Abstract
Bacteria colonize cystic fibrosis (CF) airways, and while T cells with appropriate antigen specificity are present in draining lymph nodes, they are conspicuously absent from the lumen. To account for this absence, we hypothesized that polymorphonuclear neutrophils (PMNs), recruited massively into the CF airway lumen and actively exocytosing primary granules, also suppress T-cell function therein. Programmed Death-Ligand 1 (PD-L1), which exerts T-cell suppression at a late step, was expressed bimodally on CF airway PMNs, delineating PD-L1hi and PD-L1lo subsets, while healthy control (HC) airway PMNs were uniformly PD-L1hi. Blood PMNs incubated in CF airway fluid lost PD-L1 over time, and in coculture, antibody blockade of PD-L1 failed to inhibit the suppression of T-cell proliferation by CF airway PMNs. In contrast with PD-L1, arginase 1 (Arg1), which exerts T-cell suppression at an early step, was uniformly high on CF and HC airway PMNs. However, arginase activity was high in CF airway fluid and minimal in HC airway fluid, consistent with the fact that Arg1 activation requires primary granule exocytosis, which occurs in CF, but not HC, airway PMNs. In addition, Arg1 expression on CF airway PMNs correlated negatively with lung function and positively with arginase activity in CF airway fluid. Finally, combined treatment with arginase inhibitor and arginine rescued the suppression of T-cell proliferation by CF airway fluid. Thus, Arg1 and PD-L1 are dynamically modulated upon PMN migration into human airways, and, Arg1, but not PD-L1, contributes to early PMN-driven T-cell suppression in CF, likely hampering resolution of infection and inflammation.
INTRODUCTION
CF is a life-shortening genetic disease affecting approximately 70,000 people worldwide (1). CF is caused by recessive mutations in the CF transmembrane conductance regulator (cftr), which primarily affect epithelial cell function. While CFTR dysfunction leads to multi-organ disease, morbidity and mortality in CF are primarily due to airway pathology (2). Hallmarks of CF airway pathology are impaired mucociliary clearance, bacterial colonization of the airway lumen, and chronic inflammation (3). CF airway inflammation is characterized by early, massive and sustained recruitment of blood PMNs through the submucosa into the lumen (4). Despite the constant influx of PMNs into the lumen, several types of bacterial and fungal pathogens evade eradication and colonize the CF airways, resulting in chronic infections that may persist for years. The inability of the immune system to switch off inflammation may suggest an overall defect, or dampening, of the adaptive arm of the immune response.
In recent years, several mechanisms that modulate the adaptive immune response have been identified in PMNs (5). We have previously shown that while CF airway fluid contains many dead PMNs, it also contains a sizeable population of live PMNs that are not committed to rapid death, as previously thought. Live CF airway PMNs exhibit a hyperexocytic phenotype with significant release of protease-rich primary granules (6), which contributes to the tissue damage observed in CF airway disease. Along with increased exocytosis, live airway PMNs are also reprogrammed along anabolic and pro-survival pathways, as compared to their circulating counterparts (7, 8). In addition, we have shown that these reprogrammed PMNs express receptors generally associated with antigen-presenting functions, including class II MHC, CD80 and CD294 (6), suggesting that they may be capable of modulating T-cell function.
Recent data have shown that PMNs are able to suppress T cells through cell-cell contact via the surface receptor PD-L1 (9). PD-L1 binds to Programmed Death 1 (PD-1) on the surface of activated T cells, and triggers T-cell exhaustion, which is characterized by decreased proliferation, cytotoxicity and cytokine production by T cells (10). PD-L1 also interacts with CD80, blocking the secondary signal needed for T-cell activation and cytokine production (11). PD-L1 mediated T-cell suppression occurs generally at a late step, and is believed to play a major homeostatic role, preventing overarching T-cell responses.
Several studies have also demonstrated that PMNs can inhibit T-cell function through the release of suppressive molecules, such as Arg1 and reactive oxygen species (ROS), both in the context of in vitro cell cultures and in human disease (12–16). Previous studies have shown that arginase activity is increased in CF airways (17, 18), suggesting a potential role for the suppression of T-cell function by this enzyme in CF airway disease. Arg1 is stored in the primary and tertiary granules of human PMNs and requires the release of primary granules to become fully active (13, 19, 20). Arg1 cleaves the amino acid arginine to form ornithine and urea. Arginine is necessary for the expression of the invariant ζ-chain of the T-cell receptor (TCR) complex, and in environments of depleted arginine, T-cell function is inhibited (14, 21, 22). Arg1-mediated T-cell suppression occurs at an early step and may thus play a pathological role if it prevents T-cells from contributing to the normal course of an immune response.
It was previously shown that T cells with appropriate antigenic specificity to luminal pathogens (23) are present in high numbers in the CF airway submucosa, alveolar septa and draining lymph nodes (24–27). However, there is a striking paucity of T cells in the lumen itself (24, 27), suggesting that even though appropriate T cells develop in CF patients, they may be unable to access the lumen and/or indeed access the lumen but are rapidly and thoroughly eliminated from this compartment by an as yet unknown mechanism. These intriguing data demonstrate the existence of a strict compartmentalization of PMNs and T-cells in CF airways, with PMNs accumulating in the lumen, while T-cells stay in the submucosa and lymph nodes and are excluded from the lumen preventing them from exerting key regulatory functions therein.
To account for the relative absence of T cells in the lumen of CF airways, we hypothesized that PMNs, the most dominant immune cell in CF airways, are modulated upon entry into the lumen of the lung to suppress T-cell function. We demonstrate that PMNs in the CF airway lumen significantly modulate Arg1 and PD-L1 on their surface compared to matched blood PMNs. We determined that CF airway PMNs did not mediate T-cell apoptosis or decrease T-cell proliferation through PD-L1 signaling, and this may be due to cleavage or reuptake of PD-L1 from the surface of PMNs in CF airways. However, we found that CF patient airway fluid supernatant (hereafter referred to as ASN) completely suppressed T-cell proliferation in vitro. This suppression was largely caused by the presence of Arg1, since a combination of exogenous arginine and arginase inhibitor significantly rescued T-cell proliferation. The increased expression of Arg1 on the surface of airway PMNs positively correlated with Arg1 activity in the sputum and negatively correlated with lung function.
Overall, our results suggest that as PMNs enter the CF airway lumen, their ability to suppress T-cell function at an early step is heightened, in part through upregulation of Arg1 function. This discovery bears pathological significance, since it helps explain a long-standing paradox of chronic CF airway disease, i.e., the compartmentalization of T cells in the submucosa and their exclusion from the lumen where pathogens thrive.
MATERIALS AND METHODS
Human subjects
This study received the approval of the Institutional Review Board at Emory University. All subjects provided written informed consent before undergoing study procedures. Healthy control (HC) subjects were >18 years of age, excluding pregnant and breast-feeding individuals. CF was diagnosed by sweat chloride (60 mEq/L), using a quantitative iontophoresis test and/or documentation of two identifiable cftr mutations. CF clinical data included age, gender, mutations, lung function, current medications and microbiology (see demographic data in Table I). CF samples were collected on outpatient visits, corresponding to routine clinical check-ups, or inpatient visits, where patients were hospitalized due to an exacerbation (samples collected 2 to 5 days after inception of oral or intravenous antibiotics treatment).
Table I.
