Abstract
Nuclear receptors such as the pregnane X receptor (PXR) and constitutive androstane receptor (CAR) are xenobiotic receptors regulating not only drug metabolism and disposition but also various human diseases such as cancer, diabetes, inflammatory disease, metabolic disease and liver diseases, suggesting that PXR and CAR are promising targets for drug discovery. Consequently, there is an urgent need to discover and develop small molecules that target these PXR- and/or CAR-mediated human-disease-related pathways for relevant therapeutic applications. This review proposes approaches to target PXR and CAR, either individually or simultaneously, in the context of various human diseases, taking into consideration the structural differences between PXR and CAR.
Keywords: PXR, CAR, drug discovery, drug targets, human diseases, nuclear receptors
Introduction
Nuclear receptors (NRs) are ligand-dependent transcription factors [1] that regulate many biological events by inducing gene transcription [2]. Binding of an agonist ligand to a NR causes conformational change of the ligand-binding domain (LBD), dissociation with co-repressors and association with co-activators, leading to activation of gene transcription. These events contribute to regulation of signal transduction pathways under physiologic and pathologic conditions, and during human disease development. For this reason, NRs have been therapeutic targets for the development of new drugs [3] for various diseases, including asthma, type 2 diabetes, atherosclerosis, osteoporosis and cancer [3,4]. Structural analysis of NRs shows that NRs consist of common structural domains with a variable N-terminal domain, a conserved DNA-binding domain (DBD) and a C-terminal LBD that can be targeted in NR drug discovery [5]. NRs have been an established therapeutic target class with many prescribed drugs already on the market [6]. Thus, understanding the network of target proteins associated with NRs and their contributions to the development of diseases will advance the development and expand the utilization of NR-targeted small molecules to cure human diseases [6].
Pregnane X receptor (PXR) and constitutive androstane receptor (CAR) belong to the NR superfamily. These xenobiotic receptors are known to bind various structurally diverse chemicals [7,8]. Because they regulate an overlapping set of target genes, it is difficult to dictate their specificity in gene regulation and associated biological functions [9]. PXR is expressed in normal tissues such as liver, intestine, colon, kidney, brain, breast, prostate, peripheral mononuclear blood cells, heart, bone marrow, spinal cord, stomach, ovary, placenta and immune cells; as well as in many human cancers, including breast, prostate, colon, osteosarcoma, ovarian and endometrial cancers, with elevated expression in some cancers [10,11]. Xenobiotic receptors are not only involved in drug metabolism but they are also involved in regulating many other signal transduction pathways and related physiological processes. Nevertheless, altered expression of PXR and CAR can lead to bone disorders, hepatic steatosis, inflammatory bowel disease and cancer [12,13]. Moreover, PXR and CAR can interact with co-activators or co-repressors, depending on the ligands the receptors bind to. Binding of agonistic ligands causes receptor conformational changes that expose the hydrophobic surface within the LBD for co-activator binding. By contrast, antagonistic ligands induce co-repressor binding, resulting in receptor deactivation [8].
Because PXR and CAR bind to many drugs and have potential roles in many physiologic and pathologic processes, defining and distinguishing the following interactions will be crucial in validating PXR and CAR as potential therapeutic targets: (i) the interactions between drugs and the signaling pathways these drugs modulate; (ii) the interactions between drugs and PXR and CAR; (iii) the interactions between PXR and CAR and the signaling pathways modulated by the drugs that also modulate PXR and CAR.
PXR and CAR in drug metabolism and drug–drug interactions
Xenobiotic receptors such as PXR and CAR have important roles in drug metabolism and drug–drug interactions (DDIs) by regulating the expression of genes encoding drug-metabolizing enzymes and transporters [14,15]. The unique structure of the ligand-binding pocket of PXR enables the pocket to accommodate molecules of various shapes and sizes [14,16]. CAR is also promiscuous in ligand binding, although to a lesser extent than PXR. PXR and CAR are highly expressed in the liver, where drug metabolism and clearance occur [13]. PXR and CAR regulate the expression of genes encoding drug-metabolizing enzymes such as cytochrome P450 (CYP)3A4, CYP2B6, CYP2C9 and CYP2C19, which are responsible for metabolizing more than 80% of clinically prescribed drugs [13]. PXR and CAR also have important roles in glucose, lipid and bile acid metabolism [17]. CYP3A4 is most highly expressed in the human liver, and its induction by PXR and CAR enhances drug metabolism. This, in turn, can contribute to DDIs, which can cause an undesired decrease in the bioavailability of administered drugs, increased hepatic clearance or accelerated formation of reactive metabolites, resulting in toxicity [18]. Agonists of human PXR (hPXR) such as rifampicin increase the metabolism of the antihypertensive drug verapamil by inducing CYP3A4 [18,19]. Long-term treatment of patients with rifampicin caused a reduction in the oral bioavailability of (S)-verapamil by 96% and abolished its antihypertensive effect [19]. Thus, PXR antagonists might be useful in preventing DDIs induced by PXR agonists such as rifampicin. Recent studies showed that treatment of rifampicin causes toxicity and liver injury [20]. Several PXR antagonists, such as ET-743, ketoconazole, FLB-12, sulforaphane, A-792611, polychlorinated biphenyls, coumestrol, aryl sulfonamides and allyl isothiocyanate, have been reported [21–29]. CAR antagonists or inverse agonists such as meclizine, clotrimazole and PK11195 have also been discovered [30,31]. Because PXR and CAR regulate the expression of an overlapping set of genes, antagonists that regulate PXR and CAR might be useful. PXR and CAR can also regulate distinctive sets of genes. For example, phase 1 CYP genes encoding CYP7A1 and CYP4F12 are regulated by PXR, whereas phase 1 CYP genes for CYP1A1 and CYP1A2 are regulated by CAR [13]. The overlapping ligands and transcriptional target genes of PXR and CAR suggest possible collaborative mechanisms for detoxifying toxic compounds [13,14,32–34], an interesting topic that will be discussed below.
