Abstract
Rheumatoid Arthritis (RA) is an inflammatory autoimmune joint disease in which the complement system plays an important role. Of the several components of complement, current evidence points to C5 as the most important inducer of inflammation. Several groups generated antibodies or siRNAs or small molecule inhibitors against C5 and C5aR1 (CD88) which have showed some efficacy in RA in animal models. However, none of these candidate therapeutics has moved from bench to bedside. Here we test in CAIA a new therapeutic strategy using a novel anti-C5ab-C5 siRNA conjugate. We first demonstrate that while C5aR2 or C5L2 (GPR77) plays no role in collagen antibody induced arthritis (CAIA), C5aR1 contributes to pathogenesis. We demonstrate that injection of siRNAs blocking either C5, C5aR1 or the combination decreased clinical disease activity (CDA) in mice with CAIA by 45%, 51% and 58%, respectively. Anti-C5 antibody (BB5.1) has only limited efficacy nonetheless significantly reduced arthritis up to 66%. We then generated a novel anti-C5aR1ab-protamine-C5siRNA conjugate. Here we show for the first time that while unconjugated antibody plus siRNAs reduce arthritis by 19%, our an anti-C5aR1ab - protamine - C5 siRNA conjugate was effective in reducing arthritis by 83% along with a parallel decrease in histopathology, C3 deposition, neutrophils and macrophages in the joints of mice with CAIA. These data suggest that by targeting anti-C5 siRNAs to the receptor for its C5a activation fragment (C5aR1), a striking clinical effect can be realized.
Keywords: complement, arthritis, immune complex, inflammation
Introduction
It is well accepted that the complement system plays an important role in the development of Rheumatoid Arthritis RA (1), and evidence suggests that the C5 component of complement may play a central role in disease progression (2). C5 is cleaved into C5a and C5b. C5a promotes inflammation via engagement of its receptors C5aR1 and C5aR2, while C5b nucleates the assembly of the membrane attack complex (MAC, C5b-9). It appears that C5aR1 signaling is crucial for the progression of RA as C5aR1−/− mice do not develop appreciable disease (3). The C5a receptor (C5aR, CD88), is expressed by immune cells such as neutrophils, dendritic cells and macrophages (4), and is also expressed by liver, kidney, brain, lung and skin (reviewed in (5)). Engagement of C5aR results in numerous pro-inflammatory processes including chemotaxis, vasodilation, enhanced secretion of inflammatory mediators and reactive substances, and enhanced phagocytosis, as well as other effects (4). A second C5a receptor, C5L2 (now known as C5aR2) has been identified but its role is controversial (6, 7).
Several groups have targeted the C5-C5aR signal transduction pathway in RA. The anti-C5 mAb, BB5.1 decreased disease in the collagen-induced arthritis (CIA) mouse model (8). Other C5 neutralizing antibodies prevented both CIA and collagen antibody-induced arthritis (CAIA) in mice (9). C5 deficient mice are highly resistant to CIA in some studies but not others (10-12). In a recent study by Macor et al. (13) an anti-C5 antibody was developed which bound to mouse, rat, and human RA tissues but not healthy tissues. Clinical effects mediated by this antibody were modest. Using the CAIA model, we have shown that C3 and C5 components of the complement cascade play an important role in disease development (3, 14, 15). Of note, we found that over 80% of C5a is derived from the alternative pathway (AP) (15).
Human trials with C5 and C5aR targeted therapeutics have been largely unsuccessful despite the abundance of C5 and C5aR1 within human RA joint tissues (16-18). Eculizumab, a humanized anti-C5 antibody, has shown excellent efficacy when used to treat paroxysmal nocturnal haemoglobinuria (19); however, its use in a phase IIb (unpublished) trial for the treatment of RA was unsuccessful (discussed in (20)). PMX53 was also unsuccessful in a small clinical trial testing its efficacy on RA patients (20).
Small interfering RNAs (siRNAs) are a new and evolving class of bio-therapeutics which is likely to find applications alongside traditionally used antibodies, fusion proteins and recombinant proteins. These double stranded RNAs, 20 – 25 bp in length, interfere with the expression of specific genes via the engagement of the RNA-inducing silencing complex (RISC), and have been applied to the treatment of various diseases including cancer, infection, and arthritis (21-23). Targeting of the siRNA along with minimization of off target effects is a major challenge. Functionalized nanoparticles have been successfully used to deliver siRNAs in collagen-induced arthritis (CIA) by targeting integrins upregulated during angiogenesis (24). Antibodies have also been useful targeting agents for siRNAs. The conjugates of an antibody-siRNA (F105 ab-protamine-siRNA HIV-gag) have been tested successfully both in vitro and in vivo (25). Polo-like kinase (PLK1) siRNA conjugated to a single chain fragmented antibody (ScFv)-protamine complex has been shown to suppress HER2+ breast cancer growth (26). Recently it has been demonstrated that an antibody-siRNA (Shamporter-siRNA nephrin or TRPC-6) conjugate could successfully inhibit gene expression in podocytes after i.v. administration in mice (27).
In this study we examined the efficacy of siRNAs targeting the C5-C5aR signaling pathway. In particular, we explored the effect of conjugating C5 siRNAs to an anti-C5aR1 blocking antibody (ab). Here we show that an anti-C5aR1ab-protamine-C5siRNA conjugate is significantly more efficacious than the combination of identical siRNAs and unconjugated anti C5aR1 antibody in the CAIA. These data provide a proof of concept that it is possible to block complement sufficiently with a bi-specific therapeutic molecule in order to effectively block disease progression. Furthermore, these data demonstrate the utility of anti-C5aR1ab-protamine-C5siRNA conjugates as potential therapeutic entities for the treatment of arthritis.
Materials and Methods
Conjugation of anti-C5aR mAb with protamine
Anti-C5aR1 mAb (clone 20/70) was purchased from LifeSpan Biosciences, Inc. (Seattle, WA). This 20/70 clone has been well described as an anti-C5aR1 blocking antibody by several investigators (28-32). It functions by binding to C5aR1 and stearically inhibiting its interaction with C5a. Protamine was conjugated by BIOO Scientific to divalent anti-C5aR1 mAb (3 mg) using the BIOO T3-Max® Conjugation kit (Austin TX) with approximately 34% efficiency according to the manufacturers’ instructions. This conjugation efficiency can vary greatly from one experiment to another. Protamine evolved to bind nucleic acids and is positively charged. Protamine plays no role in binding of the C5siRNA to cells. Instead protamine is used as linker for the binding of the C5siRNA to the anti-C5aR1 antibody. The chemistry makes use of amines such as that found on lysine side chains. Any available lysine side chain amine group may be conjugated to protamine through this process. In brief, the antibody is dialyzed and combined with kit components at a specific temperature as suggested by the manufacture of conjugation kit. The conjugation reaction runs for 14–16 hours followed by addition of buffer which both stops the reaction and places the complex in an environment suitable for siRNA loading and in vivo administration. After removal of unconjugated protamine by gel filtration chromatography, complexes are assessed by SDS-PAGE. Conjugation efficiency was determined by the amount of material which has increased in molecular weight.