Demographics of CF patients.
| Gender | Visit Type | Age (years) | CFTR mutation | FVC (L) | Azith | Tobra | S. aureus | P. aeruginosa | C. albicans | A. fumigatus |
|---|---|---|---|---|---|---|---|---|---|---|
| F | O | 24 | HZ | 2.28 | N | Y | N | N | N | N |
| M | O | 46 | HZ | 3.58 | N | N | ND | ND | ND | ND |
| F | O | 23 | HZ | 2.29 | Y | N | S | M | Y | Y |
| F | O | 40 | HZ | 2.50 | Y | Y | ND | ND | ND | ND |
| F | I | 31 | HO | 1.53 | Y | N | R | P | N | N |
| F | I | 24 | HZ | 2.94 | Y | Y | R | N | Y | N |
| M | I | 24 | OT | 2.98 | N | Y | N | M | N | N |
| M | I | 24 | HZ | 3.67 | Y | Y | S | M | N | N |
| F | I | 24 | HO | 2.15 | Y | Y | R | M | Y | N |
| M | O | 24 | HO | 2.48 | Y | Y | R | M | N | N |
| M | O | 31 | HO | 4.67 | Y | Y | ND | ND | ND | ND |
| M | I | 19 | HO | 3.34 | N | N | S | M | N | N |
| M | O | 25 | HO | 4.12 | N | N | R | N | N | N |
| M | I | 31 | HZ | 4.49 | Y | Y | N | P | Y | Y |
| F | I | 36 | HZ | 3.75 | Y | N | R | N | Y | N |
| F | I | 23 | HO | 4.20 | Y | Y | N | M | N | N |
| F | O | 25 | HO | 2.67 | Y | Y | ND | ND | ND | ND |
| F | O | 24 | HZ | 3.09 | Y | Y | R | P | N | N |
| M | O | 37 | HZ | ND | N | Y | R | M | N | N |
| M | I | 27 | HO | 2.56 | Y | Y | N | M | N | N |
| M | I | 20 | HO | 3.25 | N | Y | S | M | N | N |
| M | I | 29 | HO | ND | Y | Y | S | M | N | N |
| F | I | 31 | HO | 1.86 | N | Y | R | M | N | N |
| F | I | 29 | HZ | 1.41 | Y | N | N | M | N | N |
|
F=12 M=12 |
O=10 I=14 |
28.0 ±1.3 |
HO=12 HZ=11 OT=1 |
2.99 ±1.96 |
Y=16 N=8 |
Y=17 N=7 |
S=5 R=9 N=6 ND=4 |
P=3 M=13 N=4 ND=4 |
Y=5 N=15 ND=4 |
Y=2 N=18 ND=4 |
O=outpatient visit, I=inpatient visit, HO=delta F508 homozygote, HZ=Compound heterozygotes (comprising one delta F508 and another mutation of the CF gene), OT=two mutations other than delta F508. Infection status: for Pseudomonas aeruginosa, M=mucoid, P=planktonic; for Staphylococcus aureus, S=methicillin-sensitive, R=methicillin-resistant, N=No, Y = Yes, and ND = not determined. Azith=azithromycin and Tobra=tobramycin.
Sample collection and processing
Blood was collected by venipuncture for CF and HC subjects. Sputum was collected from CF patients by spontaneous expectoration, and from HC subjects by induction, and processed as previously described (6). In brief, sputum was mechanically dissociated using repeated passage through a 18″G needle after addition of 6 ml of PBS with 2.5 mM EDTA. Blood and dissociated sputum were then processed by dual centrifugation, generating fluid (platelet-free plasma and cell- and bacteria-free airway supernatant -ASN-, respectively) and cell fractions. Platelet-free plasma and ASN were used for ELISAs, arginase activity assay, quantification of arginine, ornithine and citrulline by mass spectrometry, and in vitro coculture assays. Blood and airway cell fractions were resuspended in PBS and used for flow cytometric analysis.
Flow cytometric analysis of PD-L1 and Arg1 surface expression
Blood and airway cell staining (in their native state or upon in vitro incubation with agents to induce granule exocytosis), flow cytometric acquisition and analysis were performed as described (8). As illustrated in Figure S1A and S1B, live PMNs were analyzed using Live/Dead (Invitrogen), and antibodies against CD15 (W6D3), CD16 (3G8), CD35 (E11), CD41a (HIP8), CD45 (HI30), CD63 (H5C6), CD66b (G10F5), and PD-L1 (29E.2A3), from BioLegend, and Arg1 (6G3, Hycult Biotech). Data were acquired on a LSRII flow cytometer (BD Biosciences), analyzed with Flowjo (Treestar) and reported as median fluorescence intensity (MFI).
Transcriptional analysis of sorted blood PMNs and monocytes
Blood PMNs and monocytes were stained as indicated above and sorted using a FACSJazz (BD Biosciences), as illustrated in Supplementary Figure S2A. Sorted fractions were reanalyzed by flow cytometry, routinely yielding levels >98% viability and purity. Sorted fractions were pelleted at 800G for 10 min at 4°C and the pellet was homogenized and RNA was stabilized using QIAShredder spin columns (QIAGEN), as per the manufacturer’s guidelines. Eluates were frozen and stored at −70°C until all sorted fractions were collected. For analysis, eluates were thawed and RNA was isolated using the RNeasy kit (QIAGEN), as per the manufacturer’s guidelines. A Fluidigm array was manufactured using a 48 target genes of interest, including several housekeeping PMN and monocyte genes (including HMOX1, SLC1A5, S100A8, S100A9, and CAMP), as well as PD-L1 and Arg1, and multiplexed qPCR was performed on mRNA from the sorted fractions as previously described (28). mRNA levels are expressed as threshold cycles (Ct), with 30 cycles being the lower detection limit, and clustering analysis was performed using JMP10 (SAS Institute), as illustrated in Supplementary Figures S2B and S2C.
T-cell activation, proliferation and apoptosis assays
PBMCs were isolated from HC blood using Ficoll-Paque PLUS (GE Healthcare) gradients. PBMCs were labeled with 2.5 μM CFSE, then cultured at 1.5–2 × 106 cells/mL in a CD3 (OKT3, 5μg/mL, eBioscience)-coated or a control 96-well plate. RPMI (containing 2mM L-arginine), with Penicillin-Streptomycin and 10% FBS, with or without CF ASN, was used to incubate the PBMCs. Cells were exposed to medium +/− CF ASN either 2 hours prior to addition to the CD3-coated plates or added directly to the plate. Some wells were supplemented daily with 1mM L-arginine. In some conditions, CF ASN was pre-treated for 10 minutes with the arginase inhibitor N-ω-Hydroxy-L-norarginine (nor-NOHA, 250 μM, Enzo Life Sciences) before incubation with PBMCs. After 96 hours, PBMCs were collected and stained for flow cytometric analysis with Live/Dead and antibodies against CD5 (UCHT2), CD56 (MEM-188), CD20 (2H7) and CD69 (FN50), from BioLegend, and gated as described in Figure S1C. To assess the pro-apoptotic effect of CF ASN on T cells, CFSE-labeled PBMCs were plated in an anti-CD3 coated 96-well plate. Forty-eight hours after plating, 1:5 CF ASN was added to the culture medium and 24 hours later Annexin V (BioLegend) staining was assessed by flow cytometry using the T-cell specific gating strategy described in Figure S1C. To assess the effect of PD-L1 on the surface of CF airway PMNs on T-cell apoptosis, CFSE-labeled PBMCs were plated in an anti-CD3 coated 96-well plate. Forty-eight hours after plating, 2 × 105 CF airway PMNs were added to the culture. Airway PMNs were either untreated or pre-treated for one hour with anti-PD-L1 (5 μg/mL, BioLegend). Twenty-four hours later Annexin V staining was assessed by flow cytometry.
