Abstract
Myosin-X (Myo10) is a motor protein well known for its role in filopodia formation. New research implicates Myo10 in a number of disease states including cancer metastasis and pathogen infection. This review focuses on these developments with emphasis on the emerging roles of Myo10 in formation of cancer cell protrusions and metastasis. A number of aggressive cancers show high levels of Myo10 expression and knockdown of Myo10 has been shown to dramatically limit cancer cell motility in 2D and 3D systems. Myo10 knockdown also limits spread of intracellular pathogens marburgvirus and Shigella flexneri. Consideration is given to how these properties might arise and potential paths of future research.
Keywords: Myosin-X, Myo10, Cancer, Metastasis, Infectious Disease, Filopodia
Introduction
Myosin-X (Myo10) is an unconventional myosin that is expressed widely in vertebrate tissues and is especially prominent in developing brain, endothelia, and many epithelia (1). Myo10 is best known for its ability to localize to the tips of filopodia (2), finger-like cellular protrusions containing a core of bundled actin filaments (3,4). Myo10 has crucial functions in the formation of filopodia, with Myo10 overexpression inducing hundreds of filopodia per cell and knockdown decreasing endogenous filopodia (5–7). Importantly, new work indicates that Myo10 has central roles in cancer invasion and metastasis. The purpose of this review is to summarize the key features of Myo10’s structure and function, and to review recent research on the roles of Myo10 in cancer. We will also briefly highlight developing work implicating Myo10 in infectious diseases. Since Myo10’s structure has been reviewed recently (8), it will only be summarized here.
Myo10 Structure
Members of the myosin superfamily are typically composed of 1) a head domain capable of binding actin, hydrolyzing ATP, and generating force, 2) a neck consisting of one or more IQ motifs, each of which binds to a calmodulin or calmodulin-like light chain, and 3) a tail that endows class specific properties such as dimerization or cargo binding. Myo10 is a member of the MyTH4-FERM family of myosins, an evolutionarily ancient family with key functions in membrane-cytoskeleton interactions (8,9). The ~240 kDa Myo10 heavy chain consists of three regions: a myosin motor domain, a neck consisting of three IQ motifs, and a ~140 kDa tail. The initial ~110 amino acids of the tail consist of an α-helical region, the first part of which forms a single α-helix (SAH) (10). The second part of the α-helical region includes a region that can form a coiled coil and allows the Myo10 heavy chain to dimerize (11). Following the α-helical region are three PEST motifs. PEST motifs are enriched in the amino acids P, E, S, and T and are implicated in cleavage by the Ca++ dependent protease calpain (1). A unique feature of the Myo10 tail is its three pleckstrin homology (PH) domains, one of which binds to the key signaling lipid, phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (12–15). The MyTH4-FERM supramodule contains a MyTH4 (myosin tail homology 4 domain) and a FERM (band 4.1, ezrin, radixin, moesin) domain. The MyTH4 domain binds to microtubules (16,17), so Myo10 is unusual among actin-based motor proteins in that its tail can provide a direct link to microtubules. Consistent with this role as a linker between actin filaments and microtubules, Myo10 is required for proper orientation of meiotic and mitotic spindles (16,18,19). The Myo10 FERM domain binds to the netrin receptor Deleted in Colorectal Cancer (DCC) (17,20,21) and to β-integrins (22). Binding to β-integrins is hypothesized to allow Myo10 to link the internal actin cytoskeleton with the extracellular matrix. Although this review will focus on full-length Myo10, a “headless” form that lacks most of the motor domain is also expressed. This “headless Myo10” is expressed in stem cells and developing brain, where it may act in part as a natural dominant negative (23).
Myo10 Form and Function
Myo10 is a motor whose structure, regulation, and function are all under active investigation. The initial sequence analysis of Myo10 identified a putative coiled-coil region, so the heavy chains were predicted to dimerize due to formation of a parallel coiled coil. Indeed, all other myosins known to form coiled coil dimers do so as parallel dimers. In the case of Myo10 this model has been called into question by Mingjie Zhang and colleagues, who demonstrated that the isolated α-helical region dimerizes with a Kd of ~0.6 μM to form an anti-parallel coiled coil (11).
