Abstract
Background
Outbred mice exhibit increased airway and intestinal immunoglobulin A (IgA) following injury when fed normal chow, consistent with humans. Parenteral nutrition (PN) eliminates IgA increases at both sites. Inbred mice are needed for detailed immunological studies; however, specific strains have not been evaluated for this purpose. BALB/c and C57BL/6 are common inbred mouse strains, but demonstrate divergent immune responses to analogous stress. This study addressed which inbred mouse strain best replicates the outbred mouse and human immune response to injury.
Methods
Intravenously cannulated mice received Chow or PN for 5 days and then underwent sacrifice at 0 or 8-hours following controlled surgical injury (BALB/c: n=16-21/group; C57BL/6: n=12-15/group). Bronchoalveolar lavage (BAL) was analyzed by ELISA for IgA, TNF-α, IL-1β and IL-6 while small intestinal wash fluid (SIWF) was analyzed for IgA.
Results
No significant increase in BAL IgA occurred following injury in chow-or PN-fed BALB/c mice (Chow: p=0.1; PN: p=0.7) despite significant increases in BAL TNF-α and SIWF IgA (Chow: 264±28 vs. 548±37, p<0.0001; PN: 150±12 vs. 301±17, p<0.0001).
Injury significantly increased mucosal IgA in chow-fed C57BL/6 mice (BAL: 149±33 vs. 342±87, p=0.01; SIWF: 236±28 vs. 335±32, p=0.006) and BAL cytokines. After injury, PN-fed C57BL/6 mice exhibited no difference in BAL IgA (p=0.9), BAL cytokines or SIWF IgA (p=0.1).
Conclusions
C57BL/6 mice exhibit similar airway responses to injury as outbred mice and humans, providing an appropriate model for studying mucosal responses to injury. The BALB/c mucosal immune system responds differently to injury and does not replicate the human injury response.
Background
Outbred Institute of Cancer Research (ICR) mice are frequently used as murine models of human responses. Our laboratory previously demonstrated similarities between humans and male ICR mice in airway mucosal immunoglobulin A (IgA) and cytokine response before and after injury.1, 2 Our work shows that route of feeding affects IgA levels within airway and intestinal secretions in both unstressed and stressed mice.3, 4 Under unstressed conditions, parenteral nutrition with decreased enteral stimulation (PN) reduces baseline levels of both airway and intestinal IgA.5 Normally, the Th2 type cytokines- interkleukin-4 (IL-4), -6, -10, and -13 stimulate production and release of IgA from plasma cells at mucosal sites.6-9 Reductions in IL-4, IL-6 and IL-10 within the intestine and specifically within the small intestine lamina propria during PN implicate loss of Th2 cytokine stimulation as one cause of reduced IgA levels in uninjured mice.10
Route of nutrition also affects immune responses to injury in male ICR mice. Clinically, lower respiratory tract levels of IgA measured in bronchoalveolar lavage (BAL) specimens of severely injured trauma patients increase soon after injury.1 Experimentally, normal chow fed mice generate a similar response which is lost if mice are pretreated with PN.11 A similar response occurs within the intestine: IgA levels increase in injured mice.4 Within the lung, this appears to be related to the generation of stress cytokines. BAL levels of TNF-α, IL-1β and IL-6 all demonstrate bimodal peaks at 3 and 8 hours after injury with a peak in BAL IgA at 8 hours.6 These cytokine increases do not correspond to serum increases, and antibodies which block TNF-α and IL-1β activity eliminate increases in airway (but not intestinal), IgA levels.2, 4, 11 Intraperitoneal administration of TNF-α, IL-1β and IL-6 also induces the airway IgA increase in vivo. While TNF-α and IL-1β do not fall under the definition of Th1 or Th2 type cytokines, they are capable of generating stress responses in a wide variety of conditions.12 These IgA responses to injury may represent innate protective mechanisms designed to prevent infection at mucosal surfaces after injury, especially the human respiratory tract. Once secreted, IgA binds to pathogens and preventing mucosal attachment and subsequent invasion.13, 14 Experimentally, PN also eliminates this protective mechanism in ICR mice.4, 11
While these immunological responses correlate between outbred mice and humans, developments in molecular genetics over the past three decades have allowed for increasingly complex genomic manipulation of murine models. Inbred mouse models allow more precise definition of molecular events while controlling for genetic diversity. They also allow cell transfer between animals.15, 16 However, different inbred strains expressing distinctly different genetic backgrounds can exhibit critically different phenotypic responses. The importance of inbred strain background on phenotypic assessment has been well reviewed in the fields of neurobiology and behavior and is expanding into the field of metabolism and endocrine disorders.17-20 These observations mandate investigation to determine which mouse strain should be employed in experiments modeling human conditions. This requires a critical evaluation of the genetic backgrounds and relevant phenotypic differences present among inbred mouse strains used in the research to increase the clinical relevance of experimental results.
