | |
|---|---|---|---|
| Gestational age, weeks | |||
| <32 | 479 (19) | 74 (20) | 553 (20) |
| 33-36 | 774 (31) | 112 (31) | 886 (31) |
| ≥37 | 1223 (49) | 181 (49) | 1404 (49) |
| Birth weight, g | |||
| <1500 | 265 (11) | 42 (11) | 307 (11) |
| 1500-2499 | 562 (23) | 100 (27) | 662 (23) |
| >2500-3499 | 1649 (67) | 224 (61) | 1873 (66) |
| Male | 1577 (64) | 204 (56) | 1781 (63) |
| Postnatal age, days | |||
| <7 | 1678 (68) | 191 (52) | 1869 (66) |
| 7-14 | 484 (20) | 106 (29) | 590 (21) |
| >14 | 319 (13) | 70 (19) | 389 (14) |
| Type of SVT | |||
| Unspecified SVT | 1907 (77) | 293 (80) | 2200 (77) |
| Atrial flutter | 395 (16) | 53 (14) | 448 (16) |
| WP W syndrome | 179 (7) | 21 (6) | 200 (7) |
| Inotropic support on day of diagnosis | 144 (6) | 36 (10) | 180 (6) |
| Mechanical ventilation on day of diagnosis | 441 (18) | 102 (28) | 543 (19) |
CHD, congenital heart disease; SVT, supraventricular tachycardia; WPW, Wolff Parkinson White.
3.2. SVT therapy
Nearly half of the infants received abortive therapy (1379/2848, 48%), including adenosine (1239/1379, 90%) and cardioversion (143/1379, 10%) (Table 2). Cardioversion was primarily used in infants with a diagnosis of atrial flutter (105/143, 76%). Only 3 infants received both adenosine and cardioversion. Abortive therapy was used less frequently in infants with versus those without CHD (155/367, 42%, vs. 1227/2481, 50%, p=0.05).
Table 2.
Type of SVT therapy, n (%)
| No CHD N= 2481 | CHD N=367 | Overall N=2848 | |
|---|---|---|---|
| Abortive therapy | 1227 (50) | 155 (42) | 1379 (48) |
| Adenosine | 1095 (89) | 144 (93) | 1239 (90) |
| Cardioversion | 132 (11) | 11 (7) | 143 (10) |
| Acute therapy | 145 (6) | 34 (9) | 179 (6) |
| Amiodarone | 66 (46) | 15 (44) | 81 (45) |
| Esmolol | 60 (41) | 13 (38) | 73 (41) |
| Procainamide | 27 (19) | 6 (18) | 33 (18) |
| Secondary prevention therapy | 2206 (89) | 317 (86) | 2523 (89) |
| Amiodarone | 135 (6) | 31 (10) | 166 (7) |
| Beta-blocker | 1035 (47) | 147 (46) | 1182 (47) |
| Digoxin | 1382 (63) | 191 (60) | 1573 (62) |
| Flecainide | 77 (3) | 9 (3) | 86 (3) |
| Sotalol | 80 (4) | 14 (4) | 94 (4) |
CHD, congenital heart disease; SVT, supraventricular tachycardia.
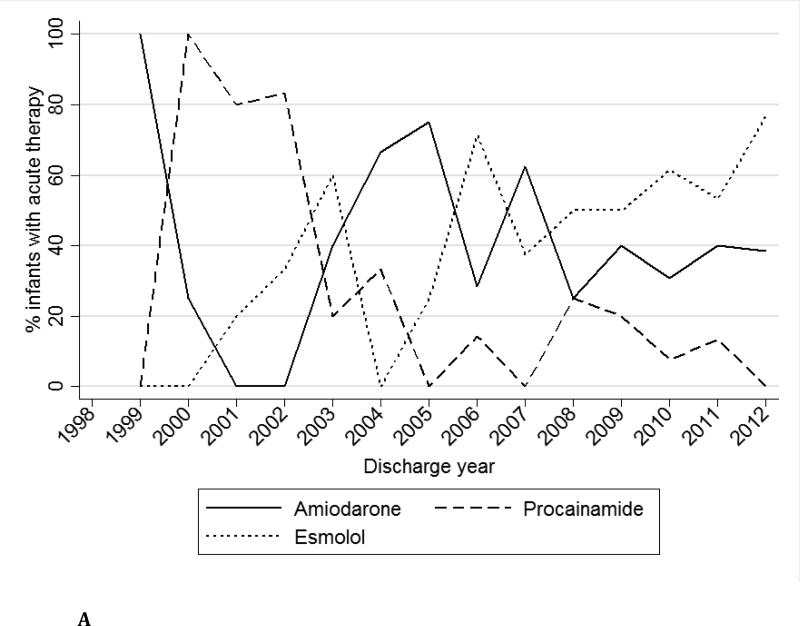
Acute therapy was used in 179/2848 (6%) infants. The most commonly used acute therapy was amiodarone (81/179, 45%), followed by esmolol (73/179, 41%) and procainamide (33/187, 18%) (Table 2). Acute therapy was used more frequently in infants with versus those without CHD (34/367, 9%, vs. 145/2481, 6%, p=0.01). Over time, the use of procainamide decreased, while esmolol and amiodarone use increased for acute therapy (Fig. 1a). Of those receiving abortive therapies, only 115/1379 (8%) also received acute therapy on the same day.