Loading of C5 siRNA to anti-C5aR-Protamine Conjugate
The conjugate of anti-C5aR1 mAb (20/70) - protamine (150μg) was incubated with Accell® C5 siRNA (8μg) or Accell® Non-Targeting (scrambled) siRNA (8μg) (Dharmacon, GE Healthcare) at 4°C for 30 min. These Accell® siRNAs are nuclease resistant and are stable in vivo (personal communication with (Dharmacon, GE Healthcare). BIOO Scientific estimates that on average, 3 protamine molecules are bound to an IgG after conjugation and that 1 protamine fragment can bind 20-30 siRNAs (Lance Ford, BIOO Scientific, personal communication). In these studies we used a mixture of four siRNAs i.e. 2μg of each to combine with anti-C5aR1-protamine. The unconjugated siRNAs were not removed due to the non-availability of this methodology as well as scientific reasons explained later on. The advantage of this method of construction was that the fewer steps were involved leading to decreased loss of material and that binding properties of the antibody generally remain unaltered.
Flow Cytometry
C5aR1 expression was measured on a macrophage cell line (RAW 264.7) using the clone 20/70-protamine conjugate at concentration of 0.4 μg /1×106 cells. Antibody conjugate binding was visualized using a FITC conjugated secondary goat-anti-rat IgG (Life Technologies, Grand Island, NY) diluted 1:400. A dose dependent curve using FACS analysis was also generated using various concentrations (1μg/ml, 0.5μg/ml, 0.250μg/ml, 0.125μg/ml, 0.0625μg/ml and 0.0312μg/ml) of the conjugated and unconjugated anti-C5aR1 ab. A matched Rat IgG2b-FITC-conjugated isotype control (BD Biosciences, San Jose, CA) was also used. C57 BL/6 WT mice (n = 3) were injected i.v. with PBS (50 μL) or anti-CII mAbs (4mg) and LPS (50 μg) and mice were sacrificed at 24 hrs. Liver and spleen were dissected, a single cell suspension made, and RBC removed. C5aR1 expression was assessed using a PE conjugated clone 20/70 mAb at a concentration of 0.2 μg/1×106 cells (BioLegend, San Diego CA) along with matched Rat IgG2b-PE conjugated isotype control (BD Biosciences) (dilution 1:2000). Liver single cell suspension might contain not only hepatocytes but also kupfer cells (macrophages). All samples were analyzed using a FC500 flow cytometry machine.
Transfection of siRNA and qRT-PCR measure of mRNA
RAW cells were cultured in 24-well plates at a density of 1×105 cells and transfected with either 1 μM (13.2 ng) or 2 μM (26.4 ng) Accell® C5 siRNA using siRNA delivery media as directed (Dharmacon, ThermoFisher Scientific, Waltham MA). RNA was extracted using the RNAeasy kit (Qiagen Inc., Valencia, CA). Separately, RNA was extracted from liver samples derived from CAIA experiments and similarly analyzed. PCR determination of mRNAs for C5 and C5aR1 from RAW cells and C57BL/6 liver as well as the mRNA for TNF-α and MMP-3 from the left knee joints were performed by RT-PCR using 40 cycles according to published methods as described (33, 34). All qRT-PCR data were analyzed using a cDNA based standard curve made by using liver and RAW cell RNA. Primer sequences are available upon request. The standard curves for mRNA encoding C5 and C5aR were constructed by using mRNA from mouse liver.
Collagen antibody-induced arthritis
To show the effect of anti-C5 antibody or C5/C5aR1 siRNAs or conjugated anti-C5aR1 mAb with or without protamine and C5siRNAs, a mouse model of RA known as collagen antibody-induced arthritis (CAIA) was used. CAIA, which represents the effector phase of inflammatory arthritis, is complement dependent and has been used to test the role of deleterious cytokines as well as the therapeutic efficacy of various drugs which are now in clinical use for the treatment of RA (35). Many biologicals which have revolutionized the treatment for RA have been tested previously in mouse models of arthritis. For example drugs such as Remicade, Humara and Enbrel has been tested using CAIA mouse model (35). Eight weeks old C57 BL/6 WT mice purchased the Jackson Laboratory (Bar Harbor, ME). Arthritis was induced in these mice using a mixture of anti-collagen antibodies and LPS according to our previously published studies (33). Four separate CAIA experiments were done and a total of 55 mice were used. For the first study, CAIA in WT and C5aR2−/− was induced according to our published studies (33) and the clinical disease activity (CDA) was examined by a blinded observer according to published studies (33, 36). These mice were originally obtained and genotyped again as C5L2−/− from Drs. Craig Gerard and Bao Lu, Boston Childern's Hospital, Boston MA. Recently, due to the change in nomenclature by the International Complement Society (ICS) (7), for this experiment and for consistency these mice have been designated as C5aR2−/−. In the second experiment, to examine the effect of an anti-C5 inhibitory antibody on arthritis, CAIA in both the cohorts of WT mice was induced according to our published studies (33, 36). Mice were injected i.p. two times i.e. at day 3 and at day 7 with an inhibitory anti-C5 antibody (BB5.1) (750μg/mouse) or IgG1. For the third experiment each mouse was injected i.v. three times i.e. at day -5, day 0 and at day 3 with a dose of the respective commercially available siRNAs. In this experiment we used a mixture of four siRNAs targeting either C5a or C5aR1. The total siRNA used for each target was 8 μg per mouse (i.e. 2μg of each). Some mice were also injected simultaneously with a combined dose of C5 and C5aR1 siRNAs (8μg + 8μg = 16 μg per mouse). For the fourth CAIA experiment, to examine the effect of anti-C5a ab - protamine - C5 siRNA conjugate on arthritis, each mouse was injected i.p. three times i.e. at day -5, day 0 and at day 3 with PBS or a conjugate of anti-C5aR1 - protamine - C5 siRNA (150μg of mAb + 8μg of C5 siRNA/mouse) (conjugated complex) or of anti-C5aR1- no protamine - C5 siRNA (150μg of mAb + 8μg of C5 siRNA/mouse) (unconjugated mixture). Mice injected with an unconjugated mixture served as negative controls. All mice were weighed before, during and after the induction of CAIA. All mice were sacrificed at day 10.
Histopathology and Immunohistochemical analysis
Knee joints from fore limbs, the right hind limb with knee joint, ankle, and paw, were fixed in 10% Neutral buffered formalin. Toluidine-blue stain was used to assess histopathology scores for inflammation, pannus formation, cartilage and bone damage as described (37). C3 immunohistochemistry was performed as described (37). Monocyte/macrophage and neutrophil infiltration was counted as described (3).
Cytokine mRNA analysis from the knee joints
The absolute levels of TNF-α, IL-1β and matrix metalloproteinase-3 (MMP-3) mRNAs were measured from knee joints of mice at day 10 treated with PBS or anti-C5aR1ab - protamine - C5siRNA or anti-C5aR1 ab - no protamine - C5 siRNA using quantitative RT-PCR according to previously described method (33). The standard curves for TNF-α, IL-1β and MMP-3 mRNAs were made using RAW cells stimulated with LPS (5ug/ml) for 24 hrs. All data were expressed in pg/ng 18S rRNA.
Statistical analysis
Normality of all data was determined by using a null hypothesis for w -statistics. P – values (p < 0.05 indicated by stars) were calculated using Student's t test (unpaired two-tailed) within GraphPad Prism® 4. The data in graphs and histograms are shown as the mean ± SEM.