Role of PXR in human diseases
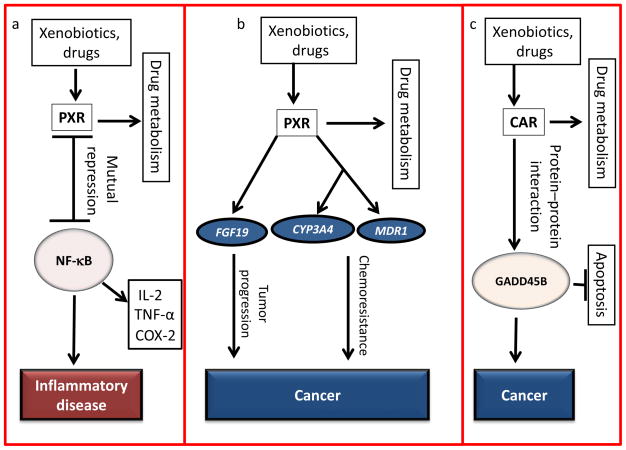
Regulation of xenobiotic metabolism provides a major pathway for detoxification and clearance of various compounds. PXR has been strongly associated with diseases such as cancer, as well as metabolic and inflammatory diseases. PXR polymorphisms have been shown to have significant effects on disease risk in patients with ulcerative colitis and Crohn’s disease [35]. Andersen et al. reported that the G allele of the PXR polymorphism rs6785049(A/G) was associated with a higher disease risk of ulcerative colitis in a case-control study of 495 Danish ulcerative colitis patients [35]. Interestingly, the A allele of the PXR polymorphism rs6785049(A/G) was associated with an increased risk of inflammatory bowel disease (IBD) in the 422 Irish patients, suggesting a positive correlation between PXR polymorphisms and interindividual susceptibility to IBD [35,36]. Mechanistically, Zhou et al. demonstrated inhibitory cross-talk between PXR and nuclear factor (NF)-κB proinflammatory pathways (Figure 1a) [37]. In human intestinal epithelial LS180 cells, the human PXR agonists clotrimazole, rifampicin and RU486 all suppressed tissue plasminogen activator (TPA)- and tumor necrosis factor (TNF)-α-induced expression of NF-κB proinflammatory target genes interleukin (IL)-2, cyclooxygenase (COX)-2, and TNF-α [37]. In mouse primary hepatocytes, the presence of TNF-α suppressed pregnenolone-16α-carbonitrile (PCN)-induced Cyp3a11 expression mediated by mouse PXR (mPXR) [37]. However, there were no changes in PXR expression, suggesting that the TNF-α-mediated effects seen were not the result of modulation of PXR expression [37]. In addition, PXR knockout (KO) mice had more proinflammatory infiltration within jejunal tissues than wild-type mice, demonstrating PXR-mediated cross-talk with NF-κB proinflammatory signaling in vitro as well as in vivo [37]. Interestingly, the authors reported lower mRNA levels of TNF-α in liver samples of patients treated with phenytoin, which positively correlated with an increase in CYP3A4 levels [37]. Shah et al. later found that activation of PXR has a protective role in a mouse model of IBD by repressing NF-κB target genes, suggesting the therapeutic potential of targeting PXR [38].
Figure 1.
Major human diseases involving pregnane X receptor (PXR) or constitutive androstane receptor (CAR). Various proteins can regulate PXR-mediated signaling pathways and thereby contribute to human diseases. PXR and CAR also regulate drug metabolism. (a) PXR activation can suppress the expression of nuclear factor (NF)-κB proinflammatory target genes interleukin (IL)-2, cyclooxygenase (COX)-2, and tumor necrosis factor (TNF)-α. Inversely, activation of NF-κB signaling and the presence of NF-κB-regulated proinflammatory mediators can suppress gene expression mediated by PXR [37]. (b) PXR activation leads to transcriptional upregulation of cytochrome P450 (CYP)3A4 and/or multidrug resistance protein (MDR)1, potentially contributing to chemoresistance [22,45]; however, PXR-mediated transcriptional upregulation of fibroblast growth factor (FGF19) can promote tumor progression, thus contributing to cancer development [46]. (c) CAR can bind to the antiapoptotic protein growth arrest and DNA-damage-inducible beta (GADD45B) to suppress apoptosis and contribute to cancer development [54]. Arrows indicate activation, stop bars indicate suppression.
PXR activation has also been linked to metabolic diseases such as diabetes, owing to the effects of PXR on glucose metabolism. A randomized open, placebo-controlled, crossover trial in healthy volunteers provided insight into postprandial glucose homeostasis and the associated effects in patients that received the human PXR agonist rifampicin versus placebo [39]. A total of 12 healthy volunteers received 600 mg of rifampicin or placebo once daily for 7 days and the oral glucose tolerance test was performed on day 8. Rifampicin treatment increased the total area under the curve for insulin by 40% and glucose by 16%, compared with placebo. The authors performed a concurrent study in rats and observed a similar increase in glucose levels during the oral glucose tolerance test in rats following administration of the rodent PXR agonist PCN compared to vehicle control [39]. Interestingly, three PXR-regulated genes involved in glucose metabolism were downregulated in the rat model that included glucose transporter 2, pyruvate dehydrogenase kinase isoenzyme 2 and glucokinase, in addition to phosphoenolpyruvate carboxykinase 1. The results corroborate previous studies reporting downregulation of phosphoenolpyruvate carboxykinase 1 with agonists of PXR and CAR [40]. Nevertheless, the authors suggested that PXR-mediated downregulation of these genes contributes to postprandial hyperglycemia and could potentially have implications in diabetes; however the clinical implications warrant further investigation.
Altered PXR expression and localization have been implicated in Barrett’s esophagus and esophageal adenocarcinoma [41]. In a previous study, van de Winkel et al. reported significant increased risk of esophageal adenocarcinoma associated with the G allele of the PXR polymorphism rs6785049(A/G), as well as the T allele of the PXR polymorphism rs2276707(C/T). Moreover, PXR mRNA levels were significantly higher in adenocarcinoma tissues and Barrett’s esophagus, suggesting a predictive correlation between PXR polymorphisms, expression and disease risk [41].
Castaño et al. reported that the G allele of PXR polymorphism rs2461823(A/G) was significantly associated with intrahepatic cholestasis of pregnancy, a disease characterized by pruritus and increased serum bile acid levels usually occurring during the third trimester of pregnancy in human patients [42]. By contrast, several studies have shown a hepatoprotective role of PXR in liver injury and cholestasis [43]. In a study reported by Staudinger et al., mouse PXR activation by the cognate ligand PCN protected mice from lithocholic-acid-induced injury that was not seen in PXR KO mice [43]. They reported that the protective effect of PXR activation was attributed to PXR-mediated repression of Cyp7a1 and induction of Na(+)-independent organic anion transporter (Oatp)2 [43]. In a similar study, Teng and Piquette-Miller showed that PXR hepatoprotection was mediated by the induction of multidrug-resistance-associated protein 3 (MRP3) and Cyp3a11 by PXR [44].
Interestingly, PXR is also known to regulate multidrug resistance protein (MDR)1, leading to chemoresistance and contributing to tumor progression (Figure 1b) [22,45]. Furthermore, Wang et al. found a link between PXR and colon cancer, in which PXR activation enhanced tumor cell growth through upregulation of fibroblast growth factor (FGF)19 [46]. Upon PXR activation, transcriptional upregulation of FGF19 can promote colon tumor progression (Figure 1b) [46]. Thus, these reports suggest the therapeutic potential of targeting PXR in various human diseases. However, the therapeutic approach of targeting PXR is disease specific. For inflammatory diseases, PXR activation has suppressive effects on NF-κB-mediated IBD, implying the therapeutic potential of PXR agonists in treating IBD. Moreover, for other benign diseases such as cholestasis, the observed association of PXR activation and hepatoprotection suggests the therapeutic potential of PXR agonists in the treatment of cholestasis. However, for malignant diseases such as colon cancer, PXR antagonists are of potential therapeutic utility owing to the role of PXR in promoting tumor cell growth, chemoresistance and malignancy. Lastly, the identification of PXR polymorphisms in various disease types will be useful in predicting disease risk, prognosis and treatment response. Figure 1a,b summarizes the regulation of gene expression, protein function and signaling pathways by PXR that are relevant to human disease.