Fig. 1a.
Acute therapy medications over time.
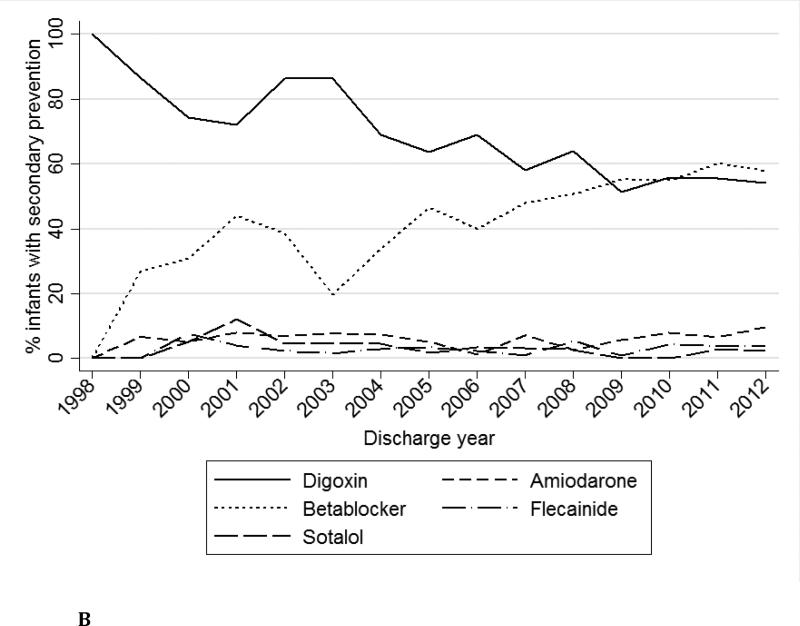
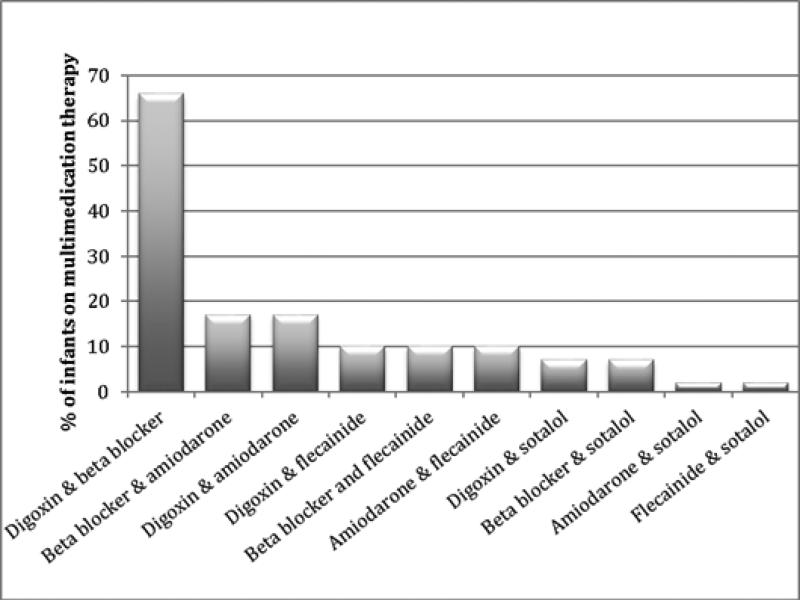
Secondary prevention therapy was used in 2523/2848 (89%) infants. The most commonly used secondary prevention therapies were digoxin (1573/2523, 62%) and beta-blockers (1182/2523, 47%), while amiodarone (166/2523, 7%), flecainide (86/2523, 3%), and sotalol (94/2523, 4%) were less commonly prescribed (Table 2). There was no difference in the frequency of secondary prevention therapy used between infants with and those without CHD (317/367, 86%, vs. 2206/2481, 89%, p=0.15). Over time, digoxin use decreased while propranolol use increased (Fig. 1b). Multidrug secondary prevention therapy was used in 490/2532 (19%) infants, and a digoxin and beta-blocker combination (321/490, 66%) was the most common (Fig. 2). Compared with infants who did not receive secondary prevention, infants on secondary prevention had lower birth weight (BW) (2948 [2195, 3505] grams vs. 3100 [2350, 3620] grams, p=0.03) while GA did not differ (p=0.58). There was no significant difference in the proportion of infants with and without CHD who received secondary prevention therapy (317/367 [86%] vs. 2206/2481 [89%], p=0.15). Hospital length of stay was longer in infants receiving secondary prevention therapy (11 [5, 24] days vs. 7 [4, 22] days, p<0.001).
Fig. 1b.
Secondary prevention medications over time.
Fig. 2.
Frequency of multidrug therapy.
3.3. Outcomes and adverse events
Overall mortality was 58/2848 (2%), and median hospital length of stay among survivors was 11 days (6, 24). Compared to infants without CHD, infants with CHD had higher mortality (21/367, 6%, vs. 37/2481, 2%, p<0.001) and longer median hospital length of stay among survivors (15 [7, 33] days vs. 11 [6, 23] days, p <0.005). SVT