Arginase activity assay
ASN was incubated at 55°C with equal volumes of 50 mM Tris-HCl, pH 7.5 and 10 mM MnCl2 for 10 min. Then, 0.5M exogenous arginine was added to the reaction and incubated at 37°C for 60 min. The reaction was stopped and the urea formed in the reaction was assessed by colorimetry. The specificity of Arg activity was confirmed by using 250 μM nor-NOHA in the ASN samples tested. Arg activity was calculated as U/mg sputum, where 1 unit is equal to the enzymatic activity necessary to produce 1 μmol urea/min.
Mass spectrometry assay for arginine, ornithine and citrulline
Arginine, ornithine and citrulline concentrations were measured by a gas chromatography / mass spectrometry kit (EZFaast, Phenomenex), according to the manufacturers’ guidelines.
Soluble PD-L1 ELISA
PD-L1 ELISA reagents, including recombinant protein for the standard curve, capture and detection antibodies were purchased from Abnova.
Data analysis
Statistical analysis was performed using JMP10 (SAS Institute). Datasets with N<20 (all in vitro data, HC in vivo data) were assessed with non-parametric statistics. Datasets with N>20 (CF in vivo data) were assessed for normality and if normally distributed, assessed with parametric statistics. Log-normally distributed data were first Log-transformed and analyzed with parametric statistics. Comparisons between HC and CF groups and between subcategories of CF patients used the Wilcoxon rank sum test. Comparisons within the CF group (e.g., expression of given markers on blood vs. airway PMNs) used the Wilcoxon signed rank test or paired t-test for non-normally and normally distributed data, respectively. Univariate correlations between continuous variables were assessed by Spearman or Pearson tests for non-normally and normally distributed data, respectively. To assess potential effects of categorical clinical variables (e.g., cftr mutations, concomitant medications, presence or absence of a given airway pathogen) on experimental outcomes (e.g., Arg1 expression by blood and airway PMNs), we used regression modeling with stepwise choice of variables by F-test. p-values < 0.05 were considered statistically significant.
RESULTS
PD-L1 expression changes upon PMN recruitment to airways in vivo
PD-L1 is a suppressor molecule involved in late homeostatic regulation of T-cell function, and which requires cell-cell contact to mediate its effects (29). Previous data have shown that PD-L1 is modulated on PMNs in chronic diseases, such as HIV, active tuberculosis and lupus (9, 30, 31), as compared to HC subjects. Here, we quantified PD-L1 expression on blood and airway PMNs from CF patients and HC subjects. Blood and airway PMNs were gated as shown in Supplementary Figure S1A and B, respectively. As we previously described (7), CF airway PMNs showed high CXCR4 expression. These cells were also CD62Llo, CD66bhi, and CD63hi (not shown), consistent with a mature PMN phenotype.
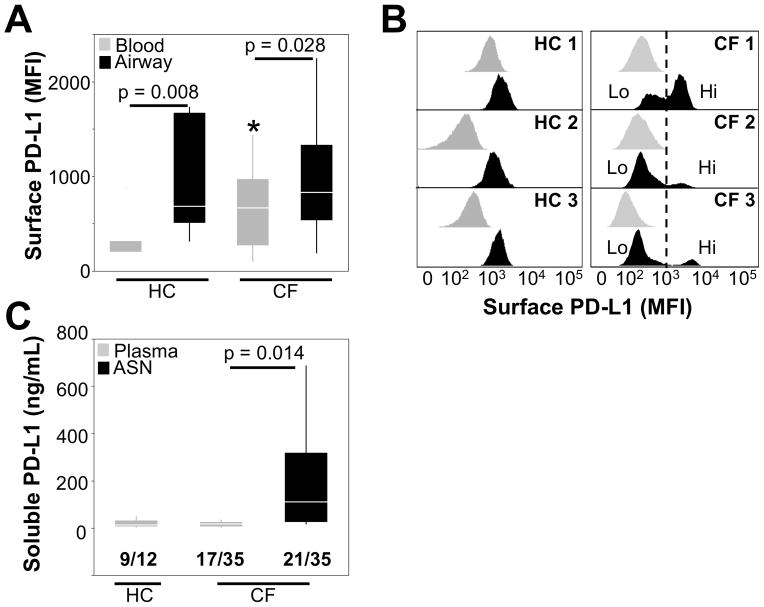
Our data show that PD-L1 is present at the protein level at the surface of blood PMNs (Figure 1A), a finding supported by qRT-PCR analysis (Supplementary Figure S2). We also found that the PD-L1 protein is expressed at slightly higher levels at the surface of CF compared to HC blood PMNs (Figure 1A). The PD-L1 protein was also present on CF and HC airway PMNs, at a higher level than on blood PMNs (Figure 1A). The majority of CF patients analyzed had two populations of PD-L1-expressing PMNs (PD-L1hi and PD-L1lo) in sputum (Figure 1B), a distribution not observed in HC subjects where airway PMNs were all PD-L1hi. The appearance of a PD-L1lo subset among CF but not HC airway PMNs may result from proteolytic cleavage (32), consistent with the high soluble PD-L1 (sPD-L1) levels measured in CF ASN (Figure 1C). However, sPD-L1 found in CF ASN may also derive from mechanisms / sources other than cleavage from the surface of airway PMNs. Taken together, these results show that PD-L1 expression is dynamically modulated at the surface of CF airway PMNs.
Figure 1. PD-L1 expression is increased on airway compared to blood PMNs in vivo.
(A) PD-L1 surface expression was quantified on live PMNs from whole blood or airway (sputum) from both HC (n=7) and CF (n=24) subjects. The median fluorescence intensities (MFI) of PD-L1 surface expression are presented as box plots. * indicates slight increase in PD-L1 expression on CF compared to HC blood PMNs (p = 0.04). (B) Representative histograms from 3 CF and 3 HC subjects show PD-L1 surface expression on matched blood (grey) and airway (black) PMNs. (C) sPD-L1 levels in plasma (HC and CF) and airway supernatant (ASN, CF only) were quantified by ELISA. The number of samples with detectable levels of sPD-L1 out of the total tested is shown below each group on the graph.
Induction of granule exocytosis modulates PD-L1 expression by PMNs in vitro
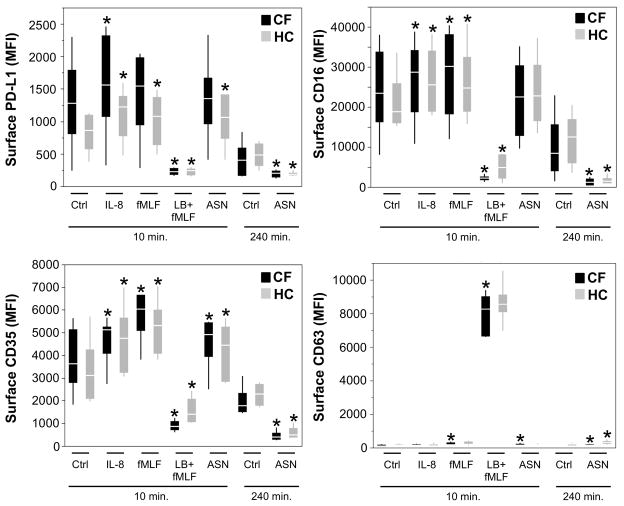
To investigate the mechanism of PD-L1 modulation on the surface of CF airway PMNs, we treated naive blood PMNs (from HC and CF subjects) with various stimuli leading to secretory vesicle, tertiary granule and secondary granule exocytosis without primary granule exocytosis (IL-8, fMLF) or with primary granule exocytosis (latrunculin B -LB- followed by fMLF) (8). Naive blood PMNs were also treated with CF ASN (limited biological material precluded similar experiments using HC ASN). Secretory vesicle and tertiary granule exocytosis were reflected by increased surface CD16 expression, while secondary granule exocytosis was reflected by increased surface CD66b expression. Primary granule exocytosis was assessed by increased surface CD63 expression, as well as decreased CD16 and CD35 expression, due to cleavage by PMN proteases and/or membrane reuptake (33, 34).
