Abstract
SLC7A11 encodes a subunit of the xCT cystine/glutamate amino acid transport system and plays a critical role in the generation of glutathione and the protection of cells from oxidative stress. Expression of SLC7A11 promotes tumorigenesis and chemotherapy resistance, but while SLC7A11 has been previously noted to be upregulated in hypoxic cells its regulation has not been fully delineated. We have recently shown that nonsense mediated RNA decay (NMD) is inhibited by cellular stresses generated by the tumor microenvironment, including hypoxia, and augments tumorigenesis. Here we demonstrate that the inhibition of NMD by various cellular stresses leads to the stabilization and upregulation of SLC7A11 mRNA and protein. The inhibition of NMD and upregulation of SLC7A11 augments intracellular cystine transport, and increases intracellular levels of cysteine and glutathione. Accordinglyy, the inhibition of NMD protects cells against oxidative stress via SLC7A11 upregulation. Together our studies identify a mechanism for the dynamic regulation of SLC7A11, through the stress-inhibited regulation of NMD, and add to the growing evidence that the inhibition of NMD is an adaptive response.
Keywords: mRNA decay, oxidative stress, transport amino acids, ER stress, gene regulation
Introduction
During tumor formation the neoplastic cell is exposed to a variety of cellular stresses including low oxygen, amino acid deprivation, and increased levels of reactive oxygen species (ROS). In addition, mitochondrial mutations as frequently found in several cancers, and several oncogenes including Myc and Ras can up-regulate ROS (1–3). Tumor cells adapt to these stresses by altering gene expression (reviewed in (4)). For example, low oxygen leads to the activation of the transcription factor HIF-1, which blunts ROS (5–7). Multiple diverse stresses also lead to phosphorylation of the eukaryotic initiation factor 2α (eIF2α), which up-regulates the transcription factor ATF-4 and upregulates genes critical for autophagy, amino acid transport, and the anti-oxidant response (8–11). Through these adaptive responses the neoplastic cell is able to grow as a three-dimensional tumor (12, 13). Understanding the molecular mechanisms underlying the adaptive response to ROS and other stresses will provide approaches to disrupt these mechanisms and form the basis of future cancer therapies.
We and others have noted that multiple cellular stresses, including amino acid deprivation, hypoxia, endoplasmic reticulum (ER) stress, and high expression of Myc leads to the inhibition of nonsense mediated RNA decay (NMD) in a mechanism dependent on the phosphorylation of eIF2α (4, 14–17). While NMD was originally thought to be responsible for the rapid degradation of mutated mRNAs, it is now appreciated that NMD also degrades select non-mutated mRNAs, including multiple mRNAs which encode proteins that play roles in cellular stress responses. For example, we have reported that the ATF-4 mRNA is a direct NMD target, and the inhibition of NMD augments the ER response pathway, the survival of cells in response to ER stress, and is necessary for the three dimensional growth of prostate cancer cells in soft agar and as explants in mice (4, 16). Thus the inhibition of NMD serves as an adaptive response in tumors. However, the mechanism by which NMD does this, other than augmenting the ER stress response, is not known.
Many of the mRNAs upregulated when NMD is disabled through gene depletion encode amino acid transporters, and the suppression of NMD increases the intracellular concentrations of many amino acids (14, 17). The regulation of amino acid metabolism plays an important role in cancer, and particularly in metabolically stressed tumors where they can serve as alternative sources of energy and, through the import of cysteine, generate glutathione to scavenge ROS (reviewed in (18)). However, the connection between NMD inhibition and amino acid transport/metabolism is complex. We recently demonstrated that many of the mRNAs encoding amino acid transporters are indirectly increased upon NMD inhibition due to the stabilization of ATF-4, and that increased mRNA expression of these transport systems does not necessarily correlate with increase protein expression and transport of amino acids (17). In addition, we also demonstrated that NMD inhibition can augment the intracellular accumulation of some amino acids indirectly through the activation of autophagy (17).
The transport and metabolism of amino acids are increasingly recognized targets for cancer therapy (19). Such targeting requires both the delineation of the complex mechanisms that regulate amino acid transport and metabolism, and the determination of the biological significance of these regulatory controls. In the current study we investigate the regulation of SLC7A11, which encodes for a subunit of the xCT amino acid transport system. xCT is rate limiting for transporting cystine into the cell and serves as the dominant means of increasing intracellular cysteine and accelerating the production of glutathione, a tripeptide synthesized comprised of cystine, gluatmic acid, and glycine. (20, 21). SLC7A11 has an established role in cancer biology and therapeutics. Reducing the amount of SLC7A11 decreases intracellular glutathione levels and increases the potency of several chemotherapeutic agents, and the high expression of a variant of the CD44 adhesion molecule stabilizes xCT to increase intracellular glutathione and promotes tumor growth (22, 23). xCT can also be regulated by the microenvironment. Glioma cells rendered hypoxic increase xCT expression and increases import of cystine and glutathione synthesis, and phosphorylation of eIF2α has been shown to be important in xCT expression, although the exact mechanism is not clear (24, 25). Because eIF2α phosphorylation, in addition to up-regulating ATF-4, also leads to the inhibition of NMD, we studied the relationship between the NMD activity and SLC7A11 regulation by cellular stress.