recurrence requiring adenosine or cardioversion beyond the first day of therapy was documented in 374/2848 (13%) infants. Incidence of arrhythmia recurrence or use of multidrug therapy did not differ between infants with and without CHD (48/367, 13%, vs. 326/2481, 13%, p=0.98, and 65/367, 18%, vs. 425/2481, 17%, p=0.78) (Table 3).
Table 3.
Outcomes, n (%)
| No CHD N = 2481 | CHD N = 367 | p value | |
|---|---|---|---|
| Mortality | 37 (2) | 21 (6) | <0.001 |
| Hospital length of staya (days) | 11 (6, 23) | 15 (7,33) | <0.005 |
| Arrhythmia recurrence | 326 (13) | 48 (13) | 0.98 |
| Multidrug therapy | 425 (17) | 65 (18) | 0.78 |
Reported as median (25th %ile, 75th %ile) among survivors.
CHD, congenital heart disease.
AEs occurred in 509/2848 (18%) infants while on secondary prevention. Median GA and BW were lower in infants who suffered at least one AE (35 [32, 38] weeks vs. 37 [34, 39] weeks, p<0.001, and 2739 [1860, 3487] grams vs. 3010 [2260, 3540] grams, p<0.001). There was no significant difference in the proportion of infants with and without CHD who suffered at least one AE (66/367 [18%] vs. 43/2481 [18%], p=0.95). Hospital length of stay was longer in infants with at least one AE (16 [7, 36] days vs. 9 [5, 19] days, p<0.001). The most common AEs were hypotension, hyperkalemia, hypoglycemia, elevated liver enzymes, and bradycardia (Table 4). Incidence of hypotension was highest on days of exposure to flecainide (143/1000 infant days) and amiodarone (161/1000 infant days) but lower on days of exposure to beta-blockers (16/1000 infant days) and sotalol (18/1000 infant days). Incidence of elevated liver enzymes was highest for days of exposure to flecainide (36/1000 infant days) and lower for days of exposure to beta-blockers (1/1000 infant days) and sotalol (0/1000 infant days).
Table 4.