As shown in Figure 2, surface PD-L1 expression was increased upon brief (10 minutes) PMN stimulation with IL-8 (HC and CF subjects), fMLF and CF ASN (HC subjects only), and drastically downregulated with LB + fMLF (HC and CF subjects). The pattern of surface PD-L1 modulation (increase or decrease) upon incubation with CF ASN and the various other stimuli used matched almost exactly the patterns of surface CD16 and CD35 modulation. In particular, the decrease in PD-L1 expression upon LB + fMLF treatment coincided with highly decreased expression of CD16 and CD35, and increased CD63 expression, reflecting primary granule exocytosis. After longer incubation with CF ASN (240 minutes), PD-L1 surface expression, along with CD35 and CD16, was also significantly decreased compared to control PMNs. These results suggest that primary granule release is necessary for acute downregulation of PD-L1 expression at the surface of PMNs.
Figure 2. PMN granule exocytosis modulates surface PD-L1 expression on PMNs in vitro.
Whole blood from HC or CF subjects was stimulated with the indicated molecules, or CF airway supernatant (ASN), diluted in RPMI. After treatment, cells were collected and stained for analysis by flow cytometry for surface expression of PD-L1, CD16, CD35 and CD63. * represents p values < 0.05 when compared to the appropriate control from the same experimental group (HC or CF) and timepoint (10 or 240 minutes). n=6 for HC and CF groups.
CF airway fluid inhibits T-cell proliferation
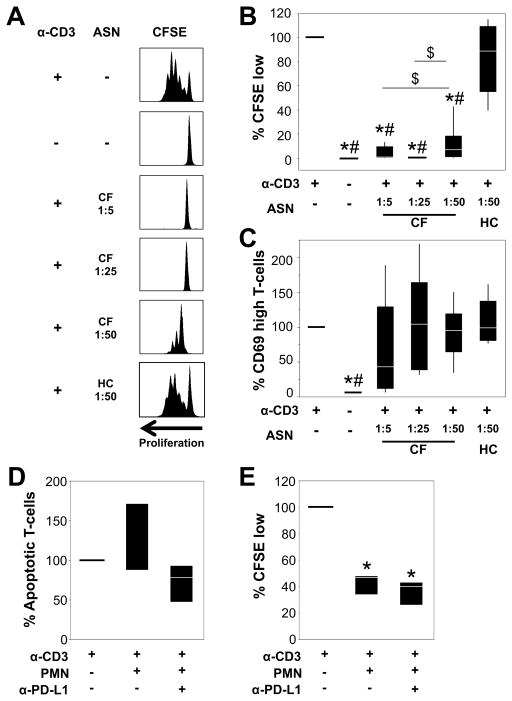
We hypothesized that soluble mediators in CF ASN, including sPD-L1, may suppress T-cell function. Therefore, we measured viability, proliferation (CFSE) and activation (CD69) of HC T cells after 96 hours of stimulation with anti-CD3 in the presence of various concentrations of CF ASN in vitro. In the absence of CF ASN, T cells underwent extensive proliferation 96 hours after plating (Figure 3). When T-cells were treated with 1:5 CF ASN, viability was significantly decreased (Supplementary Figure S3A). To determine if the 1:5 concentration of CF ASN induced T-cell apoptosis we plated PBMCs on anti-CD3-coated plates, then 48 hours later added ASN for 24 hours. This led to a significant increase in the percentage of apoptotic T cells compared to anti-CD3 stimulated T cells without CF ASN (Supplementary Figure S3B). Thus, exposure of T cells to CF ASN at 1:5 inhibits proliferation, in part, by inducing cell death.
Figure 3. Airway fluid and airway-derived PMNs from CF patients inhibit T-cell proliferation independently of PD-L1.
(A) CFSE-labeled PBMCs were cultured on control plates or anti-CD3-coated plates in the presence or absence of CF or HC ASN at the indicated dilutions. PBMCs were pre-exposed to ASN for 2 hours and plated in CD3-coated wells. After 96 hours, PBMCs were analyzed by flow cytometry. Representative histograms of CFSE proliferation are shown. Experimental conditions were compared with regards to percentages of T cells that (B) underwent 2 or more cycles of proliferation (CFSE low), (C) expressed high surface CD69 levels. Values represent the fold change compared to the control CD3-stimulated PBMCs in RPMI (set at 100%). Each box plot represents 4–8 experiments. * represents p < 0.05 when compared to CD3-stimulated cells in RPMI, # represents p < 0.05 when compared to CD3-stimulated cells in HC ASN, and $ represents p < 0.05 between CD3-stimulated cells in different concentrations of CF ASN. (D) PBMCs were cultured on control plates or anti-CD3-coated plates for 48 hours then treated with 2×105 CF airway PMNs, with or without anti-PD-L1). After 24 hours, CFSE-labeled PBMCs were collected and stained with Annexin V and Live/Dead. The percentages of (D) apoptotic T cells, and (E) CFSE-low T cells were determined by flow cytometry. The values represent the fold change compared to the control CD3-stimulated PBMCs in RPMI (set at 100%), where n=3–6 individual experiments. * represents p < 0.05 when compared to CD3-stimulated cells in RPMI.
Higher CF ASN dilutions of 1:25 and 1:50 did not impact T-cell viability (Supplementary Figure S3A), yet at these concentrations T-cell proliferation was still significantly inhibited. In contrast, T-cells exposed to HC ASN at 1:50 dilution were viable and showed normal proliferation (Figure 3). Note than no other concentration of HC ASN was tested, due to limited material. Additionally, both CF and HC ASN preserved the ability of anti-CD3 stimulated T-cells to increase expression of the early activation marker CD69 (Figure 3C). Thus, CF but not HC ASN inhibits early steps in T-cell proliferation while preserving activation.
PD-L1 on CF airway PMNs and sPD-L1 in CF ASN do not contribute to the induction of apoptosis or suppression of proliferation in PD1+ T cells
To determine if PD-L1 signaling played a role in inducing T-cell apoptosis or inhibiting T-cell proliferation, we incubated CFSE-labeled PBMCs with CF airway PMNs (putative cell-mediated effect) or CF ASN containing high levels of sPD-L1 (putative fluid-mediated effect) in the absence or presence of anti-PD-L1 antibody, which efficiently blocks PD-L1 signaling (29). Importantly, T-cells cultured with CF airway PMNs or sPD-L1 rich CF ASN upregulated PD-1 expression, confirming that signaling through the PD-L1/PD-1 pathway is possible in these conditions (Supplementary Figures S4A and B). However, addition of anti-PD-L1 to CF airway PMN/T-cell cocultures did not have a significant effect on T-cell apoptosis (Figure 3D) and while CF airway PMNs had a striking inhibitory effect on T-cell proliferation, addition of anti-PD-L1 to the cocultures did not reverse this effect (Figure 3E). Finally, addition of anti-PD-L1 to T-cells cultures in sPD-L1 rich CF ASN did not have a significant effect on T-cell apoptosis or proliferation (not shown).