Results
SLC7A11 is upregulated by cellular stress and by the inhibition of NMD
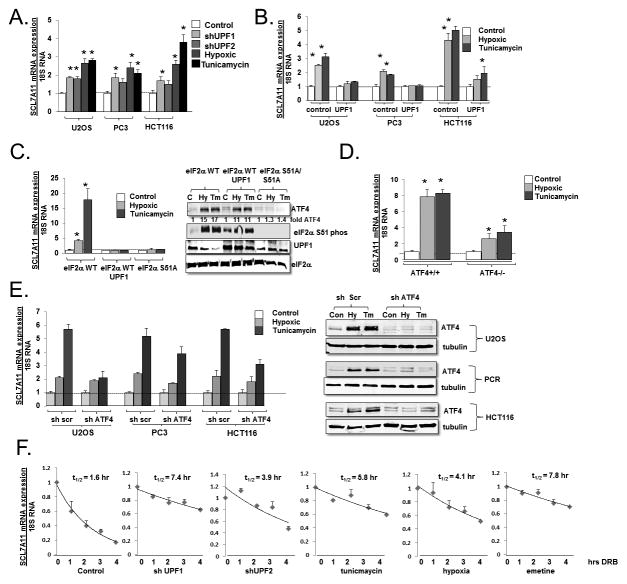
Previous work by our group and others has demonstrated that the mRNAs of multiple genes responsible for amino acid transport are increased when NMD is inhibited (14, 17). Because SLC7A11 has a well documented role in the cellular adaptation to stress and tumorigenesis, we rigorously assessed its regulation by NMD. NMD is inhibited by a wide variety of cellular stresses, including amino acid deprivation, hypoxia, and the accumulation of unfolded protein within the ER (as induced by treatment with the glycosylation inhibitor tunicamycin) (4, 15). We noted that hypoxia and tunicamycin treatment led to a significant increase in SLC7A11 mRNA in cell lines derived from ostesarcoma (U2OS), prostate cancer (PC3) and colon cancer (HCT116) (Fig 1A). A similar increase in SLC7A11 mRNA was observed with the depletion of UPF1/Rent1 or UPF2/Rent2, genes required for NMD, with previously validated effective shRNAs which up-regulate other NMD reporters and endogenous NMD targets in these cell lines (Fig 1A) (14, 26). To assess whether cellular stress increases SLC7A11 through NMD inhibition, we induced ER stress in control cells and in cells over-expressing UPF1/Rent1 (which we have previously demonstrated overcomes the stress-induced inhibition of NMD (26)). The activation of NMD by UPF1/Rent1 over-expression reversed the stress-induced up-regulation of SLC7A11 (Fig 1B) suggesting that cellular stress increases SLC7A11 through the inhibition of NMD.
Figure 1.
SLC7A11 mRNA is regulated by cellular stress and NMD. A. SLC7A11 mRNA was assessed by real-time PCR in U2OS, PC3, and HCT116 control, UPF1 depleted, UPF2 depleted, hypoxic (8 hours) or tunicamycin (Tm) (2.5 μg/ml 8 hours) cells. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. B. Control and UPF1 over-expressing U2OS, PC3, and HCT116 cells were treated with tunicamycin (8 hrs) or rendered hypoxic (8 hrs), and expression of SLC7A11 mRNA was quantitated by real-time PCR. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. C. (left panel) eIF2α wild type MEFs, either control or over-expressing UPF1, and eIF2α S51A cells were treated with cellular stress and SLC7A11 mRNA was assessed. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. (right panel) Representative immunoblots of ATF4, UPF1, phosphorylated eIF2α, and (as loading control) total eIF2α in stressed eIF2α wild-type MEFs, wild-type MEFs over-expressing UPF1/RRent1, and eIF2aα S51A/S51A MEFs. D. ATF4 wild-type and ATF4 −/− MEFs were treated with cellular stress and SLC7A11 mRNA was assessed. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. E. Control (sh Scr) and ATF4 depleted (sh ATF4) U2OS, PC3 and HCT116 cells were rendered hypoxic or treated with tunicamycin and SLC7A11 mRNA was assessed by quantitative PCR (left panel). Immunoblots of ATF4 expression in control and ATF4 depleted cells (right panel). F. U2OS cells were depleted of UPF1, UPF2, or treated with cellular stress for three hours, and then DRB was added to cease new RNA synthesis. SLC7A11 mRNA was serially assessed by quantitative PCR. Experiments were repeated in triplicate.