Role of CAR in human diseases
CAR plays a part in regulating bile acid detoxification via the induction of drug-metabolizing enzymes and transporters [47,48]. Wagner et al. found that activation of CAR by phenobarbital and 1,4-bis-[2-(3,5-dichlorpyridyloxy)]benzene reduced bilirubin and bile acid serum levels in healthy mice and increased bile acid metabolism and excretion as shown by increased serum and urine levels of polyhydroxylated bile acids in cholestatic mice [49]. In a previous study, Zhang et al. reported that CAR plays a part in bile acid detoxification by inducing Cyp3a11 and MRP3 [50]. Specifically, they utilized PXR, CAR and PXR/CAR double KO mice to investigate the roles of PXR and CAR in coordinating the function of bile acid detoxification [50]. Interestingly, CAR KO mice had more hepatic necrosis than PXR KO mice. Moreover, alanine aminotransferase (ALT) levels were approximately twofold higher in CAR KO mice than in PXR KO mice [50]. The ALT measurements corresponded to an approximate fourfold increase in total serum bilirubin levels in CAR KO over those seen in PXR KO mice [50]. Nevertheless, these findings suggest an important role of CAR in bile acid detoxification and hepatoprotection, as well as the rationale for discovery of CAR agonists that could have therapeutic potential in treating cholestasis. The potential utilization of CAR activators in treating human diseases is further demonstrated in a few recent studies. Lynch et al. reported inhibition of hepatic gluconeogenesis by novel activators of human CAR [51]. Wang et al. showed that selective activation of CAR and subsequent induction of CYP2B6 in human primary hepatocytes enhanced the bioactivation of chemotherapeutic prodrug cyclophosphamide. Therefore activator of human CAR might improve the efficacy of cyclophosphamide-based chemotherapy [52].
Growth arrest and DNA-damage-inducible beta (GADD45B) is another signaling molecule that can be upregulated in response to inflammation and has been implicated in the apoptotic suppressive functions of NF-κB [53]. Recent studies identified the role of CAR in liver tumor promotion and in promoting resistance to TNF-α-induced cell death [54,55]. Yamamoto et al. found that, although phenobarbital can induce CAR-mediated Gadd45b gene expression, CAR forms a protein–protein complex with GADD45B (Figure 1c), preventing TNF-α phosphorylation of c-Jun N-terminal kinase (JNK)1, resulting in resistance to apoptosis in primary hepatocytes from mice treated with TNF-α and actinomycin D [54].
CAR has also been shown to prevent liver injury and apoptosis through another TNF receptor superfamily member: Fas (CD95/APO-1) [56]. Baskin-Bey et al. found that mice treated with the CAR agonist 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) had decreased liver injury and hepatocyte apoptosis [56]. The antiapoptotic effect was mainly induced by depleted proapoptotic Bak and Bax and increased expression of myeloid cell leukemia factor-1 mediated by direct CAR activation by TCPOBOP [56]. The antiapoptotic mechanism was not seen in CAR KO mice, suggesting the direct involvement of CAR-induced hepatoprotection [56].
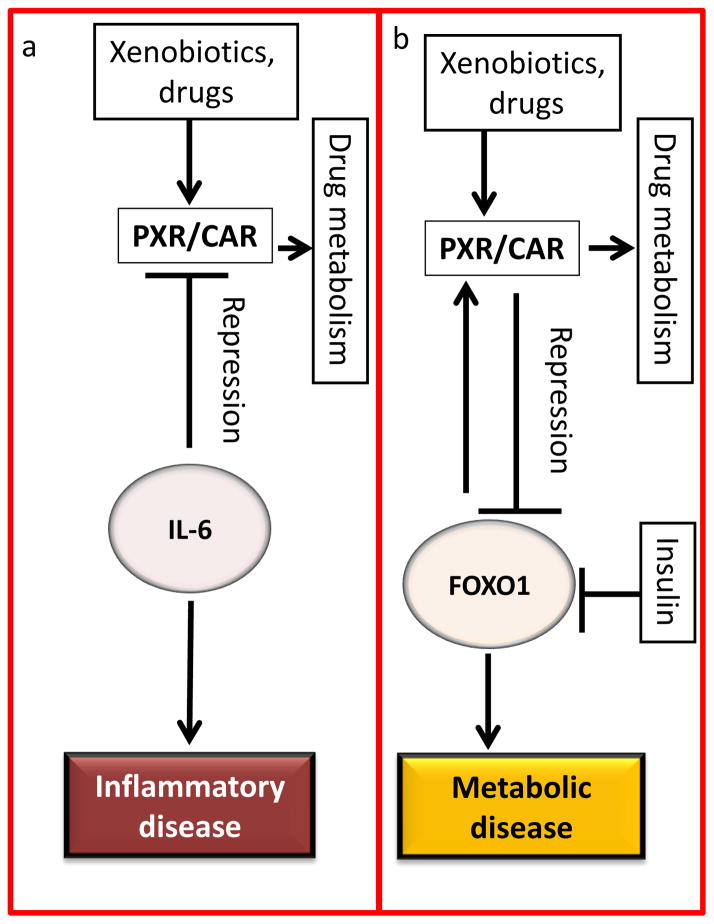
Similar to PXR, CAR has also been shown to be modulated by inflammation (Figure 2a). Pascussi et al. demonstrated the inverse relationship between inflammatory mediators, such as IL-6, and PXR and CAR mRNA expression in primary human hepatocytes [57]. Interestingly, the presence of IL-6 significantly reduced phenobarbital-mediated induction of CAR, as well as dexamethasone-mediated PXR mRNA expression, resulting in a significant decrease in the expression of CYP2B6 and various other CYP genes [57]. These findings aided in identifying a mechanism supporting the downregulation of liver drug metabolism during inflammatory diseases. Similar to PXR, the therapeutic approach to targeting CAR is disease-specific and dependent on the specific therapeutic purpose. For example, CAR agonists might have therapeutic potential in treating diseases such as cholestasis and associated liver injury, diabetes and obesity, as well as in improving the efficacy of certain prodrugs that rely on CYP2B for their bioactivation. By contrast, CAR inhibitors (antagonists or inverse agonists) might have therapeutic potential in malignant diseases such as hepatocellular carcinoma.
Figure 2.
Major human diseases involving constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Various proteins can regulate CAR and PXR-mediated signaling pathways and thereby contribute to human diseases. PXR and CAR also regulate drug metabolism. CAR and PXR activation has been shown to be modulated by inflammation. (a) The presence of inflammatory mediators such as interleukin (IL)-6 significantly reduces the expression of PXR and CAR and their target genes [38,57]. IL-6 contributes to inflammatory disease. (b) CAR and PXR activation can downregulate gluconeogenesis-associated genes via crosstalk between CAR, PXR and the transcription factor forkhead box protein O1 (FOXO1), as proposed by Kodama et al. [40]. FOXO1 is suppressed in the presence of insulin. FOXO1 is a co-activator of CAR and PXR. Inversely, CAR and PXR can repress FOXO1 activity, reducing the expression of FOXO1-mediated downstream targets providing further insight into the role of CAR in regulating metabolic diseases [40]. Arrows indicate activation, stop bars indicate suppression.