BALB/c and C57BL/6 mice are two common inbred strains used frequently in research. Several studies highlight differences in immunological response of these two strains resulting in the designation of BALB/c mice as Th2 type (e.g. IL-4, IL-6, IL-10 and IL-13) responders and C57BL/6 mice as Th1 type (TNF-β and IFN-γ) responders based on their respective cytokine profiles and responses to infectious challenges and immunizations.21-28 In general, BALB/c mice are more susceptible to viral infections while exhibiting decreased cytotoxic responses but increased humoral or immunoglobulin responses to allergens and infections consistent with its cytokine profile. In contrast, C57BL/6 mice are more resistant to viral infection exhibiting increased cytotoxic responses and decreased humoral and allergic responses.
Since Th2 cytokines favor IgA production, and PN results in a Th2 cytokine dependent reduction in respiratory and intestinal IgA, 10, 29-31 it made intuitive sense that animals such as BALB/c mice, who genetically favor Th2 cytokine production and hence IgA production would provide the most relevant model for replicating our outbred mouse work. Our primary aim is therefore to determine which inbred mouse strain (BALB/c or C57BL/6) mimics the immune responses of the outbred mouse. The present study tests the hypothesis that BALB/c mice assimilate the ICR mouse IgA response to injury and nutrition due to its preferential Th2 cytokine profile.
Materials and Methods
Animals
All protocols were approved by the Animal Care and Use Committee of the William S. Middleton Memorial Veterans Hospital-Madison and the University of Wisconsin-Madison. Male ICR mice were purchased from Harlan (Indianapolis, IN) and housed 5 per covered/filtered box under controlled temperature and humidity conditions in an American Association for Accreditation of Laboratory Animal Care-accredited facility. Animals were fed standard mouse chow (Rodent Diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) and water ad libitum for an acclimation period of 1 week prior to initiation of study protocol. After entering study protocol mice were housed individually in metal cages with wire grid floors to eliminate coprophagia.
Experimental Design
Male BALB/c and C57BL/6 mice, ages 6 to 8 weeks, were randomized to Chow (BALB/c, n=33; C57BL/6, n=29) or PN (BALB/c, n=40; C57BL/6, n=26). All animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and acepromazine (10 mg/kg), weighed, and underwent surgical central line placement of a silicon rubber catheter (0.012-inch I.D./0.025-inch O.D.; Helix Medical, Inc., Carpinteria, CA) via the right external jugular vein. The distal end of the catheter was tunneled subcutaneously over the back and exited at the midpoint of the tail. Animals were partially immobilized by tail restraint following the procedure to protect the catheter during infusion. This technique has proven to be an acceptable method of nutritional support and does not produce physical or biochemical evidence of stress.32
Mouse catheters were connected to infusion pumps and the animals received 0.9% saline at a rate of 4 mL/day and ad libitum chow and water for 48 hours of recovery. This recovery period allows serum cytokines, corticosteroid levels, and IgA secretion induced by surgical stress from catheterization to return to baseline, with resumption of regular independent oral intake. After the recovery period, experimental diets were initiated. Animals in the Chow group continued to receive 0.9% saline at 4 mL/day as well as ad libitum chow and water. PN animals received ad libitum water and PN solution through their catheters at rates of 4 mL/day (day 1), 7 mL/day (day 2) and 10 mL/day (day 3 to 5), because a graded infusion period is necessary for the mice to adapt to the changes in glucose and fluid loads. The PN solution contained 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, containing 1440 kcal/L and a non-protein calories/nitrogen ratio of 128:1. These values were calculated to meet the nutrient requirements of mice weighting 25 to 30 and metabolically scaled to the weight of our animals.33
After 5 days of experimental diet (7 days post-catheterization), mice were again anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and acepromazine (10 mg/kg). The mice were then randomized to two groups: 1) sacrifice without injury (0h) to determine baseline protein levels and 2) sacrifice8 hours following injury (BALB/c Chow 0h, n = 16; Chow 8h, n = 17; PN 0h, n = 19; PN 8h, n = 21; C57BL/6, n = 14, 15, 14, and 12). Animals in the second group (8h) underwent controlled surgical injury consisting of two wounds. First, a 3.0 cm celiotomy incision was made, and the bowel was gently eviscerated before being returned to the abdominal cavity. The second wound consisted of a 1.5 cm left neck incision with blunt dissection carried to the pretracheal tissue plane. Both incisions were then immediately sutured closed and the mice returned to their cages with water ad libitum. They remained disconnected from feeding catheters and were not provided chow. Eight hours following the controlled injury, mice were again anesthetized and sacrificed. Sacrifice was performed in both groups by exsanguination via left axillary artery transection.
Sample Collection
Immediately following sacrifice, the mouse trachea was cannulated with an 18-g angiocatheter and 1 mL of 0.9% saline was injected distally. Approximately 1 mL of bronchoalveolar lavage (BAL) fluid was then gently aspirated from the lungs. Samples were placed on ice and stored at -80°C until assayed.
Additionally, the small intestine (pylorus to terminal ileum) was removed and the lumen flushed with 20 mL Hanks Balanced Saline Solution (Bio Whittaker, Walkersville, MD). This small intestinal wash fluid (SIWF) was collected and kept on ice until they were centrifuged at 2000 × g for 10 minutes at 4°C. Supernate was stored at -80°C until assayed.