Because the phosphorylation of eIF2α is necessary for the stress induced inhibition of NMD (16, 26), we next examined whether eIF2α phosphorylation is necessary for the induction of SLC7A11. When wild-type MEFs were rendered hypoxic or treated with tunicamycin, we noted a marked increases in SLC7A11 mRNA, which was not apparent when these cells over-expressed UPF1/Rent1 (Fig 1C). SLC7A11 induction did not occur in hypoxic or tunicamycin treated eIF2α isogenic MEFS in which the eIF2α alleles are replaced with mutated alleles which cannot be phosphorylated (eIF2α S51A) (27) (Fig 1C, left panel), demonstrating that the stress-induced inhibition of NMD plays a crucial role in upregulated SLC7A11 mRNA.
The upregulation of SLC7A11 mRNA by the stress-induced inhibition of NMD could either be direct or indirect. That is, the inhibition of NMD could result in the upregulation of a transcription factor that then induces SLC7A11 transcription. Indeed, many amino acids biosynthetic enzymes are targets of the transcription factor ATF-4, and the phosphorylation of eIF2α leads to the stabilization of ATF4 through the inhibition of NMD (ATF-4 is a direct NMD target) in addition to the well described translational induction of ATF-4 (26, 28). Indeed, consistent with our previously published observations, the mild overexpression of UPF1 led to a modest decrease in ATF4 protein induction (Fig 1C, right panel). To determine the contribution of ATF4 to the induction, we rendered ATF-4 deficient MEFs hypoxic or treated them with tunicamycin. We observed a decreased, but still robust and significant upregulation of SLC7A11 in ATF-4 deficient MEFs when compared to control MEFs (Fig 1D). Similarly, SLC7A11 mRNA was upregulated when ATF4 depleted U2OS, PC3, and HCT116 cells were rendered hypoxic or treated with tunicamyicn. In addition, when we directly assessed SLC7A11 mRNA stability, by treating cells with the RNA Polymerase II inhibitor 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) and measuring SLC7A11 mRNA expression by quantitative RT-PCR, we found that the SLC7A11 half-life increased with UPF1/Rent1 depletion, UPF2/Rent2 depletion, and when cells rendered hypoxic, treated with tunicamycin or treated with the translation inhibitor emetine which potently suppresses NMD (Fig 1E). Together, these data indicate that although SLC7A11 is up-regulated by ATF-4, SLC7A11 is a direct NMD target, and the inhibition of NMD by cellular stress plays a significant contribution to the upregulation of SLC7A11 mRNA.
SLC7A11 protein is upregulated by the stress-induced inhibition of NMD
Although our data indicate that SLC7A11 mRNA is regulated by NMD activity, the finding that a mRNA is stabilized by NMD does not necessarily indicate that the encoded protein is also up-regulated. This is particularly true when NMD is inhibited by cellular stress, since the stress-induced phosphorylation of eIF2α can also suppress protein translation (16). Indeed we have demonstrated that while the SLC3A2 mRNA, which encodes the light subunit of the heterodimer responsible for large neutral amino acids, is stabilized by the inhibition of NMD, SLC3A2 protein expression and neutral amino acid transport are not increased when NMD is inhibited (17).