Results
Susceptibility of C5aR2−/− mice to CAIA
CAIA was induced as mentioned in the Materials and Methods in C5aR2−/− and WT mice (both on a C57BL/6 background). The CDA, at day 10, in C5aR2−/− and WT was 8.6 ± 0.82 and 9.0 ± 0.71 respectively (Fig 1A) and these differences were not statistically significant (p < 0.82). The prevalence, at day 10, in C5aR2−/− and WT mice was 100% (Fig 1B). These results show that C5aR2 plays no role in CAIA in contrast to C5aR1 which plays an important role in CAIA (3).
Figure 1.
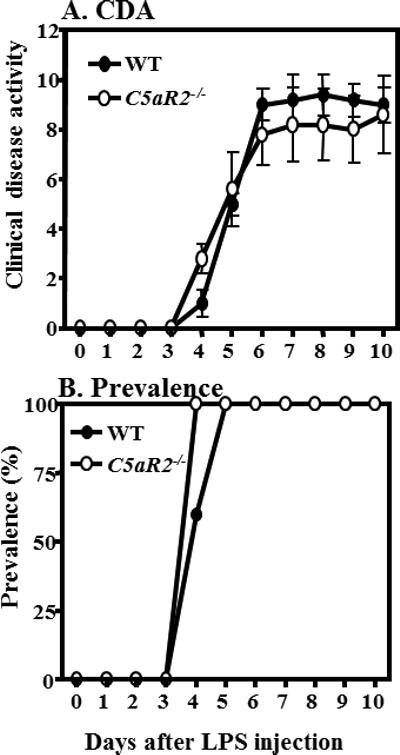
C5aR2 is not involved in CAIA. Comparing the CDA and prevalence between WT and C5aR2−/− mice, CAIA was induced in WT and in C5aR2−/− mice with anti-CII mAb 8 mg/mouse injected i.p. on day 0 followed by an i.p. injection of LPS on day 3. Mice were evaluated daily by an observer blinded to the genotype of mouse. A. Comparison of CDA between WT and C5aR2−/− mice. B. The prevalence of disease at day 10 in WT and C5aR2−/− mice was 100%. Data shown represent the mean ± SEM based on WT, n = 5 and C5aR2−/−, n = 5. No statistically significant differences, from day 4 through day 10, were seen in the CDA between WT and C5aR2−/− mice.
Effect of an anti-C5 inhibitory antibody on CAIA
We then examined the effect of an anti-C5 mAb on CAIA. CAIA was induced as described above. WT mice were injected with 750 μg of either mouse IgG1 or anti-C5 antibody on days 3 and 7 (Fig 2A). The CDA, at day 10, in WT mice treated with IgG1 and anti-C5 inhibitory antibody was 9.5 ± 0.5 and 3.22 ± 0.577 respectively (Fig 2A) and these differences were statistically significant (P < 0.0017). This decrease in the CDA of WT mice treated with anti-C5 inhibitory antibody was 66% compared with mice treated with IgG1 alone. The prevalence of disease both in IgG1 and anti-C5 mAb treated WT mice was 100% from day 5 through day 10 (data not shown). These data recapitulate in the CAIA model the concept that the C5 system plays an important role in disease progression.
Figure 2.
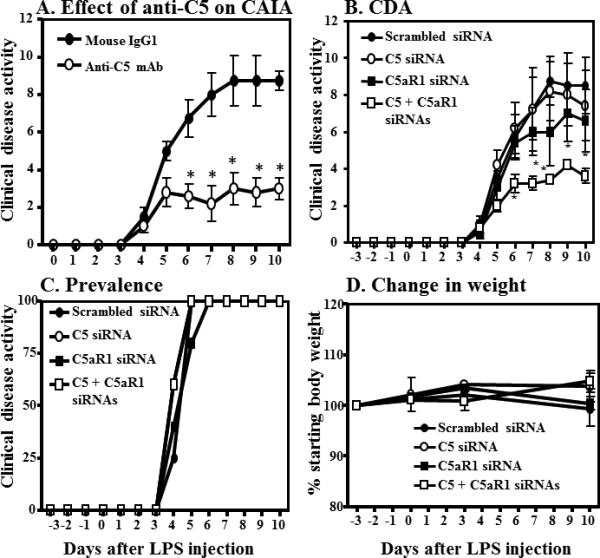
Modulation of C5 Signal Transduction in CAIA. Anti-C5 mAb, commercially available C5 and C5aR1 siRNAs affected the Clinical Disease Activity (CDA) in CAIA. CAIA was induced in WT mice as mentioned in the Materials and Methods. A. CAIA mice were injected with 750 ug of either IgG1 or anti-C5 antibody (BB5.1) on day 3 and at day 7. Data represent the mean CDA ± SEM (n = 5) *p < 0.05. B-D. Knockdown using C5siRNA and/or C5aRsiRNA. CAIA was induced as described. Groups (n = 5) were injected i.v. day -5, day 0 and at day 3 either with Scrambled siRNA, C5siRNA C5aR siRNA, or the combination of C5 and C5aR siRNAs. B) CDA, C) Prevalence, D) mice weights. All data represent the mean CDA ± SEM *p < 0.05 in comparison to scrambled siRNA injected mice
In vivo effect of C5 and C5aR1 siRNAs on CAIA
To examine the effect of C5 or C5aR1 siRNA on CAIA another CAIA experiment was performed. In this study commercially available C5, C5aR1, or scrambled siRNAs were used (Fig 2B-D). Mice were injected i.v. on days -5, day 0 and at day 3 with 2 μg of either C5 siRNA, C5aR1 siRNA, or combined (4 μg total) C5/C5aR1 siRNAs. We found that the combined use of C5/C5aR1 siRNAs vs. single C5 and C5aR1 siRNAs significantly (P < 0.05) protected mice from CAIA (Fig 2B). The CDA in WT mice treated with Scrambled siRNA, C5 siRNA, C5aR1 siRNA and combined C5/C5aR1 siRNAs was 8.50 ± 1.5, 7.4 ± 1.9, 6.6 ± 1.69 and 3.60 ± 0.40 respectively (Fig 2B). The prevalence, at day 10, in all treatment groups was 100% in all groups (Fig 2C). These CDA data validate the concept that targeting both C5 and its receptor, C5aR1 is more efficacious that targeting either singly.
Efficacy of siRNAs was tested using liver tissue from these mice upon completion of the experiment on day 10. RNA was prepared as described in Materials and Methods. C5 and C5aR1 mRNA levels were then determined by qRT-PCR using Taqman probes and absolute amounts calculated from standard curves as described above. Repeat dosing of 2 μg of C5 siRNA resulted in a final reduction of C5 mRNA by 45% (Fig 3A) with no effect on C5aR1 mRNA (Fig 3B). Conversely, repeat dosing of 2 μg of C5aR1 siRNA had no effect on C5 mRNA while reducing C5aR1 mRNA by 46.8%. Combinations of siRNAs showed similar results. These data suggest that even relatively large doses of siRNAs are only capable of reducing endogenous mRNA levels by 50% in this model.
Figure 3.