Adverse events (per 1000 infant-days) * aspartate transaminase (AST) >600 IU/L
| Digoxin | Beta-blockers | Amiodarone | Flecainide | Sotalol | |
|---|---|---|---|---|---|
| Hypotension | 49 | 16 | 161 | 143 | 18 |
| Hyperkalemia | 37 | 43 | 30 | 36 | 18 |
| Hypoglycemia | 12 | 13 | 30 | 0 | 0 |
| Elevated liver enzymesa | 14 | 1 | 10 | 36 | 0 |
| Bradycardia | 1 | 1 | 0 | 0 | 0 |
Alanine transaminase (ALT) >225 IU/L or gamma glutamyl transferase (GGT) >90 IU/L.
4. Discussion
This is the largest study to date evaluating treatment practices and in-hospital outcomes of infants with SVT. Overall, SVT in-hospital recurrence was high (13%), and almost one fifth of our cohort experienced an AE while receiving secondary prevention therapy. In addition, we found significant practice variation in medications used to treat SVT. Taken together, these findings suggest that further studies are needed to establish best practices in treatment of SVT in infants to improve outcomes and reduce risk of treatment-related AEs.
SVT is an important comorbidity in the NICU population. Although isolated SVT in otherwise healthy infants is rarely fatal, SVT may contribute to hemodynamic instability and adversely affects outcomes in children with associated comorbidities [18]. In a single-center study of 1755 patients aged ≤25 years with a discharge diagnosis of SVT, the in-hospital mortality among patients with structural heart disease was 6% compared to only 1% for those without structural heart disease. In a multicenter study of 171 infants hospitalized at a Pediatric Health Information System (PHIS)-participating institution with a primary diagnosis of SVT and no CHD, no patients died during hospitalization [16]. Results were similar in our cohort, where mortality was significantly higher in infants with SVT and CHD compared to those with SVT without CHD, even though we did observe mortalities in the latter group. Hospital length of stay in patients without CHD was also longer than reported in prior studies [9,18]. In the PHIS study of 171 infants with SVT but no CHD, median length of stay was only 4 days. Differences in age and severity of illness may explain the higher mortality and longer length of stay in both infants with and those without CHD in our cohort, as evidenced by the fact that almost half of the infants in the PHIS study were not treated in an intensive care unit, while all infants in our study were identified in a NICU.
Adenosine is the abortive therapy of choice for the majority of infants. Adenosine has been previously described as first-line abortive therapy for all types of SVT except atrial flutter, where cardioversion is favored [19–21]. Given the low rate of atrial flutter observed in our cohort, we anticipated that adenosine would be most frequently used.
Acute management therapy practices vary widely but have not changed significantly over the past decade. Acute management therapies such as amiodarone, esmolol, and procainamide are often used to treat intractable SVT that either cannot be aborted with adenosine or cardioversion, or rapidly recurs [11,22]. These medications were used on day one of therapy in 7% of the overall cohort, suggesting an overall low prevalence of intractable SVT in hospitalized infants. Acute therapy was used more frequently in infants with CHD. This may be due to increased refractoriness of SVT in these infants or to greater concerns about the risk of recurrence and associated hemodynamic compromise in this fragile population. Amiodarone was the most commonly used acute therapy in our cohort. Amiodarone therapy for acute management of recurrent adenosine-refractory SVT in children has been reported previously, including one retrospective study comparing its safety and efficacy to procainamide [10–12]. In a study of 37 patients less than 19 years old, procainamide was more effective than amiodarone in treating recurrent SVT without observed differences in adverse events [11]. However, when limiting the comparison to neonates less than 30 days of age (n=20), there was no difference in efficacy or safety between the two therapies. Of note, amiodarone was used more frequently in neonates (16/20 on amiodarone vs. 4/20 on procainamide). No other trials have evaluated safety or efficacy of acute therapies for neonatal SVT. Considering the paucity of evidence to guide the optimal therapy choice in this situation, it is not surprising to see such heterogeneity in acute SVT therapy in infants.