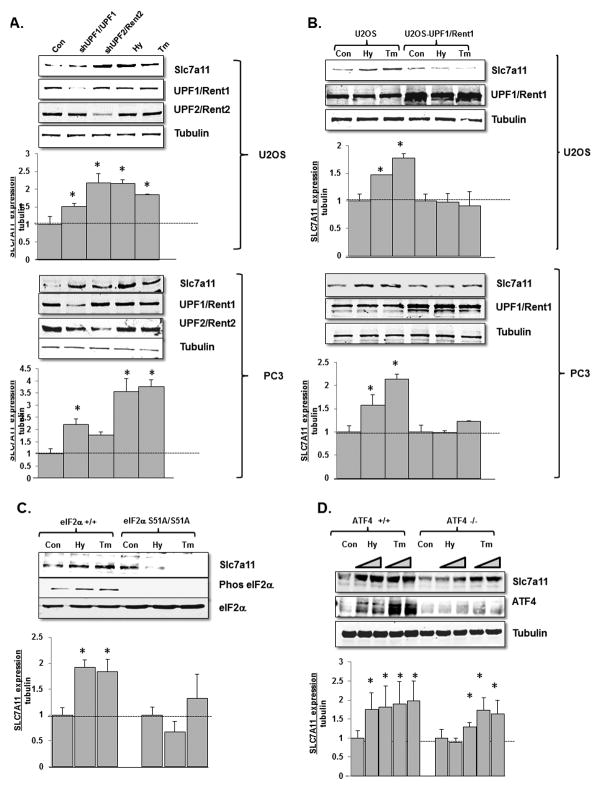
We therefore assessed whether the changes we noted with SLC7A11 mRNA in response to the stress-induced inhibition of NMD were reflected at the protein level. In addition to confirming the hypoxic up-regulation of SLC7A11 protein, we noted an increase in SLC7A11 protein in U2OS and PC3 cells treated with tunicamycin or depleted of UPF1/Rent1 or UPF2/Rent2 (Fig 2A). In concordance with our mRNA results, we also noted that UPF1/Rent1 over-expression blunted the upregulation of SLC7A11 protein in cells rendered hypoxic or treated with tunicamycin (Fig 2B) and that the stressed-induced upregulation of SLC7A11 noted in eIF2α wild-type MEFs was blunted in eIF2α S51A MEFs (Fig 2C). We also noted a diminished, but still significant increase in SLC7A11 protein in hypoxic or tunicamycin treated ATF4 deficient MEFs (Fig 2D). Thus the stress-induced changes of SLC7A11 mRNA are also reflected at the level of protein expression.
Fig 2.
SLC7A11 protein is regulated by cellular stress and NMD. A. U2OS and PC3 cells were either depleted of UPF1 or UPF2, rendered hypoxic (Hy) for 8 hours, or treated with tunicamycin (Tm) for 8 hours, and SLC7A11 protein was assessed by immunoblot. B. U2OS and PC3 cells, either expressing a control plasmid or UPF1, were rendered hypoxic or treated with tunicamycin and SLC7A11 protein was assessed. C. eIF2α wild-type or eIF2α S51A MEFs were rendered hypoxic or treated with tunicamycin for 8 hours and SLC7A11 protein was assessed. Experiments were repeated twice, and the data reflect average ± standard error. * = p<0.05 by Students T test. D. ATF4 wild-type or deficient MEFs were rendered hypoxic or treated with tunicamycin for 4 and 8 hours and SLC7A11 protein was assessed. Experiments were repeated twice and the data reflect average ± standard error. * = p<0.05 by Students T test
SLC7A11 mediated intracellular transport of cystine is regulated by stress-induced inhibition of NMD
SLC7A11 is a component of the xCT transport system, which is responsible for intracellular cystine import. We found that hyperactivation of NMD by UPF1/Rent1 over-expression significantly diminished basal intracellular cysteine levels (Fig 3A). The increase in intracellular cysteine noted with tunicamycin treatment also was significantly decreased when NMD was hyperactivated (Fig 3A), suggesting that the stress induced increase in intracellular cysteine is regulated by NMD.
Fig 3.
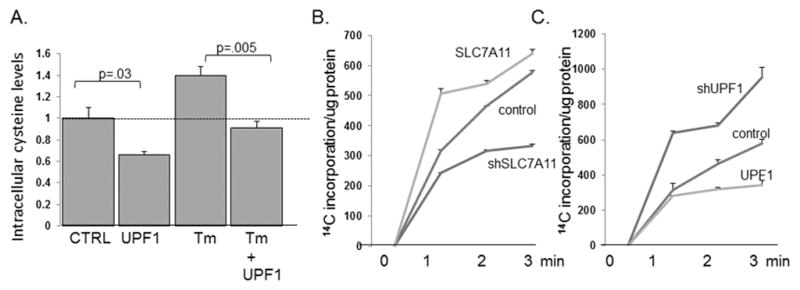
NMD activity regulates intracellular cysteine levels and cystine transport. A. Intracellular cysteine levels in control U2OS cells and in cells treated with tunicamycin, each one expressing a control plasmid or over-expressing UPF1, were assessed by HPLC/MS as described in the methods. Data reflects the average ± standard error of six replicates. B. Intracellular cystine transport was assessed as described in the text in control U2OS cells, cells over-expressing SLC7A11, cells depleted of SLC7A11, and C. cells over-expressing UPF1 or depleted of UPF1. Experiments were replicated three times and average ± standard error are displayed.