Myo10 is a low abundance protein, which has made tissue purification very difficult. Purification of full-length Myo10 via other approaches, such as baculovirus expression, has also proved difficult due to Myo10’s size and instability. The initial research to characterize the biophysical properties of Myo10 thus used baculovirus constructs consisting of the head, neck, and α-helical region, which were thought to resemble the parallel dimer formed by the heavy meromyosin (HMM) fragment of myosin II. At the sub-micromolar concentrations used in these studies, however, the “HMM-like” Myo10 constructs did not dimerize. To overcome this problem, dimerization was forced by inserting a sequence that forms a parallel coiled coil after the putative coiled-coil region. These studies found that there was a strong selective bias for Myo10 to walk processively on bundled actin filaments as opposed to single filaments (24,25). These constructs showed a relatively short step size of ~18 nm in forward displacement, but did so while walking on two filaments that were space roughly 10 nm apart (25). This was the first report of a dimeric myosin consistently straddling two different filaments as it walked along a bundle. However, different forced dimer constructs and approaches yielded motors that were not selective and walked with a step size similar to the actin helical repeat (36 nm) (26,27). This stepping pattern closely mimicked that of myosin-V, whose neck of 6 IQ motifs creates a lever arm long enough to generate 36 nm steps. Since the Myo10 neck only has 3 IQ motifs, the SAH domain after the IQ domains is thought to extend the lever arm, allowing for a step size of ~36 nm (28). If Myo10 forms anti-parallel dimers in its native state, forced parallel dimers may hold little direct relevance to Myo10’s in vivo behavior. It is fascinating, however, that in the case of the bundle-selective constructs, a forced dimer is able to actively direct the motor towards bundled actin, the primary localization site of Myo10 in cells. Research around the native state and function of this motor continues in earnest.
Importantly, Myo10 can exist as a monomer where the tail folds back onto the head and neck to form a compact and auto-inhibited “off” state (15). Binding to PIP3 at the membrane (13–15) is thought to open up the monomer and release its auto-inhibition so that it can dimerize and form an active motor. Once activated by this regulated dimerization, Myo10 can undertake its biological roles including transporting cargo and forming filopodia (2,5,29).
Myo10 and Cancer
There is growing evidence that cytoskeletal proteins and actin-based protrusions have central roles in cancer biology, particularly in metastasis. This section will discuss how Myo10 and three classes of actin-based protrusions: filopodia, invadopodia, and filopodium-like protrusions, function in cancer metastasis.
One of the first observations implicating filopodia in cancer biology was the discovery that high levels of the canonical filopodial crosslinking protein fascin provide a marker for aggressive metastatic disease and poor patient prognosis (30–32). Invasive cancer cells have also been reported to be highly filopodial and upregulation of fascin has been shown to increase in the number of filopodia (33), implicating filopodia as machinery of interest in aggressive metastatic disease. To escape the primary tumor, cancer cells also build structures called invadopodia, which are actin-rich protrusions with proteolytic activity (34) capable of digesting surrounding extracellular matrix. The Vignjevic lab has shown that both fascin and Myo10 are key components of invadopodia, with Myo10 localizing to the tips of invadopodia (34). Myo10 knockdown provided one of the strongest blocks of invadopodia function observed, with Myo10 knockdown inhibiting matrix digestion by 70%, versus 45% for fascin (34). Myo10 silencing also decreases expression of genes related to invadopodia formation and matrix metalloproteinase production (35), suggesting that Myo10 may have a role in metastasis that reaches beyond simple mechanics. The authors of that study hypothesize that since Myo10 itself does not appear to be a transcription factor, blocking invadopodia formation and morphology by Myo10 silencing may also block positive feedback from invadopodia that normally increases expression of invadopodium-related genes.