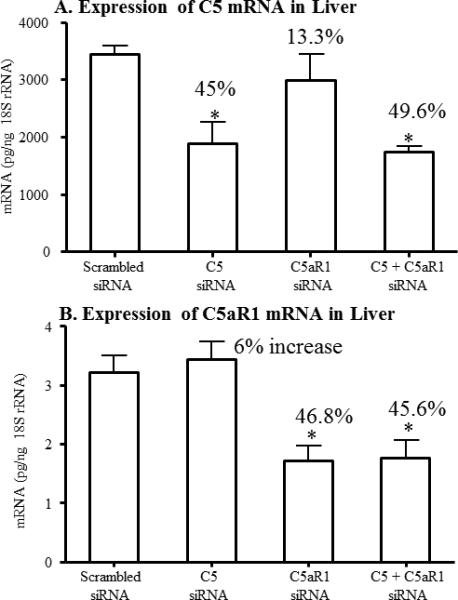
Systemic effect of specific C5 or C5aR1 siRNAs injected in vivo in mice with CAIA. The mRNA expression levels of the C5 or C5aR1, at day 10, from the liver of mice with CAIA injected with scrambled siRNAs or C5 or C5aR1 or C5 + C5aR1 siRNAs were determined using qRT PCR. All mice were injected three times with respective siRNAs i.e. at day -3, day 0 and at day 3. A. C5 mRNA levels from the liver of CAIA mice.
B. C5aR1 mRNA levels from the liver of CAIA mice. A pool of four siRNAs of each were used for these in vivo CAIA studies. All mRNA data were normalized with 18S rRNA measured in parallel from each sample. Data were expressed as mean ± SE based on n = 5 for scramble siRNA, n = 5 for C5 siRNA, n = 5 for C5aR1 siRNA and n = 5 for C5 siRNA/C5aR1 siRNA. *p < 0.05 in comparison to the scrambled siRNA treated mice.
Efficiency of conjugation of protamine with anti-C5aR1 antibody and loading of C5siRNA
The efficiency of the protamine conjugation to anti-C5aR1 antibody was determined by SDS-PAGE as described in the Materials and Methods. Both heavy and light chains were conjugated as evidenced by the upward shift in the size of these two bands (Fig 4 lane 3). Overall the conjugation efficiency of protamine to the anti-C5aR1 antibody was 35%. Unconjugated anti-C5aR1 was removed using gel filtration chromatography as described in Materials and Methods. C5siRNAs were then incubated with the conjugated anti-C5aR1 antibody for 30 min at 4°C as described in Materials and Methods and this mixture used directly for all in vitro and in vivo studies described below. Unconjugated C5siRNAs were not separated from the conjugated C5siRNA to the anti-C5aR1 ab conjugate because C5siRNA not bound to the conjugated antibody can also inhibit the C5mRNA in cells not expressing C5aR1on their surface. These data show a reasonable binding efficiency of anti-C5aR ab (20/70) to the protamine without altering the binding affinity of the antibody to its receptors.
Figure 4.

Conjugation of anti-C5aR1 ab (20/70) with protamine and C5 siRNA. Protamine functions as the C5 siRNA carrier to the target cells. Protamine-anti-C5aR mAb complexes were made by using a T3-Max® Conjugation kit from BIOO Scientific as described in Materials and Methods. Later on C5 siRNA was loaded to this complex by incubating with C5 siRNA at 4°C for 30 min. The molecular weight of the protamine-conjugated anti-C5aR mAb was increased slightly as expected. Lane 1 = Protein molecular weight marker, Lane 2 = unconjugated anti-C5aR1 mAb, Lane 3 = Conjugated anti-C5aR1 mAb, HC = heavy chain, and LC = light chain
Effect of protamine conjugation to anti-C5aR antibody on the binding affinity to C5aR1
Lysine conjugation can markedly affect the binding affinity of an antibody. We thus performed Flow Cytometry to compare the binding of our Anti-C5aR1 ab – protamine – C5siRNA conjugate to unmodified antibody. The RAW macrophage line robustly expresses C5aR1 and produces a strong fluorescence signal as compared to isotype control when unmodified antibody is used (Fig 5A). The siRNA loaded conjugated complex generates a virtually identical signal (Fig 5B). To further characterize the binding properties of conjugated anti-C5aR1 antibody, we generated full binding curves for both conjugated and unconjugated antibodies. RAW cells were incubated with varying amounts of either unconjugated or conjugated anti-C5aR1 antibody and binding was determined by FACS. Binding curves were virtually identical with a slight (not statistically significant, p > 0.05) increase for the binding of conjugated antibody (Fig S1). These data confirm that conjugation has no effect on binding affinity for this antibody.
Figure 5.
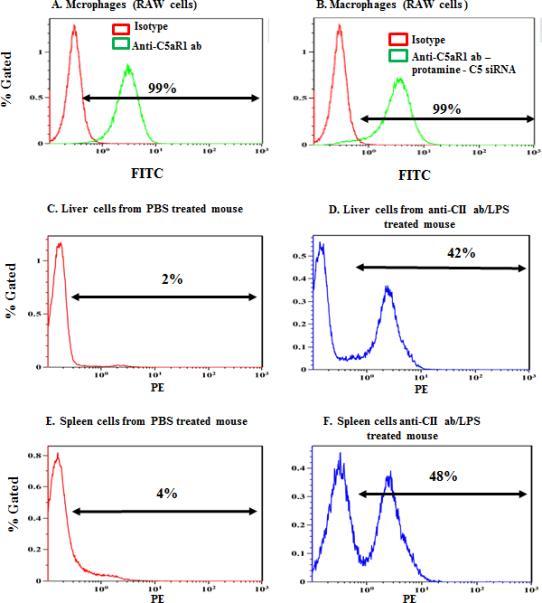
Flow cytometric analysis for C5aR1 on the surface of macrophages, liver and spleen cells. There was an increase in the surface expression of C5aR1 under inflammatory conditions. A - E Flow cytometry analysis was done using a high C5aR1 expressing macrophage cell line, RAW 264.7. (A) C5aR expressing RAW 264.7 cells were incubated with either unloaded (B) or siRNA loaded complexes followed by FITC conjugated secondary antibody as described. Binding was compared to an isotype control (shown by red line graph). Liver and spleen cells from either PBS treated mice (C, E) or anti-CII and LPS treated mice (D, F) were incubated with anti-C5aR1-PE conjugated antibody. Gates were determined using isotype controls (not shown). These experiments were repeated three times, and one representative data set is shown.
C5aR1 expression levels in vital organs increases under inflammatory conditions
To better understand the distribution of C5aR1 expression we created a state of systemic inflammation such as that seen in our arthritis model by injection of anti-collagen antibody and LPS as described in Materials and Methods. C5aR1 expression is low in liver and spleen cells derived from untreated mice (Fig 5 C and E) but is induced within 24 hours upon LPS and anti-collagen antibody treatment (Fig 5 D and F). These FACS data show that there is an increase in the expression of C5aR1 in vivo during inflammatory conditions vs. steady state.
Effect of the anti-C5aR ab – protamine-C5siRNA conjugate on C5 mRNA in a macrophage cell line
We next validated the ability of our complex to deliver siRNA to a C5aR1 expressing cell. For this experiment RAW cells were treated with C5 siRNA bound to the anti-C5aR1- protamine conjugate. Negative controls included cells treated with unconjugated anti-C5aR1 ab plus scrambled siRNA and untreated cells. The expression levels of C5 mRNA and C5aR1 mRNA were examined at 72 hrs. C5 mRNA was down regulated to 66% in RAW macropahges (Fig S2A) without affecting C5aR1 mRNA levels (Fig S2D) as measured by qRT-PCR. The effect of this conjugate on the C5 mRNA levels was also visually confirmed by running a 2% agarose gel in a semi quantitative assay (Fig S2 B) with no off target effect on the GAPDH mRNA (Fig S2C). These data confirm that our conjugate delivers functional siRNA to C5aR1 expressing cells and does not affect the expression of the targeting gene (C5aR1).