We have previously noted that increased intracellular concentrations of several neutral amino acids is not due to increased transport in or decreased transport out, but rather to the release of amino acids through autophagy (17). We thus directly examined whether the stress-induced inhibition of NMD contributes to cystine transport. We used a well described radiolabel assay to first confirm the role of SLC7A11 in cystine transport. When SLC7A11 was depleted we noted that cystine transport was decreased compared to control cells, and SLC7A11 over-expression increased intracellular cystine transport (Fig 3B). Cystine transport was increased by UPF1/Rent1 depletion, and the over-expression of UPF1/Rent1 decreased cystine transport (Fig 3C). Together these data suggest that cystine transport and intracellular cystine levels are regulated by the stress induced inhibition of NMD.
Intracellular glutathione and sensitivity to ROS is regulated by stress-induced inhibition of NMD
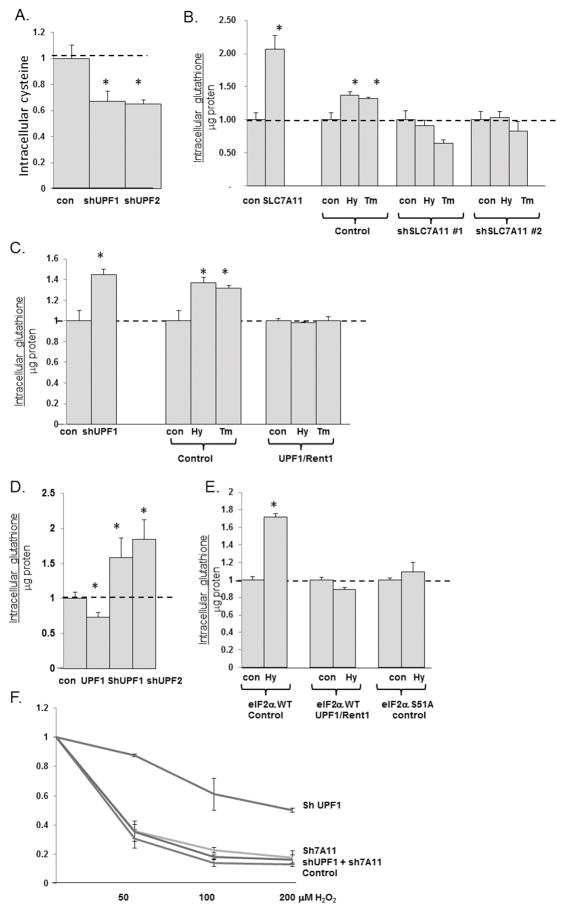
We observed that hyperactivation of NMD decreased intracellular cystine, but unexpectedly while the inhibition of NMD increased intracellular cystine transport (Fig 3C) this was not associated with an increase in the steady state intracellular levels of cysteine (Fig 4A). In fact intracellular cysteine decreased, suggesting it was being utilized. One of the critical fates of intracellular cysteine is to generate glutathione, a key element in protecting the cells against oxidative stress. SLC7A11 is rate limiting for glutathione production, and indeed, we noted that intracellular glutathione levels, assessed by a fluorimetric assay, increased with the over-expression of SLC7A11 (Fig 4A). Confirming the central role SLC7A11 regulation can play in regulating intracellular glutathione, the depletion of SLC7A11 blunted the increase in glutathione in cells rendered hypoxic or treated with tunicamycin (Fig 4B).
Fig 4.
NMD regulation of SLC7A11 determines intracellular glutathione levels and resistance to oxidative stress. A. Intracellular cysteine levels, assessed by LC/MS, were assessed in control and UPF1 and UPF2 depleted U2OS cells. Six replicates were done, and data reflects mean ± standard error. B. Glutathione levels were assessed in control U2OS cells, cells depleted over-expressing SLC7A11, and in control cells and cells depleted of SLC7A11 by two distinct shRNAs which were rendered hypoxic (Hy) for 8 hours or treated with tunicamycin (Tm) for 8 hours. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. C. Glutathione levels were measured in control cells or cells over-expressing UPF1 which were rendered hypoxic or treated with tunicamycin. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. D. Glutathione levels measured by HPLC in cells over-expressing UPF1, or depleted of UPF1 or UPF2. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. E. Glutathione levels in control and hypoxic eIF2α wild-type MEFs, wild-type MEFs rendered hypoxic, or eIF2α S51A MEFs rendered hypoxic. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test. F. Control, SLC7A11 depleted, UPF1 depleted, and SLC7A11 and UPF1 depleted U2OS cells were treated with increasing concentrations of H2O2 and survival was assessed. Experiments were repeated in triplicate, and the data reflect average ± standard error. * = p<0.05 by Students T test.