Crosstalk between PXR and CAR
PXR and CAR have significant roles in regulating the expression of drug-metabolizing enzymes and xenobiotic biotransformation. However, when targeting either CAR or PXR for novel drug discovery, the signaling crosstalk between PXR and CAR must be considered. It is known that CAR and PXR share ligands that act as dual activators of the expression of corresponding genes [32–34,58]. For example, phenobarbital, phenytoin and TCPOBOP are activators of PXR and CAR [15,58]. Furthermore, CAR and PXR modulate the gene expression of CYPs, MDR1, GSTs, UGTs and SULTs [13]. More specifically, PXR and CAR can bind to NR1, NR2, DR3, ER6, DR4, DR5 and gtPBREM motifs within the promoter regions of their targeted genes [59,60], resulting in the possibly daunting task of targeting PXR and CAR in human diseases. Recent studies have revealed the opposing effects of CAR and PXR activation in treating diabetes by modulation of hepatic gluconeogenesis. The CAR indirect activator phenobarbital was reported to enhance insulin sensitivity by decreasing glucose levels in diabetic patients [61]. A similar mechanism was reported utilizing the mouse PXR agonist PCN via suppression of phosphoenol-pyruvate carboxykinase and glucose-6-phosphatase expression [40]. Interestingly, PXR activation induced hepatic steatosis and increased serum corticosteroid levels, which was not reported with CAR activation [62,63]. However, these effects were reported primarily in mouse models, which raises questions about the translational aspect of PXR agonists in vivo and ultimately as a novel treatment modality for diabetes. Other studies have shown that the CAR indirect activator phenobarbital downregulated gluconeogenesis genes [64,65]. A recent study further showed that activation of human CAR led to inhibition of hepatic gluconeogenesis [51]. Kodama et al. observed crosstalk between PXR, CAR and forkhead transcription factor O1 (FOXO1) (Figure 2b) [40]. FOXO1 transcriptionally regulates phosphoenolpyruvate carboxykinase 1, glucose 6-phosphate and insulin-like growth factor-binding protein 1 [66–69]. FOXO1 was shown to be a co-activator of CAR by directly binding to CAR and enhancing the expression of CYP2B6, as shown by luciferase reporter activity [40]. Inversely, activation of CAR by ligands was shown to repress FOXO1 activity, providing further insight into the role of CAR in regulating metabolic diseases, particularly hyperglycemia and gestational diabetes [40]. Masuyama and Hiramatsu reported that pregnant mice treated with the CAR ligand TCPOBOP had greater glucose tolerance and suppressed hypertension and proteinuria [70]. Taken together, PXR and CAR are major regulators of genes involved in drug metabolism and transport, as well as disease progression; therefore, identifying compounds that are effective, and potentially possess dual functionality for PXR and CAR, is crucial when targeting these xenobiotic receptors involved in metabolic, benign and malignant diseases. Figure 2a,b summarizes the regulation of signaling pathways and cellular function by PXR and CAR; and the possible link of such regulation to relevant human diseases.
Structural view of small molecule modulators binding to PXR and CAR
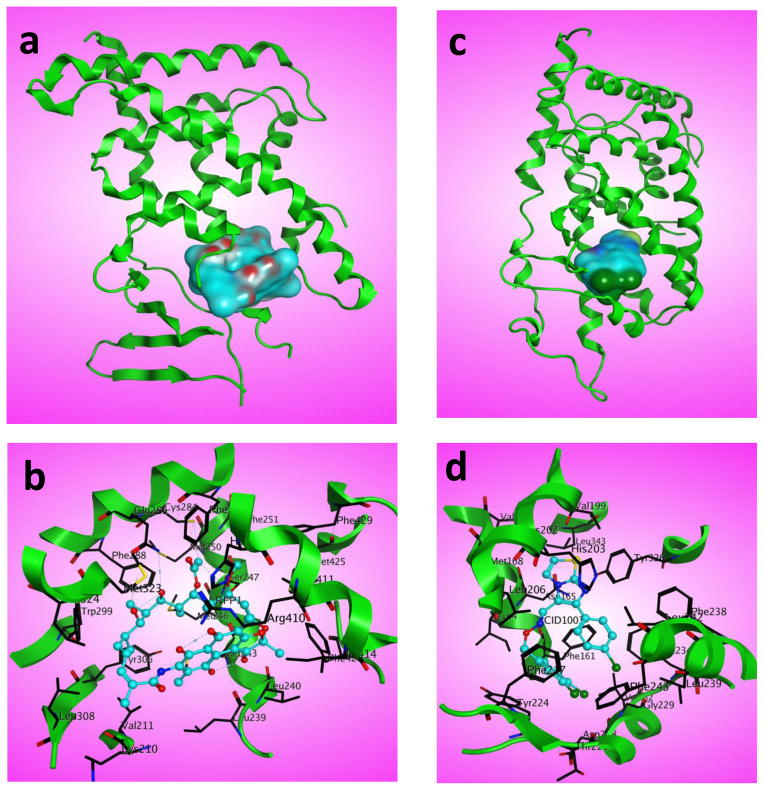
PXR and CAR have been shown to be modulated by a diverse range of structurally different molecules. The LBD of PXR contains three sets of α-helices, including α1/α3, α4/α5/α8/α9 and α7/α10, and a layer of five stranded antiparallel β-sheets [71]. PXR has two extra β-strands that comprise 60 additional residues that are absent in other NRs. The short AF-2 helix (αAF) allows co-activator binding [71]. Structural analysis showed that PXR has a large and flexible LBD that is highly hydrophobic, including a few polar amino acid residues, enabling it to accommodate various structurally diverse ligands that can bind in different orientations [71]. There are several X-ray crystal structures available for PXR LBD alone or PXR LBD with its agonists, such as hyperforin, colupulone (from hops), 17β-estradiol, SR12813, T1317, rifampicin and anti-HIV drug PNU-142721 [71–75]. The crystal structure of PXR LBD bound to rifampicin is shown in Figure 3a, and the residues important for binding are shown in Figure 3b. Structural analysis has shown that hydrophobic residues along with a few polar residues have important roles in ligand binding [75]. Although no crystal structure has been reported for PXR LBD with PXR antagonist, several reports have demonstrated how the putative antagonists might bind to PXR [76–79]. Recently, based on a novel yeast-based analysis and docking studies, Li et al. [76] proposed that a PXR antagonist such as ketoconazole might bind at the outer surface of PXR [76]. However, an antagonist specific to PXR with less cytotoxicity and with a co-crystal structure with PXR LBD is still lacking. In addition to modulating PXR, the diverse ligands (agonists and antagonists) of PXR are involved in regulating various signaling pathways. It is therefore possible that binding of these ligands to PXR could cause pleiotropic biological effects.
Figure 3.
Structural analysis of human pregnane X receptor (PXR) and human constitutive androstane receptor (CAR) ligand-binding domain (LBD). (a) X-ray crystal structure of PXR LBD with bound rifampicin (cyan surface) (PDB: 1SKX). (b) Residues of PXR LBD important for binding to rifampicin (cyan) are shown. (c) X-ray crystal structure of CAR LBD with bound CITCO (cyan surface) (PDB: 1XVP). (d) Residues of CAR important for binding to CITCO (cyan) are shown. Figure 3 was prepared using MOE software.
