Abstract
Obesity is associated with podocyte injury and the development of proteinuria. Elevated plasma free fatty acid is one of the characteristics of obesity and has been linked to podocyte dysfunction. However, the mechanisms remain unclear. In the current study, we examined the effect of saturated free fatty acid (FFA) on human podocyte apoptosis and function in vitro. The mechanism and its in vivo relevance were also determined. We found that FFA treatment induced human podocyte apoptosis and dysfunction, which was associated with increased expression of a matricellualr protein-thrombospondin1 (TSP1). FFA stimulated TSP1 expression in podocytes at the transcriptional levels through activation of MAPK pathway. Addition of purified TSP1 to cell culture media induced podocyte apoptosis and dysfunction. Tis effect is though a TGF-β independent mechanism. Moreover, peptide treatment to block TSP1 binding to its receptor-CD36 attenuated FFA induced podocyte apoptosis, suggesting that TSP1/CD36 interaction mediates FFA-induced podocyte apoptosis. Importantly, using a dietinduced obese mouse model, in vivo data demonstrated that obesity-associated podocyte apoptosis and dysfunction were attenuated in TSP1 deficient mice as well as in CD36 deficient mice. Taken together, these studies provide novel evidence that the interaction of TSP1 with its receptor CD36 contributes to obesity - associated podocytopathy.
Keywords: Podocyte apoptosis, Free fatty acid, CD36, Obesity, TSP1
1. Introduction
In the United States, about one third of adults are overweight (25 kg/m2≤body mass index (BMI) ≤29.9 kg/m2) and one third are obese (BMI ≥30 kg/m2) [1]. With the recent elevation in the prevalence of obesity worldwide, renal complication emerges to be an important issue for individuals who suffer from this disease. Obesity increases the susceptibility of chronic kidney disease (CKD) because obesity largely mediates diabetes and hypertension, which are the first two risk factors for CKD. Besides this, obesity also has its independent effects on renal damage, known as hyperleptinemia and hyperlipidemia [2]. Albuminuria is one the most common symptoms in people with obesity-associated glomerular injury, which is characterized as glomeruli enlargement, focal segmental glomerulosclerosis, basement membrane thickening, and podocyte damage [3]. Recent studies focusing on podocyte physiology have indicated that podocyte structural and functional integrity is required for preventing glomerular albumin leakage and subsequent albuminuria [4, 5]. Previous study suggested that high glucose-induced oxidative stress initiated podocyte apoptosis and depletion both in vivo and in vitro [6], which represents an early mechanism driving diabetic nephropathy. However, the exact mechanism whereby podocytes were destroyed under obese condition is still poorly understood.
Thrombospondin-1 (TSP1) is a multifunctional extracellular matrix protein and can be produced by kidney cells, including podocytes, mesangial cells and tubular cells. Accumulating evidence suggest that through activating latent transform growth factor-beta (TGF-β), TSP1 plays an important role in the development of several kidney diseases, including diabetic nephropathy, obstructive kidney disease and renal ischemia-reperfusion injury [7–10]. Our previous study demonstrated that elevated TSP1 involved in insulin resistance and adipose tissue inflammation by regulating macrophage accumulation in a diet-induced obese mouse model [11]. Moreover, we have revealed that obesity associated renal hypertrophy, albuminuria and fibrosis was abolished in TSP1 deficient mice [12], suggesting the important role of up-regulated TSP1 in obesity-associated kidney damage. Importantly, both in vivo and in vitro evidence indicated that TSP1 induces endothelial cell apoptosis through mitochondrial-dependent and -independent pathway by binding to CD36 [13, 14], which expresses on the surface of many types of cells, including podocyte [15, 16]. Based on these reports, we hypothesize that increased TSP1 mediates podocyte damage through a CD36-dependent manner under obese conditions. To test this hypothesis, in current study, saturated free fatty acid (FFA, palmitate) or human recombinant TSP1 was used to induce human podocyte injury. A peptide to block TSP1 binding to CD36 was used in the mechanism studies. Moreover, the in vivo relevance was determined by using a diet-induced obesity mouse model in either TSP1 deficient mice or CD36 deficient mice.
2. Materials and methods
2.1. Human podocyte culture and treatments
Immortalized human podocytes (generously provided by Dr. Moin Saleem from Bristol Royal Hospital for Children, Bristol, UK) were used. These cells were cultured and differentiated as described previously [17]. These podocytes were propagated at 33 °C in medium with RPMI medium1640 (Gibico), 10% fetal bovine serum (FBS, Gibico), 100U/ml penicillin-streptomyci (Gibico) and 1% insulin transferrin selenium A (Gibico). To induce differentiation, the podocytes were transferred to a 37 °C condition for 10–14 days before starting experiment.