Effect of anti-C5aR-protaimine-C5siRNA conjugate on CAIA
We then assessed the efficacy of this conjugate in vivo, again using the CAIA model. Each mouse was injected i.p. three times i.e. on days -5, day 0 and on day 3 with either Scrambled siRNA, a mixture of anti-C5aR1 ab plus C5siRNA, or the conjugate of anti-C5aR1 - protamine - C5siRNA (conjugate). As before, we measured CDA daily until day 10. At day 10 the CDAs were 2.0 ± 0.966, 9.4 ±1.60 and 11.6 ± 0.244 for conjugate, unconjugated mix, and scrambled siRNA respectively (Fig 6A). Disease prevalence was also measured daily and is shown in Figure 6B. Interestingly, 25% of the conjugate group did not show clinical evidence of disease. Thus the conjugate of anti-C5aR1 ab - protamine - C5 siRNA significantly (p < 0.05) reduced the CDA by 83% when compared to Scrambled siRNA and 79% when compared to the unconjugated mix consisting of exactly the same dose of antibody plus siRNA as in the conjugate. All mice weighed before, during and after the induction of CAIA and there was no change in weight in mice treated with the conjugate of anti-C5aR1 mAb - no protamine - C5 siRNA (data not shown). These data show that delivering C5 siRNA systemically through specific targeting inflammatory cells using C5aR1 receptors is superior to existing anti-C5 antibodies or using C5 siRNAs or C5aR1 siRNAs individually.
Figure 6.
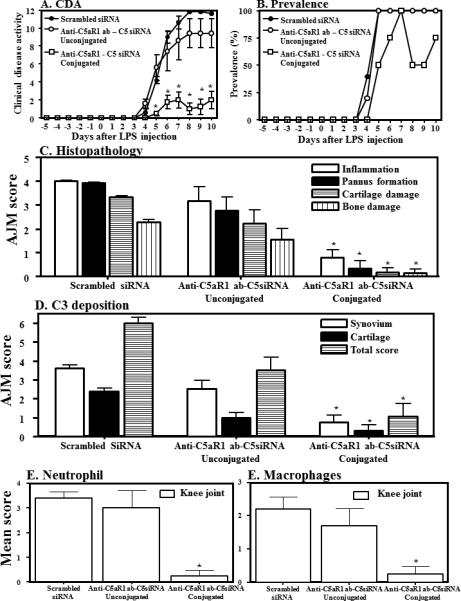
Effect of an anti-C5aR1mAb–protamine-C5siRNA complex on CAIA. WT mice with CAIA were treated with either Scrambled siRNAs, the anti-C5aR1ab-protamine-C5siRNA conjugate or with the unconjugated components (150 μg anti-C5aR1 mAb-8 μg C5 siRNA/mouse/i.p.). A) CDA. B) Disease prevalence. C) Histopathology measured in all joints (AJM) Histopathology for inflammation, pannus formation, cartilage damage and bone damage, D) AJM of C3 deposition from all joints in the synovium, on the surface of cartilage and total scores (synovium plus cartilage), E), knee joint monocyte/macrophage infiltration, and F) knee joint neutrophil infiltration. C-F measured on day 10. Mean score of macrophages and neutrophils was only from the knee joints of mice in all treatment groups at day. All data represent the mean ± SEM based on n = 5 for all groups. *p < 0.05 in comparison to the Scrambled siRNAs or anti-C5aR1 ab-protamine-no C5 siRNA
Effect of anti-C5aR1 mAb - protamine - C5 siRNA conjugate on the histopathology and C3 deposition scores in mice with CAIA
All mice were sacrificed at day 10 and joints from this study were processed for histopathology and immunohistochemical analysis. Both forelimbs and the right hind limb (five joints) were processed for histopathology and for the measurement of local C3 deposition (Fig 6C & D). Five joints from Scrambled siRNA or anti-C5aR ab-protamine-C5siRNA or unconjugated anti-C5aR plus C5siRNA treated mice were examined for inflammation, pannus formation, cartilage damage and bone damage (Fig. 6C). The mean all joint histopathology scores for all 3 groups were PBS (4.02 ± 0.02), anti-C5aR1ab-no protamine-C5siRNA (3.18 ± 0.58) and anti-C5aR1mAb-protamine-C5siRNA (0.8 ± 0.3). Individual scores for inflammation, pannus formation, cartilage and bone damage were significantly (P < 0.05) decreased in mice treated with the conjugate of anti-C5aR ab-protamine-C5siRNA compared with mice treated with Scrambled siRNA or unconjugated anti-C5aR plus C5siRNA. Overall, all joint mean (AJM) scores for histopathology were significantly (p <0.001) reduced by 85% in mice treated with conjugated anti-C5aR ab-protamine-C5siRNA as compared with Scrambled siRNA or unconjugated anti-C5aR plus C5siRNA. Representative histopathology pictures of the knee joints these mice are shown in Fig S3 (A, B and C).
C3 scores for the 3 groups were 5.98 ± 0.31, 3.5 ± 0.7, and 1.05 ± 0.68 respectively (Fig 6D). This C3 deposition in the synovium and on the cartilage surface was also significantly (P <0.05) reduced in mice treated with conjugated anti-C5aR- protamine- C5siRNA as compared with mice treated with unconjugated anti-C5aR ab plus C5siRNA or scrambled siRNA (Fig. 5D). Overall, all joint mean scores (AJM) (synovium and cartilage) for C3 deposition were reduced by 82% and 70% mice in mice treated with conjugated anti-C5aR-protamine-C5siRNA as compared to Scrambled siRNA or unconjugated anti-C5aR1 plus C5siRNA respectively. Individually C3 deposition in the synovium of mice treated with the conjugate of anti-C5aR abprotamine - C5siRNA as compared with mice treated with unconjugated anti-C5aR ab plus C5siRNA or Scrambled siRNA was decreased by 70% and 79%, respectively (Fig. 6D). Representative C3 deposition from the knee joints from mice treated with PBS or anti-C5aR-no protamine-no C5siRNA or anti-C5aR ab-protamine-C5siRNA are shown in Fig S3 (D, E and F).
Determination of macrophage and neutrophil infiltration in the synovium of knee joints from CAIA mice treated with anti-C5aR ab-protamine-C5siRNA conjugate
The infiltration of neutrophils and macrophages in knee joint synovium from mice treated with Scrambled siRNA, with unconjugated anti-C5aR ab plus C5 siRNA, or with conjugated anti-C5aR- protamine- C5siRNA was determined immunohistochemically using specific cell surface markers according to our previously published studies (3) (Fig 5E & F). The percent of neutrophils and macrophages was decreased significantly (P <0.05) in the synovium of CAIA mice treated with a conjugate of conjugated anti-C5aR- protamine- C5siRNA in comparison with the mice treated with Scrambled siRNA or with unconjugated anti-C5aR ab plus C5 siRNA (Fig. 5E & F). Neutrophil counts for the three groups were 3.4 ± .24, 3.0 ± 0.7, and 0.8 ± 0.58 respectively. The decrease in the percentages of synovial neutrophils was 88% (p < 0.005) and 85% (p < 0.001), respectively in CAIA mice treated with conjugated anti-C5aR- protamine- C5siRNA treated mice as compared with Scrambled siRNA or with unconjugated anti-C5aR ab plus C5 siRNA (Fig 5E). Macrophages showed a similar trend with counts of 2.20 ± 0.37, 1.40 ± 0.5, and 0.25 ± 0.22 respectively for the 3 groups (Fig. 5F). The decrease in synovial macrophages in conjugated anti-C5aR- protamine- C5siRNA treated mice was 82% (p < 0.005) and 66% (p < 0.001), respectively as compared with Scrambled siRNA or with unconjugated anti-C5aR ab plus C5 siRNA treated mice with CAIA (Fig 5F). Representative pictures of macrophage and neutrophil IHS from the knee joints of mice treated with PBS or anti-C5aR-no protamine-no C5siRNA or anti-C5aR ab-protamine-C5siRNA are shown in Fig S3 (D, E and F) and Fig S3 (G, H and I) and Fig S3 (J, K and L) respectively.