Contrary to PXR, which is highly inducible by agonists, CAR is constitutively active. Additionally, there are fewer agonists available for CAR. However, it has been found that some human CAR agonists are not mouse CAR agonists. Some of the CAR activators reported are phenobarbital (indirect activator), CITCO, di(2-ethylhexyl)phthalate and 6,7-dimethylesculetin [61,80–82]. CAR antagonists or inverse agonists have also been reported, such as PK11195 and meclizine [30,31,58]. X-ray crystal structures of CAR in complex with retinoid X receptor α (RXRα) and either of the two agonists CITCO and 5β-pregnanedione have been reported; and they revealed an unusual mechanism of constitutive activation of CAR by small molecules [83]. The crystal structure of CAR bound to CITCO is shown in Figure 3c. Residues of CAR important for this binding are shown in Figure 3d. From the structural analysis, it was seen that hydrophobic residues play an important part in ligand binding. However, no specific hydrogen bonds were observed between the ligand and the receptor. The imidazothiazole heterocycle of the CITCO molecule occupies a relatively polar region of the active site formed by residues such as Asn165, Val199, Cys202, His203 and Tyr326, and makes weak electrostatic interactions with His203, Asn165 and Tyr326 [83]. The crystal structure of CAR reveals a very short helix (helix X) between α10 and the AF-2 helix. The short and rigid AF-2 helix lacks the C-terminal extension present in other NRs, thus allowing the interaction between the free carboxylate and Lys195 of human CAR, leading to further stabilization of the active AF-2 confirmation and the constitutive activity of CAR. In addition, the CAR LBD is formed by helices α2-α7, α10 and β-strands 3 and 4 [83]. Because PXR and CAR share overlapping ligands and target genes, antagonists that target PXR and CAR will be useful for mechanistic investigations and clinical applications, such as effectively preventing DDIs and improving drug efficacy. The ligand-binding cavity of PXR LBD (>1150 Å3) is larger than that of CAR LBD (~675 Å3) [71,83,84]. However, whether or not binders of PXR LBD can function as PXR antagonists remains to be determined.
PXR and CAR as therapeutic targets in small-molecule-based drug discovery and development
The roles of PXR and CAR in various human diseases, as discussed above, suggest their potential as promising therapeutic targets. To validate PXR and CAR as clinically relevant and druggable targets, it is crucial to develop compounds that are effective and either selective for each receptor or dual-functional for both, based on the signaling pathways and diseases they are involved in, to minimize side-effects and improve efficacy. Tables 1 and 2 summarize the targets, signaling pathways and potential clinical implications relevant to the functions of PXR and CAR. We have also summarized the challenges associated with the particular assay described.
Table 1.
Summarized for pregnane X receptor (PXR) or constitutive androstane receptor (CAR): critical target genes or protein-protein interaction partners, clinical implications, small molecule targeting approach, assay design, assay development and possible challenges associated with the drug discovery process
| PXR or CAR | Target gene or protein– protein interaction partner of PXR or CAR | Clinical implications | Targeting approach | Assay design and development | Significance | Challenges |
|---|---|---|---|---|---|---|
| PXR | Cytorchrome P450 (CYP)3A4 | Drug metabolism Drug–drug interactions (DDIs) |
PXR agonists to enhance drug metabolism PXR antagonists to decrease drug metabolism and associated DDIs |
Small molecule HTS using biochemical time-resolved fluorescence resonance energy transfer (TR-FRET) assays [93] and cell-based luciferase reporter assays [71,92] Virtual screening of small molecules using PXR crystal structures [78,89] |
Potent and/or selective PXR modulators to regulate CYP3A4 levels | Finding potent and selective compounds Lack of X-ray crystal structure for PXR bound to antagonist |
| PXR | p53 | Compromised drug efficacy in cancer cells with mutated p53 | Small molecules that restore the inhibitory effect of mutated p53 on PXR | Cell-based reporter assays in the presence of either wild-type or mutated p53 Biochemical assays to investigate the binding of compound to PXR or p53 |
Small molecules that restore the inhibitory effect of mutated p53 on PXR might enhance drug efficacy and reduce drug resistance | No specific site known for PXR–p53 interaction No crystal structure or mechanism available on how p53 binds to PXR |
| PXR | Nuclear factor (NF)-κB | Inflammatory diseases | PXR agonists | Cell-based luciferase reporter assays to identify compounds that inhibit NF-κB activity in a PXR- dependent manner [37] | Potent and/or selective PXR agonists to inhibit NF-κB with possible therapeutic implications in treating diseases such as inflammatory bowel disorder (IBD) | Identifying specific PXR activators that are also NF-κB inhibitors |
| PXR | Fibroblast growth factor (FGF)19 | Tumor aggressiveness | PXR antagonists | Cell-based luciferase reporter assay to identify PXR antagonists that reduce the levels of FGF19 in a HTS format [46] | Potent and selective PXR antagonists to inhibit FGF19 expression with potential anticancer properties | Identifying selective antagonists to regulate FGF19 in a tumor-specific manner |
| CAR | CYP2B6 | Drug metabolism DDIs |
CAR agonists to enhance drug metabolism CAR antagonists or inverse agonists to decrease drug metabolism and associated DDIs |
HTS using cell-based luciferase reporter assays [40] Virtual screening of small molecules using CAR crystal structures [94] |
Potent selective CAR modulators to regulate CYP2B6 | Finding potent and selective compounds Lack of crystal structure for CAR bound to antagonist or inverse agonist |
| CAR | Growth arrest and DNA-damage- inducible beta (GADD45B) | Resistance to apoptosis mediated by CAR-GADD45B interaction | CAR inverse agonist that disrupts GADD45B binding [54] | *Fluorescence polarization assay using GADD45B peptide. | Disruption of CAR- GADD45B interaction can increase chemsensitivity to anticancer drugs | No specific site known for CAR –GADD45B interaction |
Table 2.