Arg1 expression is increased on the surface of both CF and HC airway PMNs but arginase activity is significantly higher in CF airway fluid
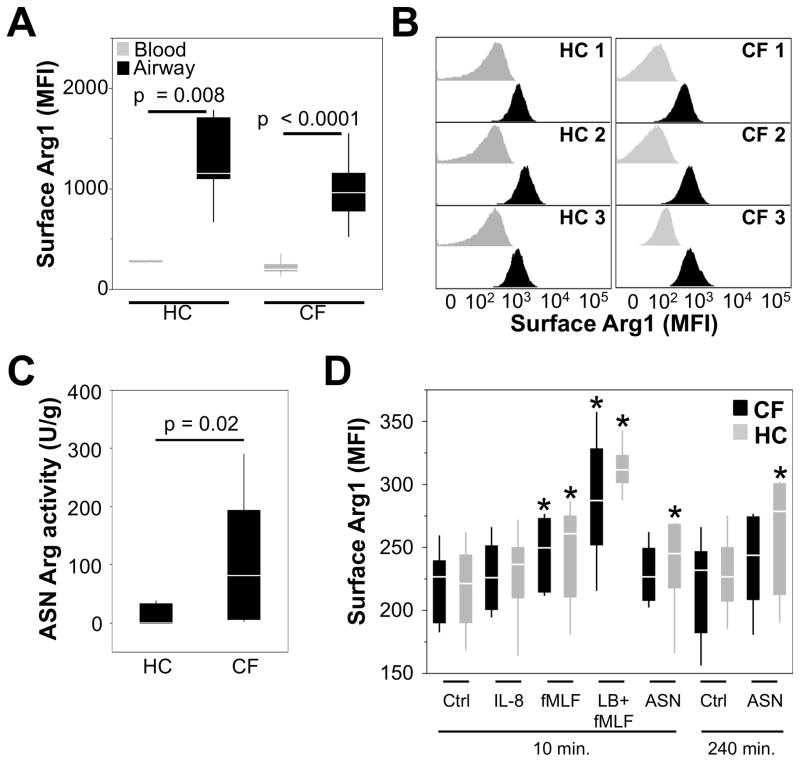
Since PD-L1 did not significantly affect T-cell function in vitro, we hypothesized that other soluble mediators in CF ASN must play a role in the observed modulation of T-cell function. Arginine is necessary for the expression of the invariant ζ-chain of the TCR complex and in environments of depleted arginine T-cell activation is inhibited (21, 35). Arg1, an enzyme stored in the primary and tertiary granules of human but not murine PMNs (19, 20), can cleave arginine and its activity is increased in CF airways (17, 18). Since previous data have shown that once released from granules, PMN effector proteins such as human neutrophil elastase (HNE) can sequester at the cell surface (36), we quantified surface Arg1 expression on blood and airway PMNs. Arg1 is present at the protein level at the surface of blood PMNs (Figure 4A), a finding supported by qRT-PCR analysis (Supplementary Figure S2), with similar expression levels in CF and HC blood PMNs. Surface Arg1 expression was increased on CF and HC airway PMNs compared to matched blood PMNs (Figure 4A). Surface expression of the Arg1 protein was upregulated unimodally in CF and HC airway PMNs (Figure 4B). Even though surface Arg1 was similar on CF and HC airway PMNs, arginase enzymatic activity was significantly higher in CF compared to HC ASN (Figure 4C). This result is consistent with the fact that, to become fully active, Arg1 requires proteolytic activation facilitated by the release of primary granule proteases (13), a process that is highly intensified in CF compared to HC airway PMNs, as we showed by single-cell analysis (6). Furthermore, the airway PMN count is 2–4 orders of magnitude higher in CF compared to HC airways, such that primary granule protease burden [proportional to the rate of release at the single-cell level and to the PMN count (6)] is dramatically higher in the CF compared to the HC airway lumen (1).
Figure 4. Arg1 expression is increased on the surface of both CF and HC airway PMNs but arginase activity is significantly higher in CF airway fluid.
(A) Arg1 surface expression was quantified on live PMNs from whole blood or airway (sputum) from both HC (n=7) and CF (n=24) subjects. The median fluorescence intensities of Arg1 surface expression are presented as box plots. (B) Representative histograms from 3 CF and 3 HC subjects show Arg1 surface expression on matched blood (grey) and airway (black) PMNs. (C) Arginase activity was measured in CF (n=20) and HC (n=5) ASN, and was calculated as units/mg sputum, where 1 unit was equal to the enzymatic activity necessary to produce 1 μmol urea/min. (D) Whole blood from HC or CF subjects was stimulated with the indicated molecules, or CF airway supernatant (ASN) diluted in RPMI (same as Fig. 2). After treatment, cells were collected and stained for analysis by flow cytometry for surface expression of Arg1. * represents p values < 0.05 when compared to the appropriate control from the same experimental group (HC or CF) and timepoint (10 or 240 minutes). n=6 for HC and CF groups.
PMN granule exocytosis increases surface Arg1 expression on PMNs in vitro
To investigate the mechanism of Arg1 upregulation on the surface of CF airway PMNs, we treated naive blood PMNs (from HC and CF subjects) with various stimuli leading to secretory vesicle and tertiary / secondary / primary granule exocytosis, as well as CF ASN (limited biological material precluded similar experiments using HC ASN). Maximal surface Arg1 expression was observed on HC and CF blood PMNs stimulated with LB + fMLF, which induces secondary and primary granule exocytosis (Figure 4D). Significant, albeit lesser, increases in surface Arg1 expression were observed when blood PMNs were stimulated with fMLF and CF ASN. These in vitro data confirm our in vivo observation made on CF airway PMNs that full PMN degranulation, including primary granules, leads to maximal Arg1 expression.
Arginase activity in CF airway fluid contributes significantly to the suppression of T-cell proliferation
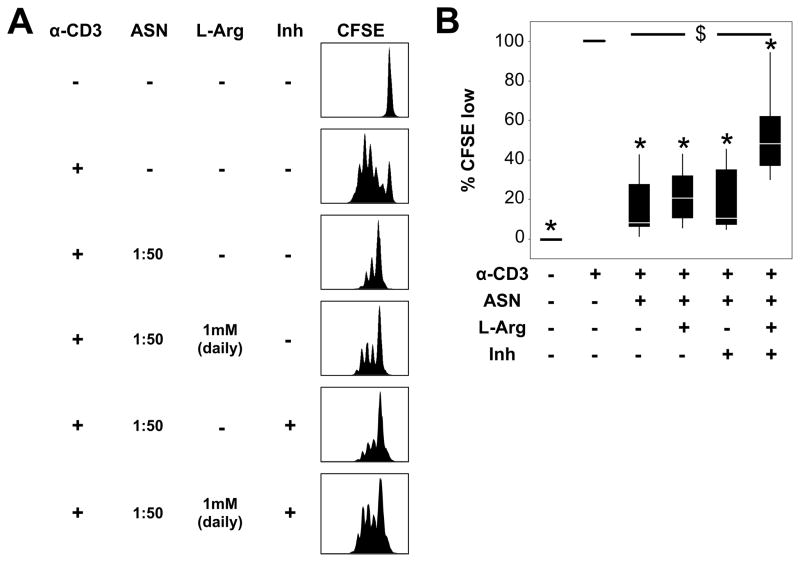
To determine if Arg1 plays a role in the inhibition of T-cell proliferation by CF ASN, anti-CD3 stimulated PBMCs were pre-treated with CF ASN in the presence of arginase inhibitor (nor-NOHA) and/or supplemented daily with L-arginine. Due to the impact of high concentrations (1:5–1:25 range) of CF ASN on T-cell viability (Supplementary Figure 3A), we determined the role of arginase using an ASN dilution of 1:50. We observed that T cells regained significant proliferative capacity when treated with both nor-NOHA and L-arginine, but not either of this treatment alone, compared to cells treated with ASN (Figures 5A and B). However, the rescue of T-cell proliferation upon combined treatment with nor-NOHA and L-arginine did not fully restore it to the level seen upon CD3 stimulation in the absence of CF ASN. Additionally, when T-cells were allowed to proliferate for 48 hours, then treated with 1:5 ASN in the presence or absence of nor-NOHA and/or L-arginine, T-cell apoptotic rates did not change (Supplementary Figure S3C). Taken together, these data suggest that arginase contributes, in part, to the inhibition of T-cell proliferation by CF ASN, and that this inhibitory effect occurs early in T-cell activation.