Glutathione exists in both reduced and oxidized forms, depending on the redox state of the cell. We found that manipulation of SLC7A11 and rendering cells hypoxic or treatment with tunicamyicn, primarily affected reduced glutathione levels, with minimal effects on oxidized glutathione levels (data not shown) and therefore assessed the role of NMD regulation in reduced glutathione regulation. Consistent with the increase in SLC7A11 mRNA and SLC7A11 protein, and cystine transport we noted with NMD inhibition, UPF1/Rent1 depletion led to an increase in reduced glutathione levels (Fig 4C). HPLC In addition, the increase in reduced glutathione levels noted in hypoxic and tunicamycin treated cells was blunted with the hyperactivation of NMD achieved by the over-expression of UPF1/Rent1 (Fig 4C). HPLC/MS confirmed that reduced glutathione was decreased in cells with UPF1/Rent1 over-expression and increased in cells with NMD inactivated by Upf1/Rent1 or UPF2/Rent2 depletion (Fig 4D). As expected with the dependency of NMD inhibition and SLC7A11 regulation on eIF2α phosphorylation, the increase in reduced glutathione noted in hypoxic eIF2α wild-type MEFs was not seen in eIF2α S51A MEFS (Fig 4E).
Because intracellular glutathione levels play an important role in scavenging ROS, we explored whether NMD inhibition contributes to the survival of cells to oxidative stress. U2OS cells depleted of UPF1/Rent1, a manipulation which increases SLC7A11 mRNA and protein expression, cysteine transport and glutathione levels, led to a significant increase in survival to 50–200 μM H2O2 (Fig 4F) The concomitant depletion of SLC7A11 totally abrogated the survival benefit to NMD inhibition, suggesting that the benefit of NMD inhibition is mediated through up-regulation of SLC7A11.
Discussion
We have previously demonstrated that NMD inhibition augments the cellular adaptation to ER stress (likely through stabilization of ATF-4) and the cellular response to metabolic stress through the activation of autophagy. In the present studies we demonstrate that the inhibition of NMD seen during cellular stress serves as an adaptive response to oxidative stress. ROS are well established to lead to eIF2α phosphorylation (15, 29). We show that the suppression of NMD by cellular stress-induced eIF2α phosphorylation leads to the stabilization of SLC7A11 mRNA and an increase in SLC7A11 protein expression, and an increase in intracellular cystine transport, a limiting amino acid in de novo glutathione synthesis (Fig 5). We demonstrate that the inhibition of NMD increases intracellular glutathione levels to promote the cellular survival to oxidative stress. Thus we have linked the inhibition of NMD to glutathione production, establishing NMD as an adaptive response to oxidative stress and providing a potential mechanism to disrupt this adaptive response.
Fig 5.
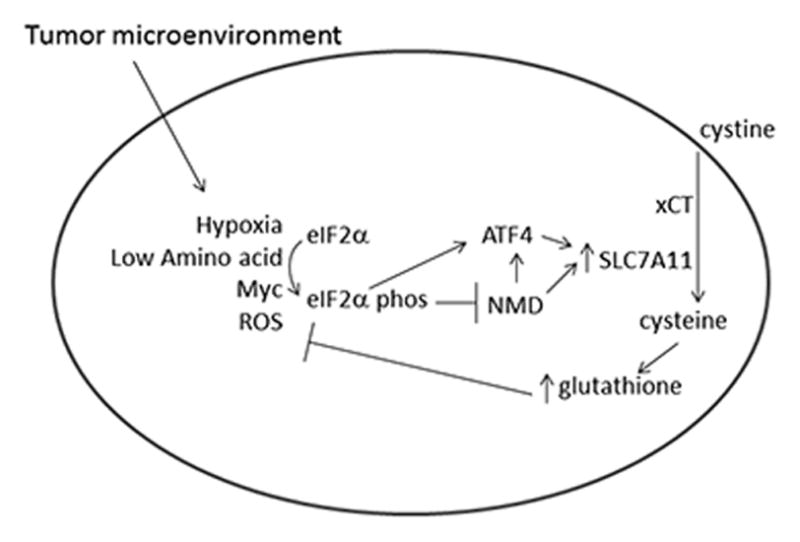
A model, based on the data in these studies as well as previously reported studies, displaying how NMD inhibition serves as an adaptive response to protect cells against oxidative stress. NMD inhibition increases cystine transport both through stabilizing the transcription factor ATF4, which is potently translationally upreuglated by eIF2α phosphorylation, and by stabilizing SLC7A11.