Pregnane X receptor (PXR) and constitutive androstane receptor (CAR): target genes or protein–protein interaction partners, clinical implications, small molecule targeting approach, assay design, assay development and possible associated challenges in the small molecule drug discovery process
| Target gene or protein–protein interaction partner of PXR and CAR | Clinical implications | Targeting approach | Assay design and development | Significance | Challenges |
|---|---|---|---|---|---|
| Forkhead transcription factor O1 (FOXO1) | Metabolic disease Diabetes |
PXR/CAR agonists to inhibit FOXO1-mediated gluconeogenesis for insulin-resistant diabetic patients [40] | Cell-based luciferase reporter assays [40] Mammalian two-hybrid assays [40] |
PXR/CAR agonists downregulate FOXO1 potentially to benefit insulin-resistant diabetic patients [40] | Identifying selective agonists of PXR and CAR |
| Multidrug resistance protein (MDR)1 | MDR1, a PXR/CAR transcriptional target, contributes to drug resistance | PXR/CAR inhibitors to decrease MDR1 levels | HTS using biochemical and cell-based assays | Inhibition of PXR/CAR-mediated induction of MDR1 can increase drug efficacy and decrease drug resistance | Selective PXR/CAR inhibitors that decrease MDR1 levels |
| Retinoid X receptor (RXR) | Drug metabolism Drug–drug interactions (DDIs) |
PXR/CAR modulators that target the PXR–RXR or CAR–RXR interactions: activators to enhance drug metabolism; inhibitors to decrease drug metabolism and associated DDIs | Cell-based assays to identify modulators for PXR/CAR that target their interactions with RXR Virtual screening of small molecules that bind to PXR or CAR surface resulting in RXR disruption CAR–RXR heterodimerization surface has been reported [83] PXR–RXR heterodimerization surface has recently been published [89] |
Modulators targeting the interactions of PXR/CAR with RXR can regulate PXR/CAR activity | Identifying specific modulators for PXR–RXR or CAR– RXR interactions |
| Cytochrome P450 (CYP)3A4 and CYP2B6 | See Table 1 | ||||
Among the targets and pathways summarized in Tables 1 and 2 is NF-κB. It has been reported that PXR plays an important part in modulating inflammation by regulating the NF-κB pathway [37]. Activation of PXR by agonists is expected to inhibit the NF-κB pathway, resulting in decreased expression of proinflammatory cytokines, therefore suggesting PXR as a novel target for IBD therapy. Because NF-κB directly interacts with RXR, which is a heterodimeric partner of PXR, inhibition of NF-κB to control inflammation could be achieved by compounds that activate either PXR or RXR or directly inhibit NF-κB [85]. PXR or CAR can also be involved in metabolic diseases such as type 2 diabetes mellitus via regulation of FOXO1. Type 2 diabetes mellitus has been characterized by impaired β cell function and insulin resistance [86]. Interestingly, whereas FOXO1 functions as a co-activator to modulate the transcriptional activity of PXR and CAR, PXR and CAR act as co-repressors of FOXO1-mediated transcriptional activity [40]. Therefore, PXR/CAR dual agonists might be therapeutically useful compounds in preventing FOXO1-mediated gluconeogenesis in diabetic patients. Recently, PXR was shown to interact with and be inhibited by wild-type p53, possibly leading to compromised drug efficacy in tumors with loss-of-function p53 [87]. Although the mechanism responsible for mutated p53 interacting with but failing to inhibit PXR is still unknown, compounds that can restore the inhibitory effect of mutated p53 on PXR might be useful in enhancing drug efficacy and reducing drug resistance. By contrast, activation of PXR induces the level of FGF19 to enhance colon cancer growth [46]. It is therefore useful to discover PXR antagonists that downregulate FGF19 and reduce colon tumor growth. To develop modulators of PXR and CAR with mechanistically and physiologically relevant properties, in silico, such as structure-based drug design, and in vitro, such as small-molecule-based HTS, approaches can be utilized (Figure 4). Enabled by the available crystal structures of PXR LBD, virtual screening approaches to identify novel modulators for PXR have been reported, although the ligand promiscuity of PXR might have been one of the limitations of such efforts [78,88,89]. Unbiased HTS might therefore provide an alternative and potentially complementary approach to identifying novel modulators of PXR and CAR [90,91]. Biochemical- and cell-based HTS approaches can be utilized and have been used previously to identify PXR modulators [92,93]. Recently, CAR activators have been identified using docking studies [94]. Although the agonist-bound structural model for PXR was not particularly useful for designing novel antagonists for PXR [79,95,96], molecular docking, ligand-based pharmacophore and in vitro studies showed that antagonists could be interacting at the AF-2 site or surrounding region on the outer surface of PXR [77–79,97]. Recent studies found a new ketoconazole-binding site near residue Ser208 which is distant from the AF-2 site, thus describing a potential second antagonist-binding site [76], which will be useful for the structure-based approach to reveal a PXR antagonist. To discover a compound that dually modulates PXR and CAR, a two-step process might be used. Because PXR appears to be more promiscuous than CAR, it might be reasonable and effective to identify PXR modulators first, followed by testing the effect of such PXR modulators on CAR.
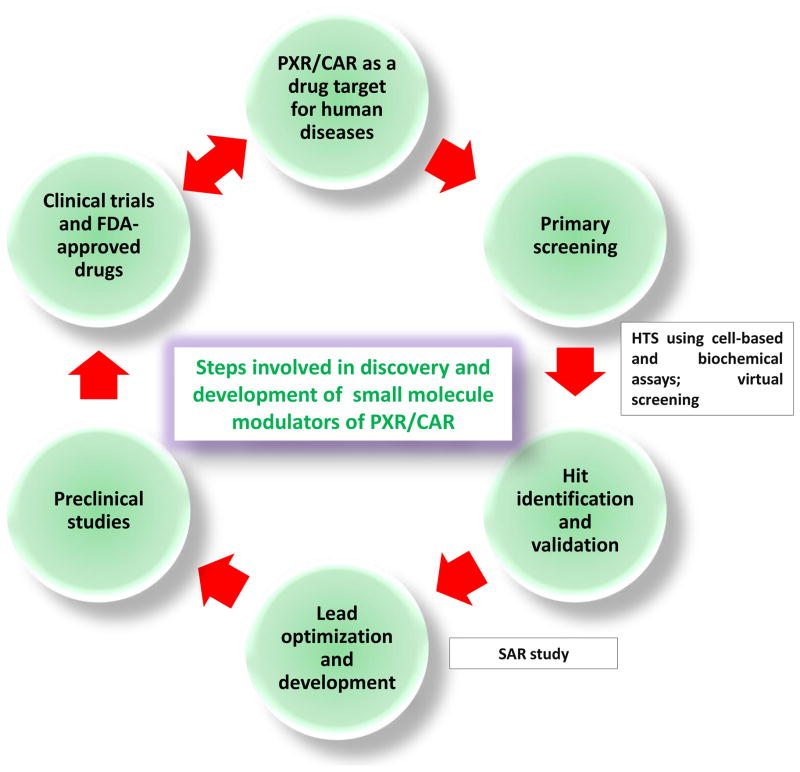
Figure 4. Pregnane X receptor.
(PXR)- and constitutive androstane receptor (CAR) -targeted drug discovery process. The proposed process involves the identification and validation of PXR and CAR as drug targets, the discovery and development of modulators of PXR and CAR, preclinical studies and clinical trials.
Concluding remarks and future directions
Targeting PXR and CAR individually or simultaneously in relevant signaling pathways and human diseases presents one of the most promising yet challenging strategies for developing novel therapeutics. PXR and CAR are involved in regulating various physiological processes in addition to drug metabolism and disposition, and can contribute to the development of various human diseases. To target PXR and CAR effectively in the context of specific and relevant pathways and diseases, it is crucial to understand their disease-specific roles and the specific therapeutic purpose, and design the appropriate assays to discover small molecules with desirable properties. Overall, the ultimate goal is to develop drugs with high specificity and potency.
Highlights.