Sample Analysis
Airway and SIWF were analyzed for IgA secretion by enzyme-linked immunosorbent assay (ELISA). Proinflammatory cytokines TNF-α, IL-1β and IL-6 were also evaluated by ELISA, but only the airway samples were assayed as previous studies indicated that respiratory IgA secretion was cytokine dependent, whereas gastrointestinal IgA secretion is cytokine independent.4, 6
Briefly, respective solid phase sandwich ELISA kits (BD Biosciences, San Diego, CA) for IgA and TNF-α, IL-1β and IL-6 were used according to manufacturer's instructions. Samples were diluted 1:5 BAL IgA quantitative analysis and 1:100 for SIWF IgA quantitative analysis. BAL samples for TNF-α, IL-1β, and IL-6 were all run neat. The absorbance at 450 nm was determined using a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). Relative concentration of secreted proteins was determined by using a 4-parameter logistic fit standard curve (SOFTmax PRO software; Molecular Devices; Sunnyvale, CA) and normalized to total luminal protein content.
Statistical analysis
Statistical analysis was performed using analysis of variance (ANOVA) and Fisher's protected least significance difference (PLSD) post hoc test corrected for multiple comparisons using Stat View (SAS Institute, Cary, NC). Differences of p< 0.05 were considered statistically significant. All results are presented as mean ± standard error of the mean.
Results
Weight Assessment
BALB/c
There were no significant differences in pre-experiment body weight between groups of BALB/c mice (p>0.05). Significantly more weight loss occurred in the PN-fed BALB/c mice than the Chow-fed BALB/c mice; however there was no difference in weight loss between Chow-fed mice with and without injury or PN-fed mice with and without injury. (Table 1)
Table 1.
BALB/c body weight and body weight change.
| Group (BALB/c) | n | Body Weight (g) | Weight Change (g) |
|---|---|---|---|
| Chow | 14 | 26.0 ± 0.4 | -2.3 ± 0.4 |
| Chow-Injury | 17 | 26.1 ± 0.3 | -1.6 ± 0.2 |
| PN | 20 | 25.8 ± 0.3 | -4.6 ± 0.4* |
| PN-Injury | 21 | 26.0 ± 0.3 | -4.7 ± 0.3* |
Values are means ± SEM. PN, parenteral nutrition.
p<0.01 versus Chow and Chow-Injury.
C57BL/6
There was no significant difference in pre-experiment body weight between groups of C57BL/6 mice (p> 0.05). Significantly more weight loss occurred in the PN-fed groups than the Chow-fed groups; however there were no differences in weight loss between injured and uninjured animals in each experimental diet group. (Table 2)
Table 2.
C57BL/6 body weight and body weight change.
| Group (C57BL/6) | n | Body Weight (g) | Weight Change (g) |
|---|---|---|---|
| Chow | 13 | 24.7 ± 0.3 | -1.2 ± 0.9 |
| Chow-Injury | 14 | 24.8 ± 0.3 | -0.9 ± 0.4 |
| PN | 13 | 24.2 ± 0.2 | -4.2 ± 0.3* |
| PN-Injury | 17 | 24.1 ± 0.4 | -4.1 ± 0.3* |
Values are means ± SEM. PN, parenteral nutrition.
p<0.01 versus Chow and Chow-Injury.
Respiratory IgA Response to Injury
BALB/c
Neither Chow-fed BALB/c mice nor PN-fed BALB/c mice demonstrated a change in respiratory IgA in BAL samples 8 hours following injury compared to baseline (Chow-Injury:190 ± 8 vs. Chow: 236 ± 24, p = 0.11; PN-Injury: 215 ± 21 vs. PN: 203 ± 18, p = 0.65). (Figure 1)
Figure 1.
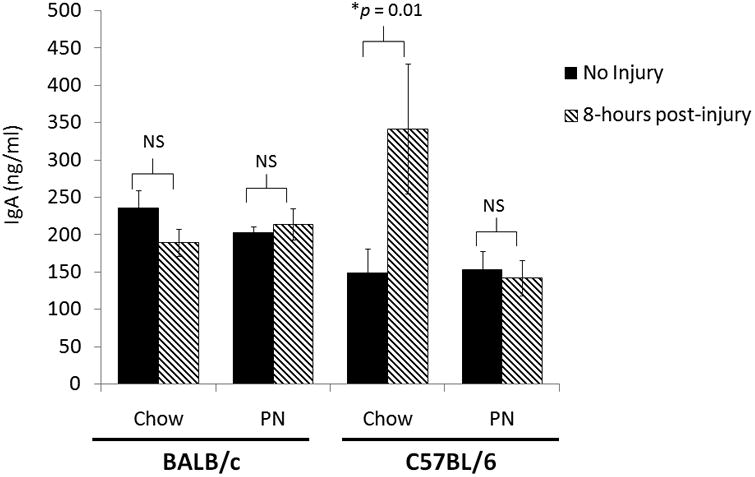
Bronchoalveolar Lavage IgA from BALB/c and C57BL/6 mice with and without Injury after Chow or Parenteral Nutrition.
IgA, immunoglobulin A; PN, parenteral nutrition; NS, non-significant (p> 0.05).