Once cancer cells have escaped from the primary tumor site they must interact with the extracellular matrix in order to survive and spread. Work from the Weinberg group has shown that a potential third type of protrusion from cancer cells, termed filopodium-like protrusions (FLPs), are required for these cells to spread and colonize (36) placing added importance on the role of actin-based protrusions in all phases of metastasis. Whether and how FLPs differ from canonical filopodia is not clear. Perhaps the most provocative result from this study was that when Myo10 is knocked down the metastatic potential of the cells is dramatically reduced in three ways: 1) the number of FLPs is reduced, 2) proliferation of the cells in tumor-like microenvironments (covered with matrigel) is decreased, and 3) the overall invasiveness of the cells is decreased (36). These data are further reinforced by recent in vitro experiments demonstrating that suppression of Myo10 almost completely blocked cancer cell outgrowth in both 2D and 3D systems (37).
Given that actin-based protrusions are important for metastatic cancer progression, it is exciting that recent studies report that Myo10 is upregulated in several aggressive metastatic cancers, including melanomas and basal-like breast carcinoma (38), and that high levels of Myo10 expression are associated with aggressiveness and metastasis in patients with breast cancer (35). These studies also reported that Myo10 is required for cancer cell invasion and dissemination using both cancer cell lines and mouse models (35,38). Other reports of high levels of Myo10 expression in cancers have been sprinkled through the literature for some time, including in acute lymphoblastic leukemia (39), and primary glioblastoma (40,41), further reinforcing the importance of Myo10 in cancer biology and expanding the apparent range of cancers that might be susceptible to Myo10 targeted therapeutics.
The loss of metastatic character caused by Myo10 silencing and subsequent disruption of filopodia, FLPs, and invadopodia may be related to the ability of these protrusions to form integrin-based adhesions. Filopodial adhesion is thought to act via β1-integrin-mediated linkage of the extracellular matrix to the internal actin cytoskeleton. Based on its domain composition, Myo10 is an excellent candidate for facilitating this interaction. Furthermore, Myo10 is implicated in transport of integrins within filopodia (22,38) and integrin signaling in FLPs is linked to cancer cell outgrowth and survival of micrometastases (36,42). Expression of a Myo10 mutant that is deficient in binding integrins is sufficient to recapitulate the arrest of cancer cell invasion seen during silencing of full-length Myo10 (38), directly showing that the integrin-Myo10 interaction is a key player in invasion.
Myo10’s importance in cancer cell biology is not limited to protrusion and migration. A hallmark of many cancers is the formation of excess centrosomes, a condition that in normal cells would be expected to result in multipolar spindles, aneuploidy, and cell death. Cancer cells must therefore cluster their excess centrosomes to allow proper spindle formation and proliferation. Myo10 has been implicated in actin-dependent centrosome clustering, and loss of Myo10 leads to an increase in cancer cells with multipolar spindles (43). These data demonstrate yet another way that loss or inactivation of Myo10 might limit cancer spread.
The importance of Myo10 in cancer progression is becoming clearer but the signaling in cancer cells that leads to its upregulation is still murky. Cancer transformation is aided by activating mutations in PI3 Kinase and/or inactivating mutations in PTEN (44). Myo10 binds and is activated by PIP3 (13,15,45), thus Myo10 functions as an effector of PI3 kinase. It is believed that upregulation of Myo10 in some aggressive carcinomas occurs via an early growth response-1 (EGR1)-dependent signaling pathway. The Myo10 gene has been reported to be a target of EGR1 regulation (46). EGR1 expression can be upregulated in mutant-EGFR (47) and mutant-p53 cancers (38). Myo10 appears to be required for p53-driven cancer cell invasion, and mutant p53 leads to upregulation of Myo10 and more aggressive cancer (48). Taken together these data show that the level of Myo10 expression affects cancer aggressiveness and that Myo10’s regulation in cancer is complex and likely differs depending on specific disease type.
Myo10 and Infectious Disease
The motor protein Myo10 and actin-based cellular protrusions known as filopodia are located at a convergence point of adhesion, motility, and signaling, and are important topics for cancer research. They are also increasingly of interest for their roles in some forms of infectious disease. For example, filoviruses, filamentous viruses with single-stranded negative-sense RNA genomes whose family members include Ebola and Marburg, may rely on Myo10 during their infection cycle. Filovirus replication takes place in the host cell cytoplasm before the viral nucleocapsids can bud out of the host cell. Myo10 and viral nucleocapsids in Marburg infected cells have been reported to co-localize during actin-dependent transport (49) and viral budding takes place through the filopodia or filopodium-like structures of infected cells (50). Further, expression of a dominant-negative Myo10 construct caused a significant reduction in virus-like particle production (50).