PXR and CAR regulate drug disposition and human diseases
PXR and CAR are potential drug targets
Small molecules modulating specific function of PXR or CAR are needed
We propose approaches to target PXR and CAR
We discuss the challenges in targeting PXR and CAR
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children’s Research Hospital and the National Institutes of Health (Grants: GM086415, GM110034 and P30-CA21765). We thank David Galloway for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukherjee S, Mani S. Orphan nuclear receptors as targets for drug development. Pharm Res. 2010;27:1439–1468. doi: 10.1007/s11095-010-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billas I, Moras D. Allosteric controls of nuclear receptor function in the regulation of transcription. J Mol Biol. 2013;425:2317–2129. doi: 10.1016/j.jmb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Sladek FM. Nuclear receptors as drug targets: new developments in coregulators, orphan receptors and major therapeutic areas. Expert Opin Ther Targets. 2003;7:679–684. doi: 10.1517/14728222.7.5.679. [DOI] [PubMed] [Google Scholar]
- 4.Carlberg C, Molnar F. Detailed molecular understanding of agonistic and antagonistic vitamin D receptor ligands. Curr Top Med Chem. 2006;6:1243–1253. doi: 10.2174/156802606777864908. [DOI] [PubMed] [Google Scholar]
- 5.Kojetin DJ, Burris TP. Small molecule modulation of nuclear receptor conformational dynamics: implications for function and drug discovery. Mol Pharmacol. 2013;83:1–8. doi: 10.1124/mol.112.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JT, et al. The nuclear receptor superfamily and drug discovery. Chem Med Chem. 2006;1:504–523. doi: 10.1002/cmdc.200600006. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee M, Chen T. Thiazide-like diuretic drug metolazone activates human pregnane X receptor to induce cytochrome 3A4 and multidrug-resistance protein 1. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee M, et al. Modulation of xenobiotic receptors by steroids. Molecules. 2013;18:7389–7406. doi: 10.3390/molecules18077389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li HS, Wang HB. Activation of xenobiotic receptors: driving into the nucleus. Expert Opin Drug Metab Toxicol. 2010;6:409–426. doi: 10.1517/17425251003598886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T. Overcoming drug resistance by regulating nuclear receptors. Adv Drug Deliv Rev. 2010;62:1257–1264. doi: 10.1016/j.addr.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, et al. The antiapoptotic role of pregnane x receptor in human colon cancer cells. Mol Endocrinol. 2008;22:868–880. doi: 10.1210/me.2007-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, et al. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YM, et al. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace BD, Redinbo MR. Xenobiotic-sensing nuclear receptors involved in drug metabolism: a structural perspective. Drug Metab Rev. 2013;45:79–100. doi: 10.3109/03602532.2012.740049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.di Masi A, et al. Nuclear receptors CAR and PXR: molecular, functional, and biomedical aspects. Mol Aspects Med. 2009;30:297–343. doi: 10.1016/j.mam.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Ekins S, et al. Challenges predicting ligand-receptor interactions of promiscuous proteins: the nuclear receptor PXR. PLoS Comput Biol. 2009;5:e1000594. doi: 10.1371/journal.pcbi.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai X, et al. Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol. 2013;9:253–266. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- 18.Ihunnah CA, et al. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim Biophys Acta. 2011;1812:956–963. doi: 10.1016/j.bbadis.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhr U. Induction of drug metabolising enzymes: pharmacokinetic and toxicological consequences in humans. Clin Pharmacokinet. 2000;38:493–504. doi: 10.2165/00003088-200038060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Li F, et al. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med. 2013;19:418–420. doi: 10.1038/nm.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh M, et al. In vivo and in vitro characterization of a first-in-class novel azole analog that targets pregnane X receptor activation. Mol Pharmacol. 2011;80:124–135. doi: 10.1124/mol.111.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Synold TW, et al. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 24.Zhou CC, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 25.Healan-Greenberg C, et al. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane x receptor. Drug Metab Dispos. 2008;36:500–507. doi: 10.1124/dmd.107.019547. [DOI] [PubMed] [Google Scholar]
- 26.Wang HW, et al. The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane x receptor. Mol Endocrinol. 2008;22:838–857. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabb MM, et al. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR) Environ Health Perspect. 2004;112:163–169. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee M, Chen T. Differential regulation of CYP3A4 promoter activity by a new class of natural product derivatives binding to pregnane X receptor. Biochem Pharmacol. 2013;86:824–835. doi: 10.1016/j.bcp.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim YP, et al. Allyl isothiocyanate (AITC) inhibits pregnane X receptor (PXR) and constitutive androstane receptor (CAR) activation and protects against acetaminophen- and amiodarone-induced cytotoxicity. Arch Toxicol. 2014 doi: 10.1007/s00204-014-1230-x. [DOI] [PubMed] [Google Scholar]
- 30.Huang WD, et al. Meclizine is an agonist ligand for mouse constitutive androstane receptor (CAR) and an inverse agonist for human CAR. Mol Endocrinol. 2004;18:2402–2408. doi: 10.1210/me.2004-0046. [DOI] [PubMed] [Google Scholar]
- 31.Li LH, et al. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–453. doi: 10.1124/mol.108.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekins S, et al. A ligand-based approach to understanding selectivity of nuclear hormone receptors PXR, CAR, FXR, LXRalpha, and LXRbeta. Pharm Res. 2002;19:1788–1800. doi: 10.1023/a:1021429105173. [DOI] [PubMed] [Google Scholar]
- 34.Maglich JM, et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 35.Andersen V, et al. Polymorphisms in NF-kappaB, PXR, LXR, PPARgamma and risk of inflammatory bowel disease. World J Gastroenterol. 2011;17:197–206. doi: 10.3748/wjg.v17.i2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dring MM, et al. The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology. 2006;130:341–348. doi: 10.1053/j.gastro.2005.12.008. quiz 592. [DOI] [PubMed] [Google Scholar]
- 37.Zhou C, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah YM, et al. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1114–1122. doi: 10.1152/ajpgi.00528.2006. [DOI] [PubMed] [Google Scholar]
- 39.Rysa J, et al. Pregnane X receptor agonists impair postprandial glucose tolerance. Clin Pharmacol Ther. 2013;93:556–563. doi: 10.1038/clpt.2013.48. [DOI] [PubMed] [Google Scholar]
- 40.Kodama S, et al. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Winkel A, et al. Expression, localization and polymorphisms of the nuclear receptor PXR in Barrett’s esophagus and esophageal adenocarcinoma. BMC Gastroenterol. 2011;11:108. doi: 10.1186/1471-230X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castano G, et al. The influence of common gene variants of the xenobiotic receptor (PXR) in genetic susceptibility to intrahepatic cholestasis of pregnancy. Aliment Pharmacol Ther. 2010;31:583–592. doi: 10.1111/j.1365-2036.2009.04210.x. [DOI] [PubMed] [Google Scholar]
- 43.Staudinger JL, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, et al. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007;67:10361–10367. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodwin B, et al. Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol. 2002;62:359–365. doi: 10.1124/mol.62.2.359. [DOI] [PubMed] [Google Scholar]