C57BL/6
Compared to uninjured controls, Chow-fed C57BL/6 mice significantly increased respiratory IgA in BAL specimens 8 hours following injury (Chow-Injury: 342 ± 87 vs. Chow: 149 ± 33, p = 0.01). PN-fed C57BL/6 mice demonstrated no increase in respiratory IgA 8 hours following injury (PN-Injury 143 ± 24 vs. PN: 153 ± 25, p = 0.90). (Figure 1)
Respiratory Cytokine Response to Injury
BALB/c
There were statistically significant increases in proinflammatory cytokines TNF-α, IL-1β and IL-6 in respiratory secretions of Chow-fed BALB/c mice 8 hours following surgical stress when compared to baseline. In PN-fed BALB/c mice, there was a significant increase in TNF-α in BAL fluid 8 hours following injury (p = 0.02). Additionally there were increases in cytokines IL-1β and IL-6 in BAL fluid of PN-fed BALB/c mice 8 hours following injury, but they did not reach statistical significance, (IL-1β: p = 0.08; IL-6:p = 0.05).(Table 3)
Table 3.
Cytokine response to injury in BAL fluid of BALB/c mice fed Chow or PN.
| Lung Cytokine | Chow | Chow-Injury | PN | PN-Injury |
|---|---|---|---|---|
| TNF-α (pg/ml) | 301 ± 39 | 512 ± 46* | 345 ± 26 | 481 ± 53† |
| IL-1β (pg/ml) | 875 ± 103 | 1550 ± 127* | 1060 ± 77 | 1349 ± 156 |
| IL-6 (pg/ml) | 44.2 ± 5.6 | 110 ± 12* | 67.7 ± 9.8 | 99.1 ± 15.8 |
Values are means ± SEM. BAL, bronchoalveolar lavage; PN, parenteral nutrition.
p<0.01 versus Chow;
p<0.05 versus PN.
C57BL/6
There were statistically significant increases in proinflammatory cytokines TNF-α, IL-1β and IL-6 in respiratory secretions of Chow-fed C57BL/6 mice 8 hours following surgical stress when compared to baseline. PN-fed C57BL/6 mice did not display change in respiratory secretion of any of these proinflammatory cytokines 8 hours following surgical stress when compared to baseline. (Table 4)
Table 4.
Cytokine response to injury in BAL fluid of C57BL/6 mice fed Chow or PN.
| C57BL/6 | Chow | Chow-Injury | PN | PN-Injury |
|---|---|---|---|---|
| TNF-α (pg/ml) | 39.8 ± 6.0 | 101.6 ± 19.6* | 56.7 ± 9.2 | 68.3 ± 11.1 |
| IL-1β (pg/ml) | 201 ± 27 | 374 ± 63* | 264 ± 39 | 316 ± 46 |
| IL-6 (pg/ml) | 15.3 ± 2.7 | 35.1 ± 5.6* | 26.8 ± 6.5 | 27.2 ± 4.0 |
Values are means ± SEM. BAL, bronchoalveolar lavage; PN, parenteral nutrition.
p<0.01 versus Chow.
Small Intestinal IgA Response to Injury
BALB/c
Compared to uninjured controls, both Chow-fed and PN-fed BALB/c mice significantly increased intestinal IgA secretion as measured by SIWF 8 hours following injury (Chow-Injury: 548 + 37 vs. Chow: 264 + 28, p< 0.0001; PN-Injury: 301 + 17 vs. PN: 150 + 12, p< 0.0001). (Figure 2)
Figure 2.
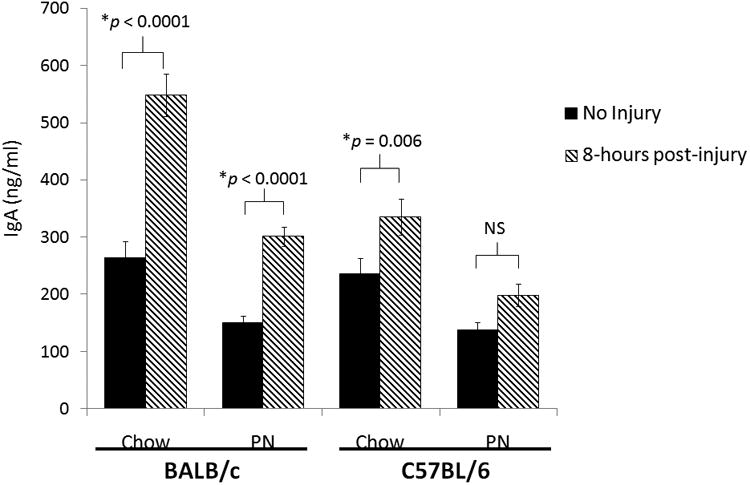
Small Intestinal Wash IgA from BALB/c and C57BL/6 mice with and without Injury after Chow or Parenteral Nutrition.
IgA, immunoglobulin A; PN, parenteral nutrition; NS, non-significant (p> 0.05).