Figure 5. Arg1 in CF airway fluid contributes to the inhibition of T-cell proliferation.
(A) CFSE-labeled PBMCs were cultured on anti-CD3-coated plates after being pre-treated with CF ASN at 1:50 in RPMI (a concentration that does not induce apoptosis, labeled as “ASN”) for 2 hours. To block arginase activity, 1:50 CF ASN was pre-treated with 250 μM nor-NOHA (labeled as “Inh”) for 10 minutes prior to incubation with CFSE-labeled PBMCs. L-arginine (LArg, 1mM) was supplemented daily by addition to the culture medium. After 96 hours, PBMCs were analyzed by flow cytometry. Representative histograms of CFSE staining are shown. (B) The percentages of T cells that underwent >2 cycles of proliferation (CFSE low) are shown. The values represent the fold change compared to the control CD3-stimulated PBMCs in RPMI (second column, set at 100%). Each box plot represents 6 experiments. * represents p < 0.05 when compared to CD3-stimulated cells in RPMI, and $ represents p < 0.05 between CD3-stimulated cells in CF ASN alone vs. CF ASN with combined nor-NOHA and L-Arg treatments.
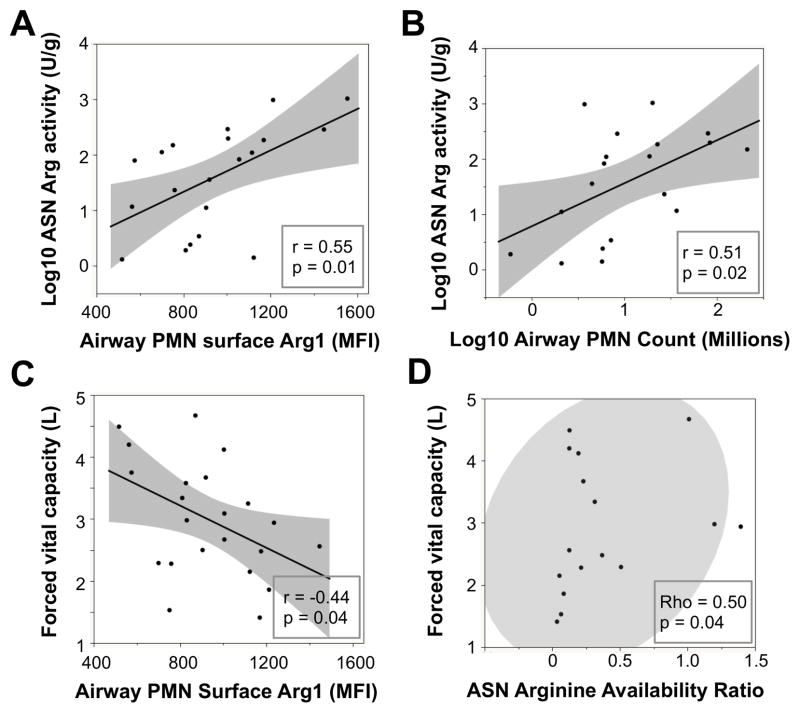
Arg1 expression on CF airway PMNs correlates positively with arginase activity in CF ASN and negatively with CF lung function
Expression of Arg1 on CF airway PMNs was not correlated with age, gender, cftr mutation, medications, microbiology, or outpatient vs. inpatient visits (regression modeling with stepwise choice of variables by F-test, not shown). However, we observed significant positive correlations between arginase activity in ASN and both surface Arg1 expression on CF airway PMNs (Figure 6A) and the overall count of airway PMNs (Figure 6B). These findings suggest that Arg1 expressed by airway PMNs in a given CF patient is functionally linked to the arginase activity of the corresponding ASN. While these findings do not exclude possible contributions from other cells (e.g., epithelium, macrophages) in the overall arginase burden in CF airways, they strongly implicate PMNs as major contributors to this enzymatic activity. In addition, we found a significant negative correlation between surface Arg1 expression on CF airway PMNs and forced vital capacity, or FVC (Figure 6C). Finally, the arginine bioavailability ratio in ASN, calculated as the quotient of arginine over its breakdown products ornithine and citrulline (37), showed a significant positive correlation with FVC (Figure 6D). These data suggest that Arg1 expression on CF airway PMNs and its impact on arginine bioavailability may modulate, not only T-cell function, but also lung function.
Figure 6. Correlations between ASN arginase activity, airway PMN surface Arg1, airway PMN count, arginine bioavailability ratio, and forced vital capacity.
Correlations were assessed using the Pearson test among (log-)normally distributed variables (A, B, C) and the Spearman test (D) due to the non-parametric distribution of ASN arginine bioavailability ratio.
DISCUSSION
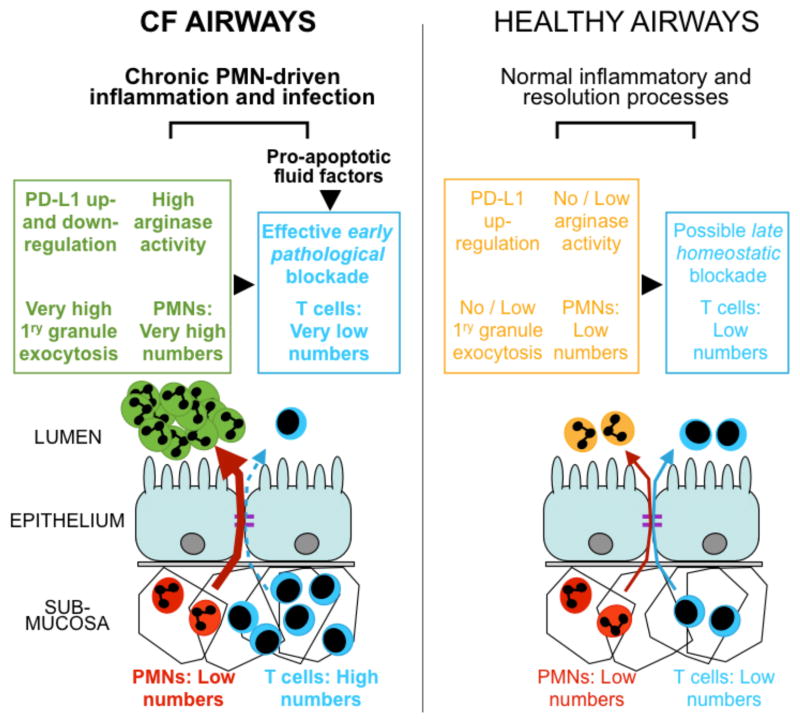
Our study is the first to demonstrate the suppressive capacity of mature CF airway PMNs and CF ASN toward the T-cell response. Prior data described a population of myeloid-derived suppressor cells (MDSCs) that inhibited T-cell proliferation in vitro in the blood of CF patients who were infected with P. aeruginosa (38). These MDSCs pelleted with PBMCs upon Ficoll separation, yet resembled PMNs on cytospins, suggesting they may be an immature population of PMNs. The population of CF airway PMNs described in our study bore characteristics of a mature population, and were present in CF patients, with or without P. aeruginosa infection. Further investigation is needed to determine if the mature CF airway PMNs observed in our study are related to the population of immature MDSCs described in CF blood (38), which may account for their similar high CXCR4 expression. While this prior study (38) suggested a systemic regulation of T cells by circulating MDSCs in CF, our study suggests that T-cell suppression is also mediated by mature PMNs at the primary disease site, i.e., the lumen of the airways, at an early Arg1-regulated step (Figure 7).