ROS are an ubiquitous byproduct of aerobic metabolism and are commonly elevated in tumors due to metabolic stress generated by the tumor microenvironment and/or because of mutations in mitochondrial and/or other genes (30). Glutathione serves as the major endogenous soluble antioxidant in cells. In addition to preventing the accumulation of damaging ROS, glutathione redox status contributes to the activation of several signaling pathways, including c-jun-N-termainal kinase (JNK) stress activated protein kinase, protein kinase C, tyrosisne kinases, and NFκB (31–33). ROS has also been shown to be necessary for myc-induced tumorignesis, in part through the stabilization of HIF-1α (3). Interestingly, in some models Myc and other oncogenes temper the production of ROS, at least in part through the transcription factor Nrf2. Thus Myc induced phosphorylation of eIF2α and inhibition of NMD may be one of several mechanism which diminish Myc’s induction of ROS and its oncogenic potential and serve in a negative feedback loop (11, 15, 34). As xCT is a cystine/glutamate antiporter, the inhibition of NMD and increase in intracellular cystine transport would be expected to be accompanied by the export of glutamate, an amino acid with an important role in cancer, and thus a link which deserves further study (35). The importance of ROS homeostasis in cancer, and the documented role xCT plays in tumor metastasis (36) suggest our insights into SLC7A11 regulation have implications in cancer biology.
Our findings also have potential implications for cancer therapy. In some models antioxidants have been shown to accelerate cancer (37). Glutathione levels have also been found to be elevated in many chemotherapy resistant tumors, and a goal in cancer therapy has been to reduce intracellular glutathione levels to increase tumor sensitivity to radiation and chemotherapy. Two such mechanisms which have been pursued are the downregulation of SLC7A11 and the inhibition of xCT (22, 38). Our data indicate that blunting the inhibition of NMD by the tumor microenvironment and cellular stress would be an additional mechanism to down-regulate SLC7A11 and its downstream effects. Although hyperactivating NMD to decrease SLC7A11 expression, cystine transport, and intracellular glutathione levels could improve the efficacy of many chemotherapeutic agents, and disrupt the neoplastic’s cell ability to adapt to a hostile microenvironment, decreased ROS could also be beneficial in a number of conditions including tumorigenesis and inflammatory diseases. While activating the enzymes required for NMD is likely more difficult than inhibiting these enzymes, several compounds have been identified that can inhibit NMD ((39, 40) and Martin et al, unpublished). It is possible that the increase of intracellular glutathione levels by effective and safe pharmacological inhibition of NMD may also have clinical utility in cancer or other pathologies.
Methods
Cell lines
U2OS, PC3, HCT116 and mouse embryo fibroblast (MEF) cells were grown in DMEM containing 10% FBS. Prior to rendering cells hypoxic, medium was changed to DMEM containing 10% FCS buffered with 25mM HEPES. Cells were incubated at 37°C, either in 5% CO2 or in a Plas-Labs environmental chamber, and oxygen concentration in the chamber was maintained at ≤0.5%.
Immunoblot, and RNA assesment
Immunoblots were prepared as previously described (41) and membranes were probed with antibodies against Slc7a11 (Novus Biologicals, NB300-318), UPF1/Rent1 (sc H-300), UPF2/Rent2 (kindly provided by J. Lykke-Andersen), Phospho-eIF2α (Epitomics, 1090-1), Total eIF2α (sc-11386), ATF4 (sc-200) and α-Tubulin (T9026, Sigma). RNA assessment: Isolation of RNA, cDNA generation, real-time PCR, DRB treatment and RNA stability experiment were done as described previously ((16). Real time primers for SLC7A11 are; human, 5′-GGGCATGTCTCTGACCATCT-3′ and 5′-TCCCAATTCAGCATAAGACAAA-3′; mouse 5′-TTGCAAGCTCACAGCAATTC-3′ and 5′-AGGGCAACCCCATTAGACTT-3′. Glutathione, cysteine transport, and cell viability assays: Intracellular glutathione was determined fluorometrically by using a Glutathione Assay Kit (Bio Vision, K264-100) and by liquid chromatography/Mass Spec (Metabolon, Durham, North Carolina) as previously described (17). Intracellular cysteine was also assessed by LC/MS by Metabolon.
Cystine transport
We utilized a well described assay (42) to assess cystine transport. Briefly, cells were washed twice with sodium free extracellular fluid (ECF) containing 122 mM Choline Chloride, 25 mM Choline Bicarbonate, 3 mM KCL, 1.4mM CaCl2, 1.2mM MgSO4, 0.4 mM K2HPO4, 100 mM D-Glucose, 10mM HEPES (pH 7.4). The cells were then incubated with sodium free ECF buffer containing C14 Cystine (0.1μCi/mL) for 0, 1, 2 and 3 minutes, during which cysteine transport was linear, and then washed twice with PBS and lysed with 500 μl of 0.2N NaOH. C14 incorporation was determined by using liquid scintillation counter (PerkinElmer, Inc). and normalized to μg protein.