C57BL/6
Chow-fed C57BL/6 mice demonstrated a significant increase in SIWF IgA 8 hours following surgical stress compared to baseline (Chow-Injury: 335 ± 32 vs. Chow: 236 ± 28, p = 0.01). PN-fed C57BL/6 animals had no difference in SIWF IgA levels 8 hours following surgical stress (PN-Injury: 198 ± 19 vs. PN: 138 ± 14, p = 0.10). (Figure 2)
Discussion
Inbred mouse strains allow sophisticated immunologic studies such as cell transfer experiments as well as host transgenic and gene knockout studies. Such work is impossible in outbred mice due to antigenic incompatibilities between individual animals. However, different inbred mouse strains may demonstrate different phenotypic responses to the same independent variables.18-21, 34-40 This work shows that two commonly used strains of inbred mice, BALB/c and C57BL/6, display dramatically different mucosal immunologic stress responses to injury, highlighting the importance of choosing an appropriate background strain of mice for relevant study. In this work we examined whether BALB/c or C57BL/6 mice immunologically resemble the respiratory and intestinal mucosal immune responses to injury noted to occur in humans and outbred ICR mice.1 The motives for this experiment were two-fold: firstly, many international laboratories studying mucosal immunity cannot obtain outbred mice and, secondly, outbred mice cannot be used for cell transfer experiments needed to isolate the source and site of immunologic problems occurring in our studies of PN.
IgA is the primary specific immunologic antiviral and antibacterial defense protecting mucosal surfaces such as the respiratory, gastrointestinal, and urinary tracts. In the respiratory tract, reduced IgA levels in the BAL fluid correlate with increased mucosal bacterial adherence and increased rates in pneumonia.41-43 Prior clinical work demonstrated that injury stimulates an innate respiratory IgA immune response in severely injured trauma patients within 30 hours of injury.1 This response can be experimentally reproduced with a controlled injury in ICR mice. The airway IgA increase occurs within 8 hours with airway IgA levels returning to baseline by 24 hours after this limited, controlled injury. Experimentally, this ICR airway response is cytokine dependent (TNF-α, IL-1β, and IL-6) in a manner consistent with localized airway increases in these cytokines in the human BAL samples.2, 6 Either pretreatment with PN or the administration of anti- TNF-α or anti- IL-1β blocking antibodies to ICR mice eliminate the airway IgA increases after injury.11 Experimentally, PN decreases BAL IgA levels and impairs both established anti-viral and anti-bacterial IgA mediated respiratory defenses, providing a cogent hypothesis for the increase in pneumonia documented in PN fed patients.44-49 The gastrointestinal tracts of mice also respond to injury with an innate IgA immune increase.4 The intestinal response is detectable within 2 hours of injury and continues to increase up to 8 hours following injury in mice. Unlike in the lung, gastrointestinal IgA production and secretion appears independent of the proinflammatory cytokines TNF-α, IL-1β and IL-6, however. Like the airway, PN eliminates this intestinal IgA increase.
In this experiment we evaluated immune response to injury in BALB/c and C57BL/6 mice subjected to controlled injury after administering experimental diets of Chow or PN for 5 days. Since Th2 cytokine responses typically upregulate IgA production, 29, 30, 50we hypothesized that BALB/c mice would display findings similar to our outbred mouse and human data as BALB/c are considered to immunologically favor Th2 responses to infection, immunization, and stress.51, 52 Contrary to our hypothesis, C57BL/6 mice more aptly resembled the ICR and human immune responses. The C57BL/6 mice exhibited the same innate respiratory and gastrointestinal IgA responses to injury seen in ICR mice during Chow feeding which were eliminated with PN. PN also eliminated the respiratory cytokine response to injury in C57BL/6 animals. BALB/c mice, however, displayed no significant change in respiratory IgA levels in either Chow or PN-fed animals following injury despite increase in the respiratory cytokine TNF-α levels following injury as well as non-significant increases in IL-1β and IL-6. Interestingly, the gastrointestinal IgA response remained intact in both the chow- and PN-fed groups.
The multitude of inbred mouse strains permits a huge variety of genetic experiments. However, the investigator faced with this array of options must determine which strain provides the appropriate isogenic environment to study the intervention of interest. Rarely do experimental publications justify the experimental and clinical rationale for the inbred species used in an experiment. This oversight may lead to contradictory results depending on the mouse strain used in the work due to lack of appreciation of differences in inbred mouse phenotypes. Data in standardized collections such as the Mouse Phoneme Database on The Jackson Laboratory website 53allows an educated experiment design and interpretation but it does not contain information on immune phenotypic responses to various stimuli. In our work investigating route of nutrition and respiratory and gastrointestinal responses to injury, C57BL/6 mice provide the appropriate model reflecting the experimental results found in our human and outbred mouse studies.
BALB/c and C57BL/6 mice infected with Leishmania major demonstrate classic differences in immunology between mouse strains with distinct genetic backgrounds. In these experiments, L. major infection assessed the role of CD4+ T-cell development in the host response to infectious disease.21, 54 The overarching conclusion from this model established that control of this infection and resolution of illness required an IFN-γ driven activation of macrophages, or Th1 response. C57BL/6 mice effectively mount this Th1 response to clear the infection, while BALB/c mice fail to mount a robust Th1 response and develop progressive disease. The weak Th1 response of BALB/c mice tips the immunologic balance in favor of a Th2 response, namely production of IL-4 and stimulation of immunoglobulin producing B cells, dampening cytolytic potential in addition to other defenses such as anti-tumor immunity.55
Other works link differences in cytokine and chemokine expression to phenotypic variations in inflammatory and cancer responses of these two mouse strains.34, 36, 56-61 For instance, BALB/c mice prove more fibrosis resistant, radiosensitive, and cancer susceptible than their C57BL/6 counterparts, again highlighting the power of the dampened cytolytic potential seen in Th2 predominant mice, providing an attractive explanation for divergent phenotypes.38, 56, 62 Recent speculation also suggests that differences in gut microbial composition between inbred mouse strains contribute to differences in metabolic, inflammatory, and immune states independent of experimental variables.63 However, directly linking genotypic variance to phenotypic differences has proved elusive. In our work the predominant Th2 response of BALB/c mice may allow them to propagate an IgA response to injury despite PN treatment. This response may be worth investigating further in the future.