Figure 7. Working model accounting for the observed exclusion / early blockade of T-cells in the CF airway lumen.
Our data are consistent with the previously observed imbalance in PMNs and T cells between the submucosa and lumen of the airways, suggesting that massive PMN recruitment to the lumen and high exocytosis of primary granules lead to high arginase activity and early, pathological blockade of T-cells, while PD-L1 on airway PMNs is first up- and then down-regulated. Our data suggest that other pro-apoptotic factors present in CF airway fluid may also block T cells, contributing to chronic inflammation and infection driven by pathological airway PMNs (dark green). By contrast, in healthy airways, PMNs are in low numbers and do not exocytose primary granules, such that arginase activity is absent or low, while PD-L1 on PMNs stays upregulated. Normal airway PMNs (light orange) do not impede T-cell responses, but may provide late homeostatic regulation of T cells via PD-L1 / PD-1 signaling, thus contributing to normal inflammatory and resolution processes.
PD-L1 expression is modulated on PMNs from patients with various chronic diseases, including HIV-1 infection and active tuberculosis where it is increased (9, 30) and active lupus where it is decreased (31), as compared to HC subjects. In the CF patients assessed in our study, surface PD-L1 levels on blood PMNs was similar to that in HC subjects. When we used a PD-L1 antibody to block PD-L1 signaling in co-culture experiments in vitro, we found that treatment with anti-PD-L1 did not rescue T-cell proliferation or inhibit T-cell apoptosis. While PD-L1 was increased unimodally on HC airway PMNs, it was expressed in a bimodal pattern on the surface of CF airway PMNs, and sPD-L1 could be detected in CF ASN, suggesting PD-L1 may be cleaved from the surface and / or internalized in CF airways, similar to CD16 and CD35 (33, 34).
Our in vitro results support this notion, since the release of primary granules induced by exogenous treatment with LB + fMLF resulted in a significant decrease in PD-L1 expression on blood PMNs, paralleling the effect seen on surface CD16 and CD35. In a previous study, sPD-L1 was decreased when matrix metalloproteinase inhibitor was added to L929 cells transfected with PD-L1 (32), supporting the notion that PMN-derived mediators may cleave PD-L1 from the cell surface. Whether sPD-L1 plays any functional role is unknown. In our studies, treatment with anti-PD-L1 antibody to inhibit sPD-L1 did not affect the induction of T-cell apoptosis or block to proliferation exerted by CF ASN, suggesting that sPD-L1 does not play a significant role in T-cell suppression in vitro. In addition, anti-PD-L1 antibody did not reverse the inhibition of T-cell proliferation in the context of T-cell / CF airway PMN cocultures. Thus, PD-L1 is likely not primarily responsible for the suppression of T-cell function in CF airways.
Unlike PD-L1, Arg1 expression was highly increased on the surface of airway PMNs from all CF patients assessed in this study. Arg1 expression on CF airway PMNs positively correlated with arginase activity in the ASN and Arg1 expression on CF airway PMNs negatively correlated with lung function, which suggests that therapeutic modulation of Arg1 in CF airway PMNs may positively impact lung function. Nitric oxide synthase (NOS) competes with Arg1 to cleave arginine, producing nitric oxide, an important mediator of smooth muscle relaxation, bronchodilation, and bacterial killing (39). Inhaled L-arginine has previously been administered to CF patients, resulting in increased nitric oxide production; however, Arg1 activity was also increased in these trials, suggesting that increasing arginine availability upregulates both NOS and Arg1 activities (40, 41). These results, along with data from our in vitro T-cell experiments, suggest that treatment with arginine combined with an arginase inhibitor may be required to maximize arginine availability for pathways other than Arg1 in CF.
Our in vivo and in vitro data support the idea that Arg1 is released from PMNs, which make up >95% of live cells in expectorated sputum, and binds to the cell surface, although we cannot rule out the possibility that other cell types in CF airways produce Arg1, or other arginases (including host-derived arginase 2, and /or bacterial arginases) in vivo. Arg1 is primarily found in human granulocytes, and to a lesser extent in monocytes / macrophages (15, 20). Of note, high Arg1 protein was detected in monocytes from trauma patients (42). In contrast to humans, Arg1 in mice is not constitutively expressed in granulocytes, but is commonly used as a marker of alternatively activated macrophages (20, 43, 44). Interestingly, Arg1 activity was recently shown to be increased in cftr−/− mouse lungs, although the actual cellular source was not identified (45). Discrepancies between humans and mice regarding Arg1 expression in the various myeloid subsets emphasize the need for further patient-based studies to better understand the regulation and role of this enzyme in CF.
Our study suggests that CF airway PMNs possess multiple mechanisms to suppress T-cell function in the lumen. Beyond PD-L1 and Arg1 described in this study, proteases and ROS from PMNs may also play a significant role in this regard. For instance, HNE, which is highly elevated in CF airways, cleaves several molecules from the surface of T cells that impact their functional capacity, including CD2, CD4, CD8 and IL-2 receptor (46–48). Additionally ROS, specifically hydrogen peroxide, suppress T-cell activation and proliferation (10, 49, 50). In our in vitro experiments, neither elastase inhibitor nor catalase (which detoxifies hydrogen peroxide) improved T-cell proliferation in the presence of CF ASN (data not shown).
Since CF airway disease is patchy, featuring highly localized disease foci interspersed with normal areas, as well as inhomogeneous infectious and inflammatory plugs (which form the basis of sputum), it is likely that incoming T cells are exposed to various levels of PMN-derived immunomodulatory factors, depending on the precise location to which they are being recruited. For this reason, it was important to test T-cell responses in the context of various ASN concentrations. This consideration also impacts the potential outcome of treatments directed at PMN-derived immunomodulatory factors, which may be active away from plugs, but not in their immediate vicinity, due to overwhelming concentrations of toxic factors.