Similar stories are developing around the role of Myo10 and filopodia in bacterial infection as well. Shigella flexneri is an intracellular pathogen that primarily infects mammalian epithelial cells. In order to enter eukaryotic cells, Shigella can be captured by filopodia, which then retract bringing the bacteria to the cell body (51). Once inside a host epithelial cell, Shigella can spread laterally from cell to cell via membranous protrusions, avoiding further exposure to the host immune system. Myo10 directly associates with Shigella as it forms these protrusions and spreads into neighboring cells (52). This Myo10 association is critical for the bacterial infection to spread, as siRNA silencing of Myo10 reduces Shigella plague formation (52), a measure of bacterial spread in cell culture systems, by over 80%. The importance of Myo10 in these viral and bacterial infection examples demonstrate its relevance to infectious disease, and as this field expands Myo10 will likely be shown to be important in infections from many other organisms and viruses.
Perspectives and the Path Forward
Loss of function of MyTH4-FERM myosins has already been linked to human disease. Mutations in myosin-VIIa cause Usher Syndrome Ib, a disease that leads to deafness and blindness early in life (53). Mutations in myosin-XV lead to unusually short stereocilia (actin-based protrusions required for hearing) and cause hereditary deafness (54,55). Based on morpholino experiments in Xenopus, it is likely that complete loss of Myo10 is embryonically lethal (56). This suggests that Myo10 is essential for survival and is consistent with its critical roles in motility, adhesion, spindle orientation (19), cell-cell junction formation (57), and endothelial cells (58). Analysis of Myo10 loss of function in specific tissues, through a conditional knockout animal model, would add greatly to our understanding of the varied and important roles of this motor. CRISPR technologies could also be useful for production of cell lines that are true Myo10 knockouts or cell lines that replace wild-type Myo10 with mutant or deletion Myo10 constructs under endogenous promoters, allowing for mechanistic analysis of Myo10 function in its critical processes. Elucidating the cellular and tissue level roles of Myo10 is an area where more work is needed. Understanding the functions of this low abundance protein and how it can play roles in such a diverse set of processes will be interesting and illuminating.
The story of Myo10 in disease is the story of cellular protrusions and how cells interact with their environment. The rapidly growing body of evidence linking Myo10 to cancer metastasis is exciting and points to Myo10 as a potential target for anti-metastatic therapeutics. Current cancer therapeutics target signaling components in cells, but the physical machinery of cellular migration has been largely ignored. Myo10 and filopodia thus provide promising targets for the development of drugs to block invasion and for the investigation of fundamental cell biology underlying both health and disease.
Figure 1.
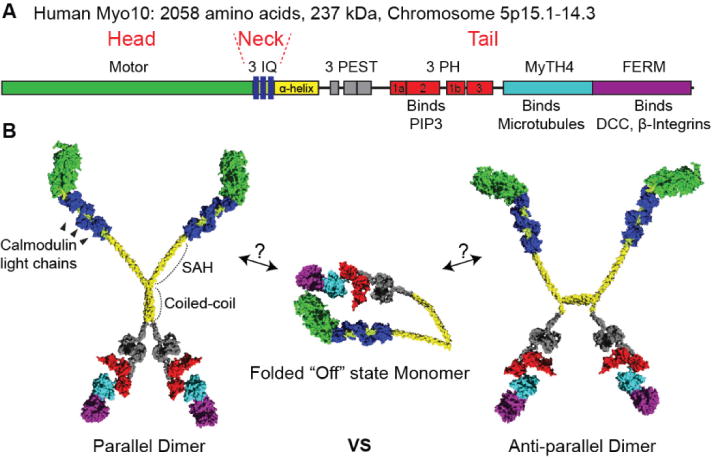
(A) Bar diagram of Myo10. The bar diagram highlights the domains of the Myo10 heavy chain. It has a head domain containing the motor, a neck domain containing three IQ motifs, and a tail domain containing numerous binding domains. The region containing 3 PH domains binds PIP3. The MyTH4-FERM domain is response for binding to microtubules and integrins. (B) Hypothetical models of Myo10 structure based on structure of individual domains. Myo10 monomers can form a folded “off” state where the tail domain folds over onto the head domain. Binding to PIP3 allows the folded monomers to form active dimers. Dimeric myosins were previously thought to form parallel heavy chain dimers, but recent data indicates that Myo10 can form anti-parallel dimers.