After starving in RPMI-1640 medium containing 0.5% bovine serum albumin (BSA, Sigma) for 24 h, podocytes were undertook the following treatment: (a) podocytes were treated with 25 µM or 125 µM of palmitate (Sigma) for 24 h and equivalent amounts of fatty acid-free BSA were added to the control group. (b) podocytes were treated with 1 µg/ml of TSP1 (R&D System) for 24 h in the presence or absence of anti-TGF-β1,2,3 antibody (15 µg/ml from R&D system), and equivalent amounts of PBS were added to the control group. (c) podocytes were pre-incubated for 2 h with the following MAPKs inhibitors: PD98059 (ERK p42/44 inhibitor, at 10−5 mol/L, Sigma), SB202190 (p38 inhibitor, at 10−5 mol/L, Sigma) and SP600125 (JNK inhibitor, at 10−5 mol/L, Sigma) and then treated with 125 µM of palmitate for 24 h. (d) podocytes were preincubated with 2 µg/ml CD36 specific peptide (YRVRFLAKENVTQDAEDN, p(93–110) [18, 19] to block TSP1 binding to CD36) or scrambled control peptide (RFAYLRKNVTENDEQAVCD) (from American Peptide Company Inc.) for 2 h, followed by treatment with 125 µM of palmitate for another 24 h. After treatment, cells were harvested for podocyte apoptotic and functional markers detection, as well as TSP1 expression in both mRNA levels and protein levels.
2.2. Isolation of murine podocytes and treatments
Isolation of podocytes from CD36 knockout mice (on C57BL6/J background, kindly provided by Dr. Deneys van der Westhuyzen at University Kentucky), TSP1 knockout mice (Jackson laboratory), or control wild type mice was performed as described previously [20, 21]. Briefly, glomeruli were isolated from mice [21] and plated on collagen type I-coated dishes at 37°C in RPMI-1640 medium with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 mM HEPES, 1mM sodium bicarbonate, and 1mM sodium pyruvate to allow glomerular podocytes to grow out. Subculture of primary podocytes was performed by detaching the glomerular cells with 0.25% trypsin-EDTA, followed by sieving thought 40 µm cell strainer, and cultured on type 1 collagen coated dishes. Podocytes of passages 1 or 2 were used for the experiments.
CD36−/− podocytes or wild type podocytes were treated with 125 µM of palmitate (Sigma) or control BSA for 24 h. After treatment, cells were harvested. TSP1 expression in both mRNA levels and protein levels were determined by PCR and western blotting, respectively. TSP1−/− podocytes or wild type podocytes were treated with 125 µM of palmitate (Sigma) or control BSA for 24 hours. After treatment, caspase 3 activity was analyzed by using caspase-3 colorimetric assay kit (R&D). BSA filter assay was also analyzed in these cells.
2.3. Experimental animals and protocols
All experiments involving mice conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Kentucky Institutional Animal Care and Use Committee. Male TSP1 −/− mice (on C57BL6/J background, kept in our lab) and CD 36 −/− mice (on C57BL6/J background, kindly provided by Dr. Deneys van der Westhuyzen at University Kentucky) at eight weeks of age and age-matched wild type littermate controls were used [11, 12, 22]. Mice were housed in a temperature controlled room with a 12 hour light/dark cycle. Mice were fed a low fat (LF) (10% kcal as fat; D12450B; research Diets, Inc, NJ) or a high fat (HF) diet (60% kcal as fat; D12492, research Diet, Inc, NJ) for 16 weeks. Each group contained 6–10 mice. The data relating to the metabolic phenotype of these mice was previously published [11, 12, 22] and the kidney samples of these mice were further analyzed in the current study.
2.4. Podocyte BSA filter assay
The transepithelial permeability of control and FFA treated podocytes was assessed by measuring the passage of BSA across the human podocyte monolayer as described previously [23]. Human podocytes at density of 5×105 were seeded into collagen-coated transwell-Col PTFE filter (3 µm pore, Corning) and cultured under differentiating conditions for 10–14 days. Cells were then treated with 125 µM of palmitate or control BSA for 24 h. After treatment, cells were washed twice with PBS. The upper compartment was then refilled with 0.5 ml RPMI 1640 and the lower compartment with 1 ml BSA medium (RPMI 1640 supplemented with 40mg/ml BSA) and incubated for 2 h or as indicated at 37°C. Total protein concentration in the upper compartment was determined using a Bio-Rad protein assay (Bio-Red Laboratories) [24].