A limitation of this study remains the inability to attribute differences in responses to specific genetic differences between these two mouse strains i.e., there is no cause and effect relationship between genetics and the outcome variables in our experiments. Other investigators struggle with the same issue, linking available cytokine milieu to effects without defining a root cause.40, 64-67 An additional limitation includes the lack of kinetic studies performed in these two inbred mouse strains. Instead, time points for sample collection were based on our findings in outbred ICR mice. The goal for this experiment was to determine if either inbred mouse strain assimilated the same response as our outbred mice and the data confirm that C57BL/6 mice exhibit the same response as outbred mice in a similar timeframe. BALB/c mice may display similar immunologic reactions to injury as outbred mice and humans but these immunologic responses would have to occur on a different time scale. Quantitative analysis of immune differences between different mouse strains was also not performed.
In conclusion, different inbred mouse strains exhibit differential phenotypic expression to stimuli which must be considered in experimental design. In this case, C57BL/6 inbred mice respond to injury in the same manner consistent with outbred ICR mouse and human data investigating respiratory and gastrointestinal tract IgA response to injury. Experimentally, PN eliminates this immune response in both male ICR and C57BL/6 mice, confirming C57BL/6 mice as a relevant model to study human mucosal immune responses after injury and nutrient manipulation. BALB/c mice respond differently than outbred ICR mice to stress and decreased enteral stimulation.
Clinical Relevancy Statement.
Animal models permit detailed mechanistic study of clinical problems such as inflammation, infection etc., which provide insights applicable to humans. Choice of a relevant model which produces similar pathophysiology is paramount to assuring relevance to the clinical condition. While outbred mice assimilate human mucosal immune responses to injury, a variety of inbred mouse strains are available to allow more detailed immunological studies but their relevance has yet to be established. This study examines the mucosal immune responses of two inbred mouse strains to injury and parenteral nutrition to determine an appropriate model which replicates human immunologic responses.
Acknowledgments
This research is supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. This research is also supported in part by National Institute of Health (NIH) Grant T32 CA090217.
Footnotes
Presented at the American Society of Parenteral and Enteral Nutrition (A.S.P.E.N.)/Clinical Nutrition Week, Las Vegas, Nevada, February 8-12, 2010.
Disclosures: The authors have no conflicts of interest to declare.
References
- 1.Kudsk KA, Hermsen JL, Genton L, Faucher L, Gomez FE. Injury stimulates an innate respiratory immunoglobulin a immune response in humans. J Trauma. 2008 Feb;64:316–323. doi: 10.1097/TA.0b013e3181627586. discussion 323-315. [DOI] [PubMed] [Google Scholar]
- 2.Jonker MA, Hermsen JL, Gomez FE, Sano Y, Kudsk KA. Injury induces localized airway increases in pro-inflammatory cytokines in humans and mice. Surg Infect. 2011 Feb;12:49–56. doi: 10.1089/sur.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano Y, Hermsen JL, Kang W, et al. Parenteral nutrition maintains pulmonary IgA antibody transport capacity, but not active transport, following injury. Am J Surg. 2009 Jul;198:105–109. doi: 10.1016/j.amjsurg.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonker MA, Hermsen JL, Sano Y, Heneghan AF, Lan J, Kudsk KA. Small intestine mucosal immune system response to injury and the impact of parenteral nutrition. Surgery. 2012 Feb;151:278–286. doi: 10.1016/j.surg.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeWitt RC, Wu Y, Renegar KB, King BK, Li J, Kudsk KA. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg. 2000 Jan;231:1–8. doi: 10.1097/00000658-200001000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonker MA, Sano Y, Hermsen JL, Lan J, Kudsk KA. Proinflammatory cytokine surge after injury stimulates an airway immunoglobulin a increase. J Trauma. 2010 Oct;69:843–848. doi: 10.1097/TA.0b013e3181c45284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okahashi N, Yamamoto M, Vancott JL, et al. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996 May;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson RJ, Marinaro M, VanCott JL, et al. Mucosal immunity: regulation by helper T cells and a novel method for detection. J Biotechnol. 1996 Jan;44:209–216. doi: 10.1016/0168-1656(95)00095-X. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Vancott JL, Okahashi N, et al. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996 Feb;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999 May;229:662–667. doi: 10.1097/00000658-199905000-00008. discussion 667-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermsen JL, Sano Y, Gomez FE, Maeshima Y, Kang W, Kudsk KA. Parenteral nutrition inhibits tumor necrosis factor-alpha-mediated IgA response to injury. Surg Infect. 2008 Feb;9:33–40. doi: 10.1089/sur.2007.029. [DOI] [PubMed] [Google Scholar]