Figure 2.
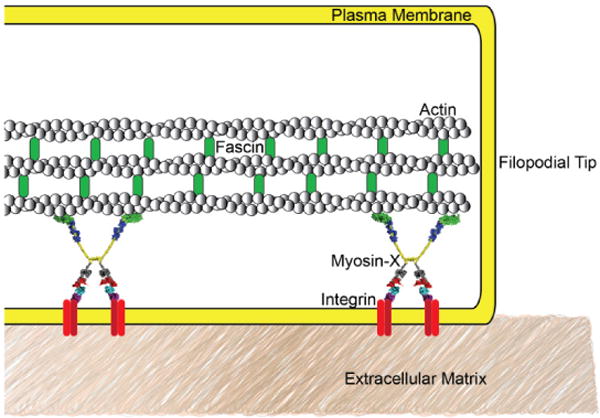
Myo10 is hypothesized to be the molecular link between the actin cytoskeleton and integrin-based adhesions to the extra-cellular matrix in cellular protrusions like filopodia and cancer cell filopodium-like protrusions. Integrin patches have been observed in cellular protrusions. Myo10 is shown linking filopodial actin to integrin patches that engage with the extracellular matrix. Filopodial adhesion can form and release rapidly, so the relative simplicity of this model is appealing. Studies of cells expressing Myo10 mutants that lack integrin binding have shown decreased filopodial adhesion and migration, further corroborating this model.
Acknowledgments
Research in the author’s lab is supported by National Institutes of Health grant R01 DC03299 and Dr. Courson is supported by NIH/NCI 5-T32-CA009156 to the Lineberger Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- 2.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 3.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 4.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokuo H, Mabuchi K, Ikebe M. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J Cell Biol. 2007;179:229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe TM, Tokuo H, Gonda K, Higuchi H, Ikebe M. Myosin-X induces filopodia by multiple elongation mechanism. J Biol Chem. 2010;285:19605–19614. doi: 10.1074/jbc.M109.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerber ML, Cheney RE. Myosin-X: a MyTH-FERM myosin at the tips of filopodia. J Cell Sci. 2011;124:3733–3741. doi: 10.1242/jcs.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breshears LM, Wessels D, Soll DR, Titus MA. An unconventional myosin required for cell polarization and chemotaxis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6918–6923. doi: 10.1073/pnas.0909796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight PJ, Thirumurugan K, Xu Y, Wang F, Kalverda AP, Stafford WF, 3rd, Sellers JR, Peckham M. The predicted coiled-coil domain of myosin 10 forms a novel elongated domain that lengthens the head. J Biol Chem. 2005;280:34702–34708. doi: 10.1074/jbc.M504887200. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Ye F, Wei Z, Wen Z, Zhang M. Antiparallel coiled-coil-mediated dimerization of myosin X. Proc Natl Acad Sci U S A. 2012;109:17388–17393. doi: 10.1073/pnas.1208642109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashanov GI, Tacon D, Peckham M, Molloy JE. The spatial and temporal dynamics of pleckstrin homology domain binding at the plasma membrane measured by imaging single molecules in live mouse myoblasts. J Biol Chem. 2004;279:15274–15280. doi: 10.1074/jbc.M312140200. [DOI] [PubMed] [Google Scholar]
- 13.Plantard L, Arjonen A, Lock JG, Nurani G, Ivaska J, Stromblad S. PtdIns(3,4,5)P(3) is a regulator of myosin-X localization and filopodia formation. Journal of cell science. 2010;123:3525–3534. doi: 10.1242/jcs.069609. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Yu J, Yan J, Wei Z, Zhang M. Structural basis of the myosin X PH1(N)-PH2-PH1(C) tandem as a specific and acute cellular PI(3,4,5)P(3) sensor. Mol Biol Cell. 2011;22:4268–4278. doi: 10.1091/mbc.E11-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umeki N, Jung HS, Sakai T, Sato O, Ikebe R, Ikebe M. Phospholipid-dependent regulation of the motor activity of myosin X. Nature structural & molecular biology. 2011;18:783–788. doi: 10.1038/nsmb.2065. [DOI] [PubMed] [Google Scholar]
- 16.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–329. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 17.Hirano Y, Hatano T, Takahashi A, Toriyama M, Inagaki N, Hakoshima T. Structural basis of cargo recognition by the myosin-X MyTH4-FERM domain. The EMBO journal. 2011;30:2734–2747. doi: 10.1038/emboj.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1-and myosin X-dependent manner. The EMBO journal. 2007;26:1487–1498. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, Du QS, Mei L, Ding YQ, Xiong WC. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9:184–192. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