Quantitative RT-PCR cytokine mRNA analysis from the knee of mice with CAIA treated with anti-C5aR ab-protamine-C5siRNA conjugate
Cytokine mRNA levels for TNF-α, IL-1β and MMP3 were determined by qRT-PCR using cDNA prepared from the mRNA from the knee joint of the left hind limb of mice treated with PBS or anti-C5aR- protamine- C5siRNA. No significant differences were seen in the levels of mRNA for TNF-α in the knee joint of CAIA mice either treated with Scrambled siRNA or with conjugated anti-C5aR- protamine- C5siRNA mice with disease (data not shown). However, a significant decrease of 76% (p < 0.033) and 78% (p < 0.04) in the mRNA levels for IL-1β and MMP3 respectively were seen in knee joints from mice treated with conjugated anti-C5aR- protamine- C5siRNA compared with mice treated with Scrambled siRNA. The absolute levels of IL-1β in the knee joint of mice treated with Scrambled siRNA or conjugated anti-C5aR- protamine-C5siRNA were 168.5 ± 40.0 and 36.7 ± 23.7 respectively. The absolute levels of MMP3 in the knee joint of mice treated with Scrambled siRNA or conjugated anti-C5aR- protamine- C5siRNA were 322.6 ± 88.8 and 78.9 ± 6.7 respectively. A baseline level of mRNA encoding IL-1β and MMP3 were seen in the knees of heathy untreated mice (data not shown). These cytokine mRNA data show that the conjugate of anti-C5aR ab-protamine-C5siRNA affected the pro-inflammatory cytokines locally in the knee joints of mice with disease.
Discussion
Previously, we found that genetic disruption of C5aR1 resulted in a virtual block of disease progression in the CAIA model with 50% of mice showing no evidence of disease and the remaining mice developing disease that was barely detectable (3). Here we focused on the possibility of translating this observation into a useful therapeutic. Before focusing on C5aR1, however; we addressed the new observation that C5L2 is a second C5a receptor (C5aR2) (38). We found no differences in the CDA between C5aR2−/− mice and WT mice in the CAIA model further demonstrating that the effects of C5a are mediated through C5aR1; consistent with our previous study (3).
These studies led us to hypothesize that a reason for the incomplete inhibition of disease progression when using antibodies to target either C5 or C5aR1 is that a small amount of signaling is sufficient to drive disease. We tested our hypothesis by considering that C5aR1 signal transduction is a function of both the concentration of ligand (C5a) and the availability of receptor (C5aR1). Indeed, using siRNAs targeted to these two mRNAs, we found that dual targeting was synergistic as compared to targeting ligand or receptor individually. The efficacy of combined siRNAs was somewhat similar to that seen with BB5.1. We cannot discount the possibility that modified siRNAs might perform with a higher efficacy. Furthermore, we cannot ensure that both antibody and siRNA dosages were at similar points in their respective dose response curves. Nevertheless, these data do support the concept that the simultaneous inhibition of both C5 and inhibition of C5a binding to C5aR1 improves efficacy, presumably through a better inhibition of C5aR1 signaling.
By blocking both C5 mRNA and binding of C5a to C5aR1, we expected to synergistically decrease the possibility of activation C5-C5aR1 axis. C5aR1(20/70) has been shown to block the binding of rC5a to C5aR1 making this a reasonable choice for our antibody (31). Concerning the use of other anti C5aR1 antibodies, we hypothesize that C5siRNA conjugated to any other C5aR1 antibody, which can bind and block the binding of rC5a to the C5aR1, will show the similar effect. Theoretically C5siRNA bound to asialo-glycoprotein receptor (ASGR) antibody should have the same effect on hepatocytes. Similarly a newly developed anti-C5aR1 inhibitory antibody by Novo Nordisk Park can be conjugated (39). Most biological activities of C5a are mediated through C5aR1 (40) and it is widely expressed in inflammatory cells and the agents that act on C5aR hold great potential as therapeutics (41).
We next considered the possibility of combining antibody mediated targeting with siRNAs. This mode of inhibition would be especially useful if C5a was being used in an autocrine fashion to drive activation. Several groups have described the use of antibodies as delivery vehicles to target siRNAs, sharing the concept of using positively charged protamine attached to the antibody to bind negatively charged siRNAs (25, 27). To our knowledge, this technology has not yet been applied as a treatment for a disease state in vivo. We chose to replicate aspects of this technology using a bivalent C5aR1 monoclonal antibody (clone 20/70) shown by several groups to block C5aR1 signal transduction (28-32). We made use of amine conjugation technology to attach protamine to the antibody. Free amine groups on lysine side chains which are accessible become conjugated. If, the complementarity determining regions (CDR) contains lysine residues this can dramatically alter the binding properties of the antibody necessitating the control experiments performed in Figures 5 and S1.
We next considered the sources of C5a. While macrophage and neutrophil within the arthritic joint are highly likely to be local sources of C5a the liver and spleen are well known systemic sources. Given that we are targeting cells expressing C5aR1, we wanted to determine the level of C5aR1 expression in these tissues. Liver and spleen cells from healthy animals expressed low levels of C5aR1 on their surface. However under inflammatory conditions such as arthritis there was a marked up-regulation of C5aR1 in crude liver and spleen preparations. This would suggest that a state of systemic inflammation is sufficient to induce C5aR1 expression in liver, in turn, indicating that our anti-C5aR1ab-protamine-C5siRNA conjugate would be targeted to liver as well as arthritic joints at very early stage of the disease initiation. A model of how the conjugate affects cells systemically and within the arthritic joint is shown (Fig 7). We have not examined the surface expression of C5aR1 in the knee joints of arthritic mice due to the non-availability of suitable anti-C5aR1 antibody for immunohistochemical staining.
Figure 7.
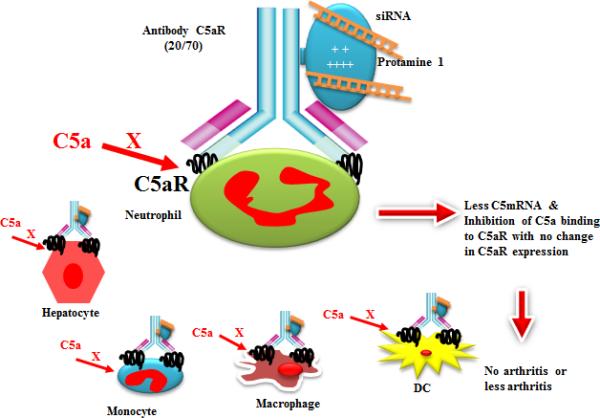
A model hypothesis showing the conjugation of anti-C5aR1 (20/70) mAb with protamine followed by conjugation with C5siRNAs. The conjugated complex has been designated as anti-C5aR1ab-protamine-C5siRNA and the unconjugated complex has been designated as anti-C5aR-no protamine -C5siRNA. A putative binding of conjugated complex to hepatocytes, neutrophils, monocytes, macrophages and dendritic cells has been shown followed by its effect on the disease phenotype.