- 12.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998 Feb;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 14.Kilian M, Russell MW. Function of mucosal immunoglobulins. In: Orga PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, CA: Academic Press; 1994. pp. 127–137. [Google Scholar]
- 15.Festing MF. Improving toxicity screening and drug development by using genetically defined strains. Methods Mol Biol. 2010;602:1–21. doi: 10.1007/978-1-60761-058-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Festing MF. Inbred strains should replace outbred stocks in toxicology, safety testing, and drug development. Toxicol Pathol. 2010 Aug;38:681–690. doi: 10.1177/0192623310373776. [DOI] [PubMed] [Google Scholar]
- 17.Crawley JN. Behavioral phenotyping of rodents. Comp Med. 2003 Apr;53:140–146. [PubMed] [Google Scholar]
- 18.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002 Nov;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J Nutr. 2004 Dec;134:3264–3269. doi: 10.1093/jn/134.12.3264. [DOI] [PubMed] [Google Scholar]
- 20.Kirk EA, Moe GL, Caldwell MT, Lernmark JA, Wilson DL, LeBoeuf RC. Hyper- and hypo-responsiveness to dietary fat and cholesterol among inbred mice: searching for level and variability genes. J Lipid Res. 1995 Jul;36:1522–1532. [PubMed] [Google Scholar]
- 21.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 22.Kirschner H, Kochen M, Hirt HM, Munk K. Immunological studies of hsv-infection of resistant and susceptible inbred strains of mice. Z Immunitatsforsch Immunobiol. 1978 Mar;154:147–154. [PubMed] [Google Scholar]
- 23.Pepose JS, Whittum-Hudson JA. An immunogenetic analysis of resistance to herpes simplex virus retinitis in inbred strains of mice. Invest Ophthalmol Vis Sci. 1987 Sep;28:1549–1552. [PubMed] [Google Scholar]
- 24.Ellermann-Eriksen S, Justesen J, Mogensen SC. Genetically determined difference in the antiviral action of alpha/beta interferon in cells from mice resistant or susceptible to herpes simplex virus type 2. J Gen Virol. 1986 Sep;67:1859–1866. doi: 10.1099/0022-1317-67-9-1859. [DOI] [PubMed] [Google Scholar]
- 25.Autenrieth IB, Beer M, Bohn E, Kaufmann SH, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994 Jun;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autenrieth IB, Bohn E, Beer M, Preger S, Heinze G, Heesemann J. Role of T-helper-cell subtypes and cytokines in immunity to Yersinia enterocolitica in susceptible and resistant strains of mice. Contrib Microbiol Immunol. 1995;13:203–206. [PubMed] [Google Scholar]
- 27.Okano M, Nishizaki K, Abe M, et al. Strain-dependent induction of allergic rhinitis without adjuvant in mice. Allergy. 1999 Jun;54:593–601. doi: 10.1034/j.1398-9995.1999.00063.x. [DOI] [PubMed] [Google Scholar]
- 28.Karupiah G. Type 1 and type 2 cytokines in antiviral defense. Vet Immunol Immunopathol. 1998 May;63:105–109. doi: 10.1016/s0165-2427(98)00086-5. [DOI] [PubMed] [Google Scholar]
- 29.Kramer DR, Sutherland RM, Bao S, Husband AJ. Cytokine mediated effects in mucosal immunity. Immunol Cell Biol. 1995 Oct;73:389–396. doi: 10.1038/icb.1995.61. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay AJ. Genetic approaches to the study of cytokine regulation of mucosal immunity. Immunol Cell Biol. 1995 Dec;73:484–488. doi: 10.1038/icb.1995.78. [DOI] [PubMed] [Google Scholar]
- 31.Lebman DA, Coffman RL. The effects of IL-4 and IL-5 on the IgA response by murine Peyer's patch B cell subpopulations. J Immunol. 1988 Sep;141:2050–2056. [PubMed] [Google Scholar]
- 32.Sitren HS, Heller PA, Bailey LB, Cerda JJ. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983 Nov-Dec;7:582–586. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 33.National Academy of Science. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy of Science; 1978. National Research Publication No. 10. [Google Scholar]
- 34.Hayashi M, Higashi K, Kato H, Kaneko H. Assessment of preferential Th1 or Th2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol Appl Pharmacol. 2001 Nov;177:38–45. doi: 10.1006/taap.2001.9286. [DOI] [PubMed] [Google Scholar]
- 35.Mähler M, Bristol IJ, Leiter EH, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998 Mar;274:G544–551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- 36.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005 Jun;288:G1328–1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 37.Ward WF, Sharplin J, Franko AJ, Hinz JM. Radiation-induced pulmonary endothelial dysfunction and hydroxyproline accumulation in four strains of mice. Radiat Res. 1989 Oct;120:113–120. [PubMed] [Google Scholar]
- 38.Zhang SB, Maguire D, Zhang M, et al. Mitochondrial DNA and functional investigations into the radiosensitivity of four mouse strains. Int J Cell Biol. 2014 doi: 10.1155/2014/850460. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asanuma H, Fujihashi K, Miyakoshi T, et al. Long- and short-time immunological memory in different strains of mice given nasally an adjuvant-combined nasal influenza vaccine. Vaccine. 2007 Sep;25:6975–6980. doi: 10.1016/j.vaccine.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 40.Güler ML, Gorham JD, Hsieh CS, et al. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996 Feb;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 41.Annane D, Clair B, Mathieu B, et al. Immunoglobulin A levels in bronchial samples during mechanical ventilation and onset of nosocomial pneumonia in critically ill patients. Am J Respir Crit Care Med. 1996 May;153:1585–1590. doi: 10.1164/ajrccm.153.5.8630606. [DOI] [PubMed] [Google Scholar]