- 21.Wei Z, Yan J, Lu Q, Pan L, Zhang M. Cargo recognition mechanism of myosin X revealed by the structure of its tail MyTH4-FERM tandem in complex with the DCC P3 domain. Proc Natl Acad Sci U S A. 2011;108:3572–3577. doi: 10.1073/pnas.1016567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 23.Raines AN, Nagdas S, Kerber ML, Cheney RE. Headless Myo10 is a negative regulator of full-length Myo10 and inhibits axon outgrowth in cortical neurons. J Biol Chem. 2012;287:24873–24883. doi: 10.1074/jbc.M112.369173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy S, Ricca BL, Norstrom MF, Courson DS, Brawley CM, Smithback PA, Rock RS. A myosin motor that selects bundled actin for motility. Proc Natl Acad Sci U S A. 2008;105:9616–9620. doi: 10.1073/pnas.0802592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricca BL, Rock RS. The stepping pattern of myosin X is adapted for processive motility on bundled actin. Biophys J. 2010;99:1818–1826. doi: 10.1016/j.bpj.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi Y, Farrow RE, Billington N, Nagy A, Batters C, Yang Y, Sellers JR, Molloy JE. Myosin-10 produces its power-stroke in two phases and moves processively along a single actin filament under low load. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1833–1842. doi: 10.1073/pnas.1320122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Sato O, Ruhnow F, Arsenault ME, Ikebe M, Goldman YE. Single-molecule stepping and structural dynamics of myosin X. Nat Struct Mol Biol. 2010;17:485–491. doi: 10.1038/nsmb.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baboolal TG, Sakamoto T, Forgacs E, White HD, Jackson SM, Takagi Y, Farrow RE, Molloy JE, Knight PJ, Sellers JR, Peckham M. The SAH domain extends the functional length of the myosin lever. Proc Natl Acad Sci U S A. 2009;106:22193–22198. doi: 10.1073/pnas.0909851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerber ML, Jacobs DT, Campagnola L, Dunn BD, Yin T, Sousa AD, Quintero OA, Cheney RE. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Curr Biol. 2009;19:967–973. doi: 10.1016/j.cub.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC cancer. 2006;6:241. doi: 10.1186/1471-2407-6-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, Tubbs RR, Adams JC, Hicks DG. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:186–192. [PubMed] [Google Scholar]
- 32.Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S, Higashiyama M, Watanabe G, Imamura M, Shimada Y. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2597–2605. doi: 10.1158/1078-0432.CCR-04-1378. [DOI] [PubMed] [Google Scholar]
- 33.Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, Ben-Ze’ev A, Robine S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer research. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 34.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao R, Chen J, Zhang X, Zhai Y, Qing X, Xing W, Zhang L, Malik YS, Yu H, Zhu X. Elevated expression of myosin X in tumours contributes to breast cancer aggressiveness and metastasis. British journal of cancer. 2014;111:539–550. doi: 10.1038/bjc.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer discovery. 2012;2:706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon A, Geraldo S, Maiuri P, Funfak A, Bibette M, Vignjevic D. The role of filopodia in directed cancer cell migration in 2D and 3D. ASCB Annual Meeting; Philadelphia, PA. 2014. [Google Scholar]
- 38.Arjonen A, Kaukonen R, Mattila E, Rouhi P, Hognas G, Sihto H, Miller BW, Morton JP, Bucher E, Taimen P, Virtakoivu R, Cao Y, Sansom OJ, Joensuu H, Ivaska J. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. The Journal of clinical investigation. 2014;124:1069–1082. doi: 10.1172/JCI67280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross ME, Zhou X, Song G, Shurtleff SA, Girtman K, Williams WK, Liu HC, Mahfouz R, Raimondi SC, Lenny N, Patel A, Downing JR. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood. 2003;102:2951–2959. doi: 10.1182/blood-2003-01-0338. [DOI] [PubMed] [Google Scholar]