Secondary prevention therapies vary widely, and medication preferences have changed over time. In our cohort, beta-blockers appear to be replacing digoxin as the medication of choice in recent years. Digoxin and beta-blockers are generally considered first-line treatment for secondary prevention of SVT [4,7,16]. However, there is no evidence-based explanation for the marked increase in beta-blocker use and decrease in digoxin use over time. Indeed, the 2012 Study of Antiarrhythmic Medications in Infancy (SAMIS) trial found no significant difference in efficacy between the two medications [9]. Notably, this trial was stopped prematurely due to difficulty with patient accrual, limiting the interpretability of its findings [9]. A retrospective comparative effectiveness study using a subset of the Pediatrix cohort (n = 457) described here corroborated the trend observed in the SAMIS trial and demonstrated that digoxin was more efficacious than beta-blockers with no difference in incidence of adverse events [23]. Similar to prior studies and provider surveys, therapies that are normally considered second-line, such as amiodarone, flecainide, or sotalol, were used less frequently and were more often prescribed as part of a multidrug regimen [7,16]. In the PHIS study, 44% of infants had received second-line therapy and 45% had received multidrug therapy at the time of discharge [16]. The combination of digoxin and beta-blockers was the most common multidrug therapy, followed by combinations that included amiodarone. We observed similar distribution of multidrug combinations in our study, with digoxin and beta-blockers being the most common, but fewer infants (17%) received multidrug therapy overall. The rate of multidrug therapy in the PHIS study was high and, as the authors concluded, may have been related to the severity of SVT episodes or to prior failures of outpatient therapies. Our cohort consisted exclusively of infants admitted to NICUs, most of which would not yet have had a trial of outpatient therapy.
None of the secondary prevention therapies that we studied demonstrated a markedly different safety profile. Overall, AEs were seen in almost 20% of infants while being treated with a secondary prevention therapy. There was a higher incidence of hypotension in infants on flecainide and amiodarone, and infants on flecainide also had significantly higher incidence of elevated liver enzymes. Assessment of AEs in a retrospective study is difficult due to comorbidities and use of concurrent medications; however, these data can be important for detecting rare events and for comparing therapies. Of note, hypotension is a known side effect of amiodarone [24,25], and the higher incidence in infants on amiodarone may indicate a need to be especially cautious in hemodynamically unstable infants. Additionally, the higher incidence of elevated liver enzymes in patients on flecainide may warrant further study. In previous pharmacokinetic studies in infants, hepatic function was not measured or recorded as an adverse event [14,26]. However, in adult studies, elevated liver enzymes have been reported [27]. The mechanism is unclear but tends to be transient and self-resolving [27].
The strengths of our study include its large sample size and the detailed clinical data provided by the Pediatrix Clinical Data Warehouse. In contrast to an administrative database, we were able to extract daily medication usage, diagnoses, and laboratory and clinical adverse events. This allowed us to provide a detailed description of timing and nature of pharmacotherapy in infants with SVT. In addition, we were able to provide additional information about the safety profile of secondary prevention therapies in infants, an important aspect given the high rate of spontaneous resolution of SVT in infancy and the associated need to minimize medication-related harm. Our study is limited to information on infants hospitalized in the NICU. We lack data on SVT recurrence, changes in medication regimens, and adverse events experienced by infants in the outpatient setting. Diagnoses are made based on bedside provider documentation, and we lack electrocardiogram results to confirm diagnoses of SVT and provide a more specific categorization of SVT types by underlying pathophysiologic mechanism. Such a categorization would frequently require consultation with a pediatric cardiologist or electrophysiologist, and possibly include invasive diagnostic testing. While this may be more specific, we believe that our classification based on the documentation of bedside providers may provide a more realistic picture of the daily management of infants with SVT. We do not have information on the occurrence and timing of any cardiac surgical intervention. This precludes any description or comments about perioperative arrhythmia incidence and pharmacologic practice variation during that time. We also do not have drug dosing information, which may affect outcomes and safety. Some AEs, such as abnormal thyroid function or ventricular arrhythmias, and effects on ECG parameters such as QRS duration and QT interval, were not captured. Despite these limitations, our study provides valuable insights into SVT treatment in infants.
In summary, this study is the largest to date evaluating current practices and outcomes of SVT in infants. Overall, there was significant variation in prescribing habits for both acute management and secondary prevention therapies. This variation likely reflects a general lack of evidence in management of SVT and is especially worrisome in tenuous infants with CHD or other serious comorbidities where SVT is associated with a relatively high mortality rate. Furthermore, there remains room for improvement in reducing arrhythmia recurrence and the incidence of adverse events. Considering the significant variation in SVT treatment and suboptimal outcomes, further clinical trials are warranted for determining best practices in treating SVT in infants.
Highlights.
- In-hospital recurrence of supraventricular tachycardia in infants is common.
- Antiarrhythmic therapy practices vary widely, especially for drugs used as secondary prevention.