- 42.Niederman MS, Merrill WW, Polomski LM, Reynolds HY, Gee JB. Influence of sputum IgA and elastase on tracheal cell bacterial adherence. Am Rev Respir Dis. 1986 Feb;133:255–260. doi: 10.1164/arrd.1986.133.2.255. [DOI] [PubMed] [Google Scholar]
- 43.Niederman MS, Mantovani R, Schoch P, Papas J, Fein AM. Patterns and routes of tracheobronchial colonization in mechanically ventilated patients. The role of nutritional status in colonization of the lower airway by Pseudomonas species. Chest. 1989 Jan;95:155–161. doi: 10.1378/chest.95.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996 Jun;223:629–635. doi: 10.1097/00000658-199606000-00001. discussion 635-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renegar KB, Kudsk KA, Dewitt RC, Wu Y, King BK. Impairment of mucosal immunity by parenteral nutrition: depressed nasotracheal influenza-specific secretory IgA levels and transport in parenterally fed mice. Ann Surg. 2001 Jan;233:134–138. doi: 10.1097/00000658-200101000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renegar KB, Johnson CD, Dewitt RC, et al. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001 Jan;166:819–825. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 47.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999 Feb;229:272–278. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992 May;215:503–511. doi: 10.1097/00000658-199205000-00013. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991 Aug;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 50.Lebman DA, Coffman RL. Cytokines in the mucosal immune system. In: Orga PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, CA: Academic Press; 1994. pp. 243–249. [Google Scholar]
- 51.Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014 Mar;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 52.Iwakabe K, Shimada M, Ohta A, et al. The restraint stress drives a shift in Th1/Th2 balance toward Th2-dominant immunity in mice. Immunol Lett. 1998 May;62:39–43. doi: 10.1016/s0165-2478(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 53.Laboratory TJ. Mouse Phenome Database Web Site. [Accessed August 11, 2014]; http://www.jax.org/phenome.
- 54.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002 Dec;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Morgan R, Podack ER, Rosenblatt J. B cell regulation of anti-tumor immune response. Immunol Res. 2013 Dec;57:115–124. doi: 10.1007/s12026-013-8472-1. [DOI] [PubMed] [Google Scholar]
- 56.Kolb M, Bonniaud P, Galt T, et al. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of “fibrosis-prone” and “fibrosis-resistant” mouse strains. Am J Respir Cell Mol Biol. 2002 Aug;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 57.Melgar S, Drmotova M, Rehnström E, Jansson L, Michaëlsson E. Local production of chemokines and prostaglandin E2 in the acute, chronic and recovery phase of murine experimental colitis. Cytokine. 2006 Sep;35:275–283. doi: 10.1016/j.cyto.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Koo GC, Gan YH. The innate interferon gamma response of BALB/c and C57BL/6 mice to in vitro Burkholderia pseudomallei infection. BMC Immunol. 2006;7:19. doi: 10.1186/1471-2172-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B, Koo GC, Yap EH, Chua KL, Gan YH. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect Immun. 2002 Feb;70:504–511. doi: 10.1128/IAI.70.2.504-511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giavina-Bianchi P, Kalil J, Rizzo LV. Development of an animal model for allergic conjunctivitis: influence of genetic factors and allergen concentration on immune response. Acta Ophthalmol. 2008 Sep;86:670–675. doi: 10.1111/j.1600-0420.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 61.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006 Jul;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koch J, Hau J, Pravsgaard Christensen J, Elvang Jensen H, Bagge Hansen M, Rieneck K. Immune cells from SR/CR mice induce the regression of established tumors in BALB/c and C57BL/6 mice. PLoS One. 2013 doi: 10.1371/journal.pone.0059995. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulati AS, Shanahan MT, Arthur JC, et al. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS One. 2012 doi: 10.1371/journal.pone.0032403. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charles PC, Chen X, Horwitz MS, Brosnan CF. Differential chemokine induction by the mouse adenovirus type-1 in the central nervous system of susceptible and resistant strains of mice. J Neurovirol. 1999 Feb;5:55–64. doi: 10.3109/13550289909029746. [DOI] [PubMed] [Google Scholar]
- 65.Mähler M, Bristol IJ, Sundberg JP, et al. Genetic analysis of susceptibility to dextran sulfate sodium-induced colitis in mice. Genomics. 1999 Jan;55:147–156. doi: 10.1006/geno.1998.5636. [DOI] [PubMed] [Google Scholar]
- 66.Gorham JD, Güler ML, Steen RG, et al. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci U S A. 1996 Oct;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imai Y, Nagai R, Ono Y, et al. Production of secretory immunoglobulin A against Shiga toxin-binding subunits in mice by mucosal immunization. Infect Immun. 2004 Feb;72:889–895. doi: 10.1128/IAI.72.2.889-895.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