- 48.Echchgadda I, et al. The xenobiotic-sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4alpha in the regulation of human steroid-/bile acid-sulfotransferase. Mol Endocrinol. 2007;21:2099–2111. doi: 10.1210/me.2007-0002. [DOI] [PubMed] [Google Scholar]
- 49.Wagner M, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, et al. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517–49522. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- 51.Lynch C, et al. Activation of the constitutive androstane receptor inhibits gluconeogenesis without affecting lipogenesis or fatty acid synthesis in human hepatocytes. Toxicol Applied Pharmacol. 2014;279:33–42. doi: 10.1016/j.taap.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D, et al. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 2013;121:329–338. doi: 10.1182/blood-2012-06-436691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papa S, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto Y, et al. Nuclear receptor CAR represses TNFalpha-induced cell death by interacting with the anti-apoptotic GADD45B. PLoS One. 2010;5:e10121. doi: 10.1371/journal.pone.0010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto Y, et al. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- 56.Baskin-Bey ES, et al. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- 57.Pascussi JM, et al. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000;274:707–713. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- 58.Moore LB, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson SS, et al. Regulation of human CYP2C9 by the constitutive androstane receptor: discovery of a new distal binding site. Mol Pharmacol. 2002;62:737–746. doi: 10.1124/mol.62.3.737. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Negishi M. Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab. 2003;4:515–525. doi: 10.2174/1389200033489262. [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou J, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 63.Zhai Y, et al. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol Endocrinol. 2007;21:138–147. doi: 10.1210/me.2006-0291. [DOI] [PubMed] [Google Scholar]
- 64.Argaud D, et al. Inhibition of gluconeogenesis in isolated rat hepatocytes after chronic treatment with phenobarbital. Biochem J. 1991;280:663–669. doi: 10.1042/bj2800663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueda A, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Nakae J, et al. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 68.Schmoll D, et al. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 69.Yeagley D, et al. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J Biol Chem. 2001;276:33705–33710. doi: 10.1074/jbc.M101215200. [DOI] [PubMed] [Google Scholar]
- 70.Masuyama H, Hiramatsu Y. Treatment with constitutive androstane receptor ligand during pregnancy prevents insulin resistance in offspring from high-fat diet-induced obese pregnant mice. Am J Physiol Endocrinol Metab. 2012;303:E293–300. doi: 10.1152/ajpendo.00167.2012. [DOI] [PubMed] [Google Scholar]
- 71.Watkins RE, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–2333. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 72.Watkins RE, et al. 2.1 A crystal structure of human PXR in complex with the St. John’s wort compound hyperforin. Biochemistry. 2003;42:1430–1438. doi: 10.1021/bi0268753. [DOI] [PubMed] [Google Scholar]
- 73.Chrencik JE, et al. Structural disorder in the complex of human pregnane X receptor and the macrolide antibiotic rifampicin. Mol Endocrinol. 2005;19:1125–1134. doi: 10.1210/me.2004-0346. [DOI] [PubMed] [Google Scholar]
- 74.Xue Y, et al. Crystal structure of the pregnane X receptor-estradiol complex provides insights into endobiotic recognition. Mol Endocrinol. 2007;21:1028–1038. doi: 10.1210/me.2006-0323. [DOI] [PubMed] [Google Scholar]
- 75.Cheng Y, Redinbo MR. Activation of the human nuclear xenobiotic receptor PXR by the reverse transcriptase-targeted anti-HIV drug PNU-142721. Protein Sci. 2011;20:1713–1719. doi: 10.1002/pro.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H, et al. Novel yeast-based strategy unveils antagonist binding regions on the nuclear xenobiotic receptor PXR. J Biol Chem. 2013;288:13655–13668. doi: 10.1074/jbc.M113.455485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekins S, et al. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]
- 78.Ekins S, et al. Computational discovery of novel low micromolar human pregnane X receptor antagonists. Mol Pharmacol. 2008;74:662–672. doi: 10.1124/mol.108.049437. [DOI] [PubMed] [Google Scholar]
- 79.Kortagere S, et al. Ligand- and structure-based pregnane X receptor models. Methods Mol Biol. 2012;929:359–375. doi: 10.1007/978-1-62703-050-2_15. [DOI] [PubMed] [Google Scholar]
- 80.Maglich JM, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 81.DeKeyser JG, et al. Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2. Mol Pharmacol. 2009;75:1005–1013. doi: 10.1124/mol.108.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang WD, et al. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu RX, et al. A structural basis for constitutive activity in the human CAR/RXRalpha heterodimer. Mol Cell. 2004;16:919–928. doi: 10.1016/j.molcel.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 84.Wu B, et al. 3D structures and ligand specificities of nuclear xenobiotic receptors CAR, PXR and VDR. Drug Discov Today. 2013;18:574–581. doi: 10.1016/j.drudis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Cheng J, et al. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci. 2012;33:323–330. doi: 10.1016/j.tips.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 87.Elias A, et al. Tumor suppressor protein p53 negatively regulates human pregnane X receptor activity. Mol Pharmacol. 2013;83:1229–1236. doi: 10.1124/mol.113.085092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matter H, et al. Development of in silico filters to predict activation of the pregnane X receptor (PXR) by structurally diverse drug-like molecules. Bioorg Med Chem. 2012;20:5352–5365. doi: 10.1016/j.bmc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 89.Wallace BD, et al. Structural and functional analysis of the human nuclear xenobiotic receptor PXR in complex with RXRalpha. J Mol Biol. 2013;425:2561–2577. doi: 10.1016/j.jmb.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brenk R, et al. Here be dragons: docking and screening in an uncharted region of chemical space. J Biomol Screen. 2005;10:667–674. doi: 10.1177/1087057105281047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paiva AM, et al. Inhibitors of dihydrodipicolinate reductase, a key enzyme of the diaminopimelate pathway of Mycobacterium tuberculosis. Biochim Biophys Acta. 2001;1545:67–77. doi: 10.1016/s0167-4838(00)00262-4. [DOI] [PubMed] [Google Scholar]
- 92.Shukla SJ, et al. Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos. 2011;39:151–159. doi: 10.1124/dmd.110.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shukla SJ, et al. Identification of pregnane X receptor ligands using time-resolved fluorescence resonance energy transfer and quantitative high-throughput screening. Assay Drug Dev Technol. 2009;7:143–169. doi: 10.1089/adt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynch C, et al. Identification of novel activators of constitutive androstane receptor from FDA-approved drugs by integrated computational and biological approaches. Pharm Res. 2013;30:489–501. doi: 10.1007/s11095-012-0895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue Y, et al. Crystal structure of the PXR-T1317 complex provides a scaffold to examine the potential for receptor antagonism. Bioorg Med Chem. 2007;15:2156–2166. doi: 10.1016/j.bmc.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lemaire G, et al. Discovery of a highly active ligand of human pregnane x receptor: a case study from pharmacophore modeling and virtual screening to “in vivo” biological activity. Mol Pharmacol. 2007;72:572–581. doi: 10.1124/mol.106.033415. [DOI] [PubMed] [Google Scholar]
- 97.Biswas A, et al. Elucidating the ‘Jekyll and Hyde’ nature of PXR: the case for discovering antagonists or allosteric antagonists. Pharm Res. 2009;26:1807–1815. doi: 10.1007/s11095-009-9901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]