- Adverse events are common in infants exposed to antiarrhythmic drugs.
ACKNOWLEDGMENTS
Funding disclosure
P.Y.C. and R.H.C. do not have any financial disclosures. P.B.S. receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (HHSN2752010000031 and 1R01-HD081044-01) and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). K.D.H. and C.P.H. receive salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). These sponsors played no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Appendix
Appendix.
Cardiac lesions in infants with CHD
| Infants with CHD N=367 (%) | |
|---|---|
| Aortic valve anomaly | 9 (2) |
| Atrial septal defect | 122 (33) |
| Atrioventricular canal defect | 25 (7) |
| Double outlet right ventricle | 11 (3) |
| Ebstein's anomaly | 36 (10) |
| Hypoplastic right ventricle | 3 (1) |
| Interrupted aortic arch | 2 (<1) |
| Pulmonary atresia | 18 (5) |
| Pulmonary valve stenosis | 13 (4) |
| Total anomalous pulmonary venous return | 5 (1) |
| Transposition of the great arteries | 16 (4) |
| Tricuspid atresia | 5 (1) |
| Truncus arteriosus | 5 (1) |
| Ventricular septal defect | 142 (39) |
| Other, non-cyanotic | 32 (9) |
| Other, cyanotic | 7 (2) |
Diagnoses are not mutually exclusive.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no potential conflict of interest.
REFERENCES
- 1.Nadas AS, Daeschner CW, Roth A, Blumenthal SL. Paroxysmal tachycardia in infants and children: study of 41 cases. Pediatrics. 1952;9:167–81. [PubMed] [Google Scholar]
- 2.Lundberg Å. Paroxysmal tachycardia in infancy: a clinical and experimental study. Acta Paediatr. 1963;52:192–5. doi: 10.1111/j.1651-2227.1963.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 3.Garson A, Gillette PC. Electrophysiologic studies of supraventricular tachycardia in children. I. Clinical-electrophysiologic correlations. Am Heart J. 1981;102:233–50. doi: 10.1016/s0002-8703(81)80015-4. [DOI] [PubMed] [Google Scholar]
- 4.Weindling SN, Saul JP, Walsh EP. Efficacy and risks of medical therapy for supraventricular tachycardia in neonates and infants. Am Heart J. 1996;131:66–72. doi: 10.1016/s0002-8703(96)90052-6. [DOI] [PubMed] [Google Scholar]
- 5.Tanel RE, Walsh EP, Triedman JK, Epstein MR, Bergau DM, Saul JP. Five-year experience with radiofrequency catheter ablation: implications for management of arrhythmias in pediatric and young adult patients. J Pediatr. 1997;131:878–87. doi: 10.1016/s0022-3476(97)70037-4. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi A, Black GB, Park YM, Jerrell JM. Factors associated with the occurrence and treatment of supraventricular tachycardia in a pediatric congenital heart disease cohort. Pediatr Cardiol. 2014;35:368–73. doi: 10.1007/s00246-013-0784-3. [DOI] [PubMed] [Google Scholar]
- 7.Wong KK, Potts JE, Etheridge SP, Sanatani S. Medications used to manage supraventricular tachycardia in the infant a North American survey. Pediatr Cardiol. 2006;27:199–203. doi: 10.1007/s00246-005-1126-x. [DOI] [PubMed] [Google Scholar]
- 8.Tavera MC, Bassareo PP, Neroni P, Follese C, Manca D, Montis S, et al. Supraventricular tachycardia in neonates: antiarrhythmic drug choice dilemma. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):30–3. doi: 10.3109/14767058.2010.517937. [DOI] [PubMed] [Google Scholar]
- 9.Sanatani S, Potts JE, Reed JH, Saul JP, Stephenson EA, Gibbs KA, et al. The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984–91. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 10.Dilber E, Mutlu M, Dilber B, Aslan Y, Gedik Y, Celiker A. Intravenous amiodarone used alone or in combination with digoxin for life-threatening supraventricular tachyarrhythmia in neonates and small infants. Pediatr Emerg Care. 2010;26:82–4. doi: 10.1097/PEC.0b013e3181ce2f6a. [DOI] [PubMed] [Google Scholar]