Cell viability
cells were cultured in 6 well dishes and incubated with H2O2 for 8hrs. After incubation, medium was replaced with fresh medium and cells viability was measured after 7 days as previously described (16).
Acknowledgments
This work was supported by RO1DK081641 (L.B.G.), a generous gift from Mr. Arthur Levine and Ms. Diane Lederman, and the NYU Cancer Institute. LBG was the Saul Farber Associate Professor of Medicine.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest
References
- 1.Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15(1):100–9. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 3.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12(3):230–8. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7(13):1916–24. doi: 10.4161/cc.7.13.6203. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283(16):10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11(5):407–20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–22. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 9.Krokowski D, Han J, Saikia M, Majumder M, Yuan CL, Guan BJ, et al. A self-defeating anabolic program leads to beta-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J Biol Chem. 2013;288(24):17202–13. doi: 10.1074/jbc.M113.466920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Li Y, Lopez AB, Chiang CM, Kaufman RJ, Snider MD, et al. Temporal regulation of Cat-1 (cationic amino acid transporter-1) gene transcription during endoplasmic reticulum stress. Biochem J. 2010;429(1):215–24. doi: 10.1042/BJ20100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122(12):4621–34. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29(12):2082–96. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24(19):3470–81. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36(10):1073–8. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Wengrod J, Gardner LB. Overexpression of the c-myc oncogene inhibits nonsense-mediated RNA decay in B lymphocytes. J Biol Chem. 2011;286(46):40038–43. doi: 10.1074/jbc.M111.266361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Zavadil J, Martin L, Parisi F, Friedman E, Levy D, et al. Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol Cell Biol. 2011;31(17):3670–80. doi: 10.1128/MCB.05704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wengrod J, Martin L, Wang D, Frischmeyer-Guerrerio P, Dietz HC, Gardner LB. The inhibition of nonsense mediated RNA decay activates autophagy. Mol Cell Biol. 2013 doi: 10.1128/MCB.00174-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2(10):881–98. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18(3):207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261(5):2256–63. [PubMed] [Google Scholar]
- 21.Lo M, Wang YZ, Gout PW. The x(c)− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215(3):593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005;65(16):7446–54. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- 23.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Ogunrinu TA, Sontheimer H. Hypoxia increases the dependence of glioma cells on glutathione. J Biol Chem. 2010;285(48):37716–24. doi: 10.1074/jbc.M110.161190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem. 2009;284(2):1106–15. doi: 10.1074/jbc.M807325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner LB. Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Mol Cell Biol. 2008;28(11):3729–41. doi: 10.1128/MCB.02284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 28.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283(45):31153–62. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–6. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest. 1993;69(3):261–74. [PubMed] [Google Scholar]
- 32.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a coordinately regulated defence against oxidative stress. Free Radic Res. 1999;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez PC, Machado J, Jr, Heussler VT, Botteron C, Palmer GH, Dobbelaere DA. The inhibition of NF-kappaB activation pathways and the induction of apoptosis by dithiocarbamates in T cells are blocked by the glutathione precursor N-acetyl-L-cysteine. Biol Chem. 1999;380(12):1383–94. doi: 10.1515/BC.1999.178. [DOI] [PubMed] [Google Scholar]
- 34.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26(9):877–90. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene. 2009;28(4):599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- 37.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 38.Timmerman LA, Holton T, Yuneva M, Louie RJ, Padro M, Daemen A, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–65. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keeling KM, Wang D, Dai Y, Murugesan S, Chenna B, Clark J, et al. Attenuation of nonsense-mediated mRNA decay enhances in vivo nonsense suppression. PLoS ONE. 2013;8(4):e60478. doi: 10.1371/journal.pone.0060478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durand S, Cougot N, Mahuteau-Betzer F, Nguyen CH, Grierson DS, Bertrand E, et al. Inhibition of nonsense-mediated mRNA decay (NMD) by a new chemical molecule reveals the dynamic of NMD factors in P-bodies. J Cell Biol. 2007;178(7):1145–60. doi: 10.1083/jcb.200611086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin L, Kimball SR, Gardner LB. Regulation of the unfolded protein response by eif2bdelta isoforms. J Biol Chem. 2010;285(42):31944–53. doi: 10.1074/jbc.M110.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomi M, Hosoya K, Takanaga H, Ohtsuki S, Terasaki T. Induction of xCT gene expression and L-cystine transport activity by diethyl maleate at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2002;43(3):774–9. [PubMed] [Google Scholar]