Arthritis is a heterogeneous disease and its origin is considered to be systemic but the outcome is local inflammation in a subset joints. We think the therapeutic effect of anti-C5aR1 ab-protamine-C5siRNA is systemic because it might have inhibited C5mRNA systemically on all C5aR1 expressing cells present in the liver, spleen and macrophages, neutrophils present locally in the knee joints. However, low C3 deposition and the presence of low levels of C5mRNA in the knee joints of mice treated with this conjugate indicate that the effects of the conjugate were local because infiltration of macrophage and neutrophil was also decreased significantly. This can only be possible with low generation of C5a systemically and locally.
We interpret our results as consistent with the hypothesis that there exists an autocrine or paracrine loop involving C5aR1 signaling and C5 production within the affected joint. Other interpretations are possible however and should be considered. For example, it is possible that antibody mediated delivery of the C5a siRNA is the critical therapeutic process and the actual choice of antibody is less important. It may be the case that any antibody capable of interacting with Fc receptors (expressed on follicular dendritic cells, macrophages, neutrophils and mast cells) will suffice. Alternatively, the targeting of some macrophage receptor other than C5aR1 may suffice. In FACS experiments we find that the inclusion of Fc block has no effect on conjugated anti C5aR1 binding. Given this, it is likely that the majority of interactions of the conjugated antibody in vivo will be with C5aR1 receptors. Since, as shown in Fig 6A, the unconjugated mixture of anti C5aR1 antibody and siRNA is far less effective than the conjugated complex, it is reasonable to assume that the siRNA is being targeted primarily to C5aR1 expressing cells with only a minor component being targeted to Fc receptors. We have not addressed the degree to which our conjugate is interacting with Fc receptors in this study. Future experiments will directly test these questions to establish the degree of flexibility that this therapeutic strategy may entertain.
In summary, we have shown that C5aR2 does not seem to play a role in arthritis disease progression while C5aR1 and C5 appear to be central to disease development. Targeting both C5 ligand and C5aR1 simultaneously is clearly an improvement over targeting either component separately which may have led to inhibition of therapeutic use for the treatment of RA. This suggests that only small amounts of C5aR1 signaling were required to drive disease. As C5aR1 activation would be proportional to the product of the concentration of ligand times the concentration of receptor, it is perhaps not surprising that a combination approach would be more effective. What is surprising is that conjugation would play such a critical role in efficacy for the treatment of arthritis. Future work will determine if this vastly improved efficacy is due to increased siRNA delivery via antibody uptake, the targeting of siRNAs to locally C5aR1 expressing cells (as opposed to non-specific uptake via Fc receptors), or the combined block of C5aR1 signaling and C5a production in the same cell populations. C5siRNA might get into C5aR1 positive cells through C5aR1 receptor-mediated endocytosis but the exact mechanism(s) is unknown. Given that the use of antibody based therapeutics in the clinic has been refined over several decades we envision that this strategy shows promise as a potential new therapeutic entity for the treatment of Rheumatoid Arthritis.
Supplementary Material
Acknowledgements
The authors thank Ms. Umarani Pugazhenthi, University of Colorado Anschutz Medical Campus PCR core for performing qRT-PCR from the cell lines and knee joints of mice with CAIA. The authors also thank Karen Helm flow cytometry core University of Colorado Anschutz Medical Campus flow cytometry core for performing flow cytometry to examine the expression of C5aR on various cells. Protamine was conjugated to anti-C5aR (20/70) MAb by BIOO Scientific and we are thankful to Drs. Lance P. Ford, Suresh Subramanya, BIOO Scientific for providing scientific and technical expertise related to the conjugation of siRNA to anti-C5aR MAbprotamine conjugated molecules. We thank Drs. Craig Gerard and Lu Bao, Boston's Children Hospital, Boston MA for providing C5aR2−/− (formerly named and obtained as C5L2−/−) mice on C57BL/6 background for these studies. Note that we have used a new nomenclature of C5aR2 in lieu of C5L2 designated/and or suggested by the 2014 Complement Nomenclature Committee (CNC) Board, the International Complement Society (ICS) and the European Complement Network (ECN) Board.
This work was supported by NIH grants 2R01AR51749 to PI, VMH and COI, NKB
Abbreviations used in this paper
- AJM
all joint mean
- AP
alternative pathway
- CAIA
collagen antibody-induced arthritis
- CIA
collagen-induced arthritis
- CP
classical pathway
- LP
lectin pathway
- TP
Terminal pathway
- CII
type II collagen
- CDA
clinical disease activity
- MBL
Mannose-binding lectin
- WT
wild type
- siRNAs
C5, Complement 5
- C5aR1 or CD88
Receptor for complement anaphylatoxin C5a
- C5aR2 or C5L2 (C5a receptor - like 2) or GPR77
an orphan or decoy receptor for complement anaphylatoxin C5a; Small interfering RNAs
- Anti-C5aR1mAb – no protamine-C5 siRNA
Unconjugated complex or Unconjugated
- Anti-C5aR1 mAb – protamine-C5 siRNA
Conjugated complex or conjugated
Footnotes
Disclosure
All other authors have no conflict of interest.