- 40.Mischel PS, Shai R, Shi T, Horvath S, Lu KV, Choe G, Seligson D, Kremen TJ, Palotie A, Liau LM, Cloughesy TF, Nelson SF. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene. 2003;22:2361–2373. doi: 10.1038/sj.onc.1206344. [DOI] [PubMed] [Google Scholar]
- 41.Choe G, Mischel PS. Molecular subtypes of primary glioblastoma identified by gene expression profiling. The Korean Journal of Pathology. 2002;36:328–337. [Google Scholar]
- 42.Shibue T, Brooks MW, Weinberg RA. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer cell. 2013;24:481–498. doi: 10.1016/j.ccr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, Pellman D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes & development. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews. Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox D, Berg JS, Cammer M, Chinegwundoh JO, Dale BM, Cheney RE, Greenberg S. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol. 2002;4:469–477. doi: 10.1038/ncb805. [DOI] [PubMed] [Google Scholar]
- 46.Cermak V, Kosla J, Plachy J, Trejbalova K, Hejnar J, Dvorak M. The transcription factor EGR1 regulates metastatic potential of v-src transformed sarcoma cells. Cell Mol Life Sci. 2010;67:3557–3568. doi: 10.1007/s00018-010-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maegawa M, Arao T, Yokote H, Matsumoto K, Kudo K, Tanaka K, Kaneda H, Fujita Y, Ito F, Nishio K. EGFR mutation up-regulates EGR1 expression through the ERK pathway. Anticancer research. 2009;29:1111–1117. [PubMed] [Google Scholar]
- 48.Arjonen A, Kaukonen R, Mattila E, Rouhi P, Hognas G, Sihto H, Miller BW, Morton JP, Bucher E, Taimen P, Virtakoivu R, Cao Y, Sansom OJ, Joensuu H, Ivaska J. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. The Journal of clinical investigation. 2014 doi: 10.1172/JCI67280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schudt G, Kolesnikova L, Dolnik O, Sodeik B, Becker S. Live-cell imaging of Marburg virus-infected cells uncovers actin-dependent transport of nucleocapsids over long distances. Proc Natl Acad Sci U S A. 2013;110:14402–14407. doi: 10.1073/pnas.1307681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolesnikova L, Bohil AB, Cheney RE, Becker S. Budding of Marburgvirus is associated with filopodia. Cellular microbiology. 2007;9:939–951. doi: 10.1111/j.1462-5822.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 51.Romero S, Grompone G, Carayol N, Mounier J, Guadagnini S, Prevost MC, Sansonetti PJ, Van Nhieu GT. ATP-mediated Erk1/2 activation stimulates bacterial capture by filopodia, which precedes Shigella invasion of epithelial cells. Cell host & microbe. 2011;9:508–519. doi: 10.1016/j.chom.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishai EA, Sidhu GS, Li W, Dhillon J, Bohil AB, Cheney RE, Hartwig JH, Southwick FS. Myosin-X facilitates Shigella-induced membrane protrusions and cell-to-cell spread. Cellular microbiology. 2013;15:353–367. doi: 10.1111/cmi.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 54.Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- 55.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–172. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang YS, Luo T, Xu Y, Sargent TD. Myosin-X is required for cranial neural crest cell migration in Xenopus laevis. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2522–2529. doi: 10.1002/dvdy.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu KC, Jacobs DT, Dunn BD, Fanning AS, Cheney RE. Myosin-X functions in polarized epithelial cells. Mol Biol Cell. 2012;23:1675–1687. doi: 10.1091/mbc.E11-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]