Overall, our data support the notion that CF airway PMNs suppress local T-cell responses. Therefore, even if antigen-specific T cells proliferate within the draining lymph nodes of the lung, they may not reach their final target due to the inhospitable environment created by the high numbers of CF airway PMNs. Our data are consistent with the notion that in CF airways, abundant numbers of mature PMNs exocytose primary granules, potentiating arginase activity and leading to an early, pathological T-cell suppression, while surface PD-L1 is gradually lost (Figure 7). By contrast, in HC airways, low numbers of mature PMNs do not exocytose primary granules, keeping arginase activity low while preserving high surface PD-L1, such that T-cell activation may proceed, if needed, until its late, homeostatic downregulation.
Since T cells are abundant in the CF airway submucosa, it is likely that PMN-driven exclusion of T cells from the CF airway lumen affects their transepithelial migration and/or survival in the lumen, although we cannot discriminate between these possibilities at this time. The therapeutic implications of our findings suggest that modulating arginine metabolism in CF airways may boost T-cell immunity. Further studies are required to determine whether mature airway PMNs with similar T-cell suppressive functions are present and consequential in other diseases, including chronic obstructive pulmonary disease and severe asthma.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. J. Alvarez for help with CF sample accrual and access to demographic data, Drs. A. Fitzpatrick, S. Stephenson and J. Dodds for their help accruing HC induced sputum samples, the Emory+Children’s Pediatric Research Center Biomarkers Core (F. Harris, Dr. L. Brown) and Flow Core (A. Rae, Dr. D. Archer) for access to mass spectrometry and flow cytometry systems, and Dr. A. Gaggar for helpful discussions.
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 (V.T. and R.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by grants from the Center for CF and Airways Disease Research (R.T.), the Emory URC/ACTSI Program (R.T.), the Cystic Fibrosis Foundation (V.T.), and the NIH T32 Training Program T32AA013528 (S.A.I.).
References
- 1.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashlock MA, Olson ER. Therapeutics development for cystic fibrosis: a successful model for a multisystem genetic disease. Ann Rev Med. 2011;62:107–125. doi: 10.1146/annurev-med-061509-131034. [DOI] [PubMed] [Google Scholar]
- 3.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 5.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Ann Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Davies ZA, Herzenberg LA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci USA. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, Dunn CE, Davies ZA, Moss RB, Herzenberg LA, Herzenberg LA, Tirouvanziam R. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc Natl Acad Sci USA. 2009;106:5779–5783. doi: 10.1073/pnas.0813410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laval J, Touhami J, Herzenberg LA, Conrad C, Taylor N, Battini JL, Sitbon M, Tirouvanziam R. Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J Immunol. 2013;190:6043–6050. doi: 10.4049/jimmunol.1201755. [DOI] [PubMed] [Google Scholar]
- 9.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Path. 2014;10:e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 11.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, Dallegri F, Bronte V, Ferrini S, Barbieri O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–727. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 14.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 15.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thewissen M, Damoiseaux J, van de Gaar J, Tervaert JW. Neutrophils and T cells: bidirectional effects and functional interferences. Mol Immunol. 2011;48:2094–2101. doi: 10.1016/j.molimm.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respi Crit Care Med. 2005;172:1523–1528. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BS, Bush HM, Sundareshan V, Davis C, Hagadone J, Cory TJ, Hoy H, Hayes D, Jr, Anstead MI, Feola DJ. Characterization of macrophage activation states in patients with cystic fibrosis. J Cyst Fibros. 2010;9:314–322. doi: 10.1016/j.jcf.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen LC, Theilgaard-Monch K, Christensen EI, Borregaard N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood. 2007;109:3084–3087. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 20.Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, Luckner C, Doschko G, Soler G, Eichmann K, Muller FM, Ho AD, Goerner M, Modolell M. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 21.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Natt Rev Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 22.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108:329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 24.Regamey N, Tsartsali L, Hilliard TN, Fuchs O, Tan HL, Zhu J, Qiu YS, Alton EW, Jeffery PK, Bush A, Davies JC. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax. 2012;67:164–170. doi: 10.1136/thoraxjnl-2011-200585. [DOI] [PubMed] [Google Scholar]
- 25.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, Pilewski JM, Kolls JK. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol. 2013;131:1117–1129. 1129 e1111–1115. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute JK, Geiser M, Pier GB, Friedel G, Barr ML, Schuster A, Meyer KC, Ratjen F, Bjarnsholt T, Gulbins E, Doring G. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, Gaillard D. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nath AP, Arafat DD, Gibson G. Using blood informative transcripts in geographical genomics: impact of lifestyle on gene expression in Fijians. Frontiers Genet. 2012;3:243. doi: 10.3389/fgene.2012.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Ann Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNab FW, Berry MP, Graham CM, Bloch SA, Oni T, Wilkinson KA, Wilkinson RJ, Kon OM, Banchereau J, Chaussabel D, O’Garra A. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur J Immunol. 2011;41:1941–1947. doi: 10.1002/eji.201141421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozaffarian N, Wiedeman AE, Stevens AM. Active systemic lupus erythematosus is associated with failure of antigen-presenting cells to express programmed death ligand-1. Rheumatology. 2008;47:1335–1341. doi: 10.1093/rheumatology/ken256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Tosi MF, Zakem H. Surface expression of Fc gamma receptor III (CD16) on chemoattractant-stimulated neutrophils is determined by both surface shedding and translocation from intracellular storage compartments. J Clin Invest. 1992;90:462–470. doi: 10.1172/JCI115882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadallah S, Hess C, Miot S, Spertini O, Lutz H, Schifferli JA. Elastase and metalloproteinase activities regulate soluble complement receptor 1 release. Eur J immunol. 1999;29:3754–3761. doi: 10.1002/(SICI)1521-4141(199911)29:11<3754::AID-IMMU3754>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Grohmann U, Bronte V. Control of immune response by amino acid metabolism. Immunol Rev. 2010;236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 36.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers F, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 38.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol. 2013;190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 39.Grasemann H, Ratjen F. Nitric oxide and L-arginine deficiency in cystic fibrosis. Curr Pharm Des. 2012;18:726–736. doi: 10.2174/138161212799315911. [DOI] [PubMed] [Google Scholar]
- 40.Grasemann H, Tullis E, Ratjen F. A randomized controlled trial of inhaled l-Arginine in patients with cystic fibrosis. J Cyst Fibros. 2013;12:468–474. doi: 10.1016/j.jcf.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Grasemann H, Kurtz F, Ratjen F. Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:208–212. doi: 10.1164/rccm.200509-1439OC. [DOI] [PubMed] [Google Scholar]
- 42.Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME, Rockich AK, Tsuei BJ, Boulanger BR, Kearney PA, Morris SM., Jr Arginase I expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Ann Rev immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 45.Jaecklin T, Duerr J, Huang H, Rafii M, Bear CE, Ratjen F, Pencharz PB, Kavanagh BP, Mall MA, Grasemann H. Lung Arginase Expression and Activity is Increased in Cystic Fibrosis Mouse Models. J Appl Phys. 2014;117:284–288. doi: 10.1152/japplphysiol.00167.2014. [DOI] [PubMed] [Google Scholar]
- 46.Doring G, Frank F, Boudier C, Herbert S, Fleischer B, Bellon G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J Immunol. 1995;154:4842–4850. [PubMed] [Google Scholar]
- 47.Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz HJ, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytok Res. 1999;19:1277–1287. doi: 10.1089/107999099312957. [DOI] [PubMed] [Google Scholar]
- 48.Lungarella G, Cavarra E, Lucattelli M, Martorana PA. The dual role of neutrophil elastase in lung destruction and repair. Int J Biochem Cell Bio. 2008;40:1287–1296. doi: 10.1016/j.biocel.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.