References
- 1.Ballanti E, Perricone C, Greco E, Ballanti M, Di Muzio G, Chimenti MS, Perricone R. Complement and autoimmunity. Immunol Res. 2013;56:477–491. doi: 10.1007/s12026-013-8422-y. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz S, Wetsel R, Arend WP, Holers VM. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol. 2012;188:1469–1478. doi: 10.4049/jimmunol.1102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, Whitfeld PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol Cell Biol. 2008;86:153–160. doi: 10.1038/sj.icb.7100166. [DOI] [PubMed] [Google Scholar]
- 5.Schieferdecker HL, Schlaf G, Jungermann K, Gotze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol. 2001;1:469–481. doi: 10.1016/s1567-5769(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013;27:855–864. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 7.Kemper C, Pangburn MK, Fishelson Z. Complement Nomenclature 2014. Molecular Immunology. 2014;61:56–58. doi: 10.1016/j.molimm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci U S A. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandakumar KS, Jansson A, Xu B, Rydell N, Ahooghalandari P, Hellman L, Blom AM, Holmdahl R. A recombinant vaccine effectively induces c5a-specific neutralizing antibodies and prevents arthritis. Plos One. 2010;5:e13511. doi: 10.1371/journal.pone.0013511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson M, Goldschmidt TJ, Michaelsson E, Larsson A, Holmdahl R. T-cell receptor V beta haplotype and complement component C5 play no significant role for the resistance to collagen-induced arthritis in the SWR mouse. Immunology. 1991;73:191–196. [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee S, Anderson GD, Luthra HS, David CS. Influence of complement C5 and V beta T cell receptor mutations on susceptibility to collagen-induced arthritis in mice. J Immunol. 1989;142:2237–2243. [PubMed] [Google Scholar]
- 12.Spinella DG, Jeffers JR, Reife RA, Stuart JM. The role of C5 and T-cell receptor Vb genes in susceptibility to collagen-induced arthritis. Immunogenetics. 1991;34:23–27. doi: 10.1007/BF00212308. [DOI] [PubMed] [Google Scholar]
- 13.Macor P, Durigutto P, De Maso L, Garrovo C, Biffi S, Cortini A, Fischetti F, Sblattero D, Pitzalis C, Marzari R, Tedesco F. Treatment of experimental arthritis by targeting synovial endothelium with a neutralizing recombinant antibody to C5. Arthritis Rheum. 2012;64:2559–2567. doi: 10.1002/art.34430. [DOI] [PubMed] [Google Scholar]
- 14.Banda NK, Kraus D, Vondracek A, Huynh LH, Bendele A, Holers VM, Arend WP. Mechanisms of effects of complement inhibition in murine collagen-induced arthritis. Arthritis Rheum. 2002;46:3065–3075. doi: 10.1002/art.10591. [DOI] [PubMed] [Google Scholar]
- 15.Banda NK, Levitt B, Wood AK, Takahashi K, Stahl GL, Holers VM, Arend WP. Complement activation pathways in murine immune complex-induced arthritis and in C3a and C5a generation in vitro. Clin Exp Immunol. 2010;159:100–108. doi: 10.1111/j.1365-2249.2009.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, Judex M, Kullmann F, Mountz JD, Scholmerich J, Gay S, Muller-Ladner U. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–945. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 17.Onuma H, Masuko-Hongo K, Yuan G, Sakata M, Nakamura H, Kato T, Aoki H, Nishioka K. Expression of the anaphylatoxin receptor C5aR (CD88) by human articular chondrocytes. Rheumatol Int. 2002;22:52–55. doi: 10.1007/s00296-002-0199-6. [DOI] [PubMed] [Google Scholar]
- 18.Yuan G, Wei J, Zhou J, Hu H, Tang Z, Zhang G. Expression of C5aR (CD88) of synoviocytes isolated from patients with rheumatoid arthritis and osteoarthritis. Chin Med J (Engl) 2003;116:1408–1412. [PubMed] [Google Scholar]
- 19.Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, Mojcik CF, Rother RP. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:2559–2565. doi: 10.1182/blood-2005-02-0564. [DOI] [PubMed] [Google Scholar]
- 20.Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, Sanders ME, Reedquist KA, Tak PP. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 2007;46:1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]
- 21.Apparailly F, Jorgensen C. siRNA-based therapeutic approaches for rheumatic diseases. Nature reviews. Rheumatology. 2013;9:56–62. doi: 10.1038/nrrheum.2012.176. [DOI] [PubMed] [Google Scholar]
- 22.Resnier P, Montier T, Mathieu V, Benoit JP, Passirani C. A review of the current status of siRNA nanomedicines in the treatment of cancer. Biomaterials. 2013;34:6429–6443. doi: 10.1016/j.biomaterials.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Rossi JJ. Current progress in the development of RNAi-based therapeutics for HIV-1. Gene therapy. 2011;18:1134–1138. doi: 10.1038/gt.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheinman RI, Trivedi R, Vermillion S, Kompella UB. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine (Lond) 2011;6:1669–1682. doi: 10.2217/nnm.11.90. [DOI] [PubMed] [Google Scholar]
- 25.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 26.Yao YD, Sun TM, Huang SY, Dou S, Lin L, Chen JN, Ruan JB, Mao CQ, Yu FY, Zeng MS, Zang JY, Liu Q, Su FX, Zhang P, Lieberman J, Wang J, Song E. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci Transl Med. 2012;4:130ra148. doi: 10.1126/scitranslmed.3003601. [DOI] [PubMed] [Google Scholar]
- 27.Hauser PV, Pippin JW, Kaiser C, Krofft RD, Brinkkoetter PT, Hudkins KL, Kerjaschki D, Reiser J, Alpers CE, Shankland SJ. Novel siRNA delivery system to target podocytes in vivo. Plos One. 2010;5:e9463. doi: 10.1371/journal.pone.0009463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godau J, Heller T, Hawlisch H, Trappe M, Howells E, Best J, Zwirner J, Verbeek JS, Hogarth PM, Gerard C, Van Rooijen N, Klos A, Gessner JE, Kohl J. C5a initiates the inflammatory cascade in immune complex peritonitis. Journal of Immunology. 2004;173:3437–3445. doi: 10.4049/jimmunol.173.5.3437. [DOI] [PubMed] [Google Scholar]
- 29.Shagdarsuren E, Bidzhekov K, Mause SF, Simsekyilmaz S, Polakowski T, Hawlisch H, Gessner JE, Zernecke A, Weber C. C5a receptor targeting in neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. 2010;122:1026–1036. doi: 10.1161/CIRCULATIONAHA.110.954370. [DOI] [PubMed] [Google Scholar]
- 30.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, Gessner JE. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. The Journal of clinical investigation. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunology letters. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Lu B, Gerard C, Gerard NP. Disruption of the complement anaphylatoxin receptor C5L2 exacerbates inflammation in allergic contact dermatitis. Journal of Immunology. 2013;191:4001–4009. doi: 10.4049/jimmunol.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banda NK, Mehta G, Kjaer TR, Takahashi M, Schaack J, Morrison TE, Thiel S, Arend WP, Holers VM. Essential Role for the Lectin Pathway in Collagen Antibody-Induced Arthritis Revealed through Use of Adenovirus Programming Complement Inhibitor MAp44 Expression. Journal of Immunology. 2014;193:2455–2468. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Moore A, Allden S, Bourne T, Denis MC, Kranidioti K, Okoye R, Sotsios Y, Stencel Z, Vugler A, Watt G, Shaw S. Collagen II antibody-induced arthritis in Tg1278TNFko mice: optimization of a novel model to assess treatments targeting human TNFa in rheumatoid arthritis. J Transl Med. 2014;12:285. doi: 10.1186/s12967-014-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, Holers VM. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177:1904–1912. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 37.Banda NK, Mehta G, Kjaer TR, Takahashi M, Schaack J, Morrison TE, Thiel S, Arend WP, Holers VM. Essential Role for the Lectin Pathway in Collagen Antibody-Induced Arthritis Revealed through Use of Adenovirus Programming Complement Inhibitor MAp44 Expression. Journal of Immunology. 2014 doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Molecular Immunology. 2000;37:407–412. doi: 10.1016/s0161-5890(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 39.Andersson C, Wenander CS, Usher PA, Hebsgaard JB, Sondergaard BC, Rono B, Mackay C, Friedrichsen B, Chang C, Tang R, Hornum L. Rapid-onset clinical and mechanistic effects of anti-C5aR treatment in the mouse collagen-induced arthritis model. Clinical and experimental immunology. 2014;177:219–233. doi: 10.1111/cei.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allegretti M, Moriconi A, Beccari AR, Di Bitondo R, Bizzarri C, Bertini R, Colotta F. Targeting C5a: recent advances in drug discovery. Current medicinal chemistry. 2005;12:217–236. doi: 10.2174/0929867053363379. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforovich GV, Marshall GR, Baranski TJ. Modeling molecular mechanisms of binding of the anaphylatoxin C5a to the C5a receptor. Biochemistry. 2008;47:3117–3130. doi: 10.1021/bi702321a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