- 11.Chang PM, Silka MJ, Moromisato DY, Bar-Cohen Y. Amiodarone versus procainamide for the acute treatment of recurrent supraventricular tachycardia in pediatric patients. Circ Arrhythm Electrophysiol. 2010;3:134–40. doi: 10.1161/CIRCEP.109.901629. [DOI] [PubMed] [Google Scholar]
- 12.Etheridge SP, Craig JE, Compton SJ. Amiodarone is safe and highly effective therapy for supraventricular tachycardia in infants. Am Heart J. 2001;141:105–10. doi: 10.1067/mhj.2001.111765. [DOI] [PubMed] [Google Scholar]
- 13.Ferlini M, Colli AM, Bonanomi C, Salvini L, Galli MA, Salice P, et al. Flecainide as first-line treatment for supraventricular tachycardia in newborns. J Cardiovasc Med (Hagerstown) 2009;10:372–5. doi: 10.2459/JCM.0b013e328329154d. [DOI] [PubMed] [Google Scholar]
- 14.Knudson JD, Cannon BC, Kim JJ, Moffett BS. High-dose sotalol is safe and effective in neonates and infants with refractory supraventricular tachyarrhythmias. Pediatr Cardiol. 2011;32:896–903. doi: 10.1007/s00246-011-0010-0. [DOI] [PubMed] [Google Scholar]
- 15.Price JF, Kertesz NJ, Snyder CS, Friedman RA, Fenrich AL. Flecainide and sotalol: a new combination therapy for refractory supraventricular tachycardia in children <1 year of age. J Am Coll Cardiol. 2002;39:517–20. doi: 10.1016/s0735-1097(01)01773-9. [DOI] [PubMed] [Google Scholar]
- 16.Seslar SP, Garrison MM, Larison C, Salerno JC. A multi-institutional analysis of inpatient treatment for supraventricular tachycardia in newborns and infants. Pediatr Cardiol. 2013;34:408–14. doi: 10.1007/s00246-012-0474-6. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system—tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Salerno JC, Garrison MM, Larison C, Seslar SP. Case fatality in children with supraventricular tachycardia in the United States. Pacing Clin Electrophysiol. 2011;34:832–6. doi: 10.1111/j.1540-8159.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 19.Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337–82. doi: 10.1093/europace/eut082. [DOI] [PubMed] [Google Scholar]
- 20.Texter KM, Kertesz NJ, Friedman RA, Fenrich AL., Jr Atrial flutter in infants. J Am Coll Cardiol. 2006;48:1040–6. doi: 10.1016/j.jacc.2006.04.091. [DOI] [PubMed] [Google Scholar]
- 21.Van Gelder IC, Tuinenburg AE, Schoonderwoerd BS, Tieleman RG, Crijns HJ. Pharmacologic versus direct-current electrical cardioversion of atrial flutter and fibrillation. Am J Cardiol. 1999;84:147R–51R. doi: 10.1016/s0002-9149(99)00715-8. [DOI] [PubMed] [Google Scholar]
- 22.Manole MD, Saladino R. Emergency department management of the pediatric patient with supraventricular tachycardia. Pediatr Emerg Care. 2007;23:176–85. doi: 10.1097/PEC.0b013e318032904c. [DOI] [PubMed] [Google Scholar]
- 23.Hornik CP, Chu PY, Li JS, Clark RH, Smith PB, Hill KD. Comparative effectiveness of digoxin and propranolol for supraventricular tachycardia in infants. Pediatr Crit Care Med. 2014;15:839–45. doi: 10.1097/PCC.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [September 3, 2014]; Amiodarone IV Label. Available at: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=990c1cb3-3370-75e0-ff2abb51379f5dab.
- 25. [September 3, 2014]; Amiodarone PO label. Available at: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08641e51-abca-4c1c-ba19-d24435332018.
- 26.Läer S, Elshoff J-P, Meibohm B, Weil J, Mir TS, Zhang W, et al. Development of a safe and effective pediatric dosing regimen for sotalol based on population pharmacokinetics and pharmacodynamics in children with supraventricular tachycardia. J Am Coll Cardiol. 2005;46:1322–30. doi: 10.1016/j.jacc.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 27. [May 16, 2014]; NIDDK LiverTox Clinical and Research Information on Drug-Induced Liver Injury. Sotalol. Available at: http://livertox.nlm.nih.gov/Sotalol.htm. [PubMed]