2.5. mRNA stability assay
After starving for 24 h, human podocytes were treated with 125µM of palmitate or control BSA for another 24 h. Then media were removed and actinomycin D (5 µg/ml, Sigma) was added (designated as t=0). After different periods of incubation, cells were harvested. Real-time PCR analysis for TSP1 mRNA was performed. Measurement of the ratio of TSP1/18S at t=0 (from actinomycin D treatment) was assigned a relative value of 100% as we did before [25].
2.6. TSP1 promoter-reporter constructs and luciferase assay
TSP1 promoter-luciferase reporter constructs [TSP1 (−2,033)] were generated using pGL3-basic vector (Promega) as described previously [26]. Human podocytes were cultured in 12-well plate and transiently transfected using Effectene transfection reagent (Qiagen) with 0.3 µg of TSP1 promoter luciferase reporter plasmid. pRL-SV40 (0.006 µg, Promega) was used as an internal control for transfection. Transfected cells were treated with 125 µM of palmitate or control BSA for 24 h, and luciferase activities were analyzed using the dual-luciferase assay kit (Promega) according the manufacture’s protocol as described previously [25].
2.7. Renal immunohistochemical and immunofluorescence staining
For immunohistochemical staining: kidney tissue sections were deparaffinized in xylene, and were rehydrated in graded mixtures of ethanol/water. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min at room temperature. The slides were placed in PBS buffer containing 5% Bovine Serum Albumin (BSA) for 30 min. Anti-cleaved caspase-3 (Cell signaling) antibody was applied for 1 hour at room temperature. A negative control was included by substituting control IgG for the primary antibody. After washing with PBS, biotinylated secondary antibody was applied for 30 min. After another 15 min washing, an avidin-biotinperoxidase complex was applied to the slides for 30 min. The slides were washed once again with PBS before color development with DAB using Vectastain ABC system (Vector Lab). For immunofluorescence staining: frozen kidney samples were sectioned and fixed in cold acetone for 10–15 minutes. After blocking in 5% BSA, slides were incubated with anti-WT1 antibody (Santa Cruz) for 1 hour and then with Alex Fluor 568 conjugated secondary antibody (Life Technologies) for 45 minutes. After washing, slides were incubated with DAPI for 5 minutes and coverslipped. The images were taken under fluorescent microscope and WT1 positive cells in glomerulus were counted.
2.8. Apoptosis detection
TUNEL staining
Apoptotic nuclei were detected using a transferase-mediated dUTP nick-end labeling (TUNEL) staining of cultured podocyte or kidney sections. TUNEL staining was performed according to the manufacturer's instructions (Roche). For podocyte staining, slides with cells were rinsed with PBS. 50 µl of TUNEL reaction mixture or labelled solution was added on the section and incubated for 60min at 37°C in the dark. After washing with PBS, sections were mounted with DAPI mounting medium and then analyzed under a fluorescence microscope. For kidney tissue, 4 µm paraffin-embedded kidney sections were deparaffinized with xylene and rehydrated through a graded series of ethanol. After blocking in 0.1 M Tris-HCl containing 3% BSA and 20% FBS at room temperature for 30 min, 50 µl of TUNEL reaction mixture or labelled solution was added on the section and incubate for 60min at 37°C in a humidified atmosphere in the dark. After counter staining for nuclei, slides were dehydration, cleared and mounted. Cells with brown granules in nuclei were TUNEL positive cells and TUNEL positive podocytes were counted in 30 glomeruli of kidney section. The average numbers of TUNEL-positive podocytes per glomerulus was calculated.
Annexin V and propidium iodide (PI) staining
After treatment, cells were harvested and washed in PBS and suspended in Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4). Annexin V Alexa Flour 488 (Life technology) was added and cells were incubated in the dark for 15 minutes at room temperature. Then, 1: 10 diluted propidium iodide (Sigma) were added into cells and incubated in the dark for 15 minutes at room temperature. After washing, cells were collected and analyzed by flow cytometry (using service from the Flow Cytometry Core Facility at University of Kentucky). Annexin V positive and PI negative cells were considered apoptotic.
2.9 Caspase-3 activity detection
Caspase-3 activity was analyzed by using caspase-3 colorimetric assay kit (R&D). Add 50 µl of cell lysate or kidney cortex homogenate (100–200 µg of total protein) into each well of a 96 well flat bottom microplate and 50 µl 2 × reaction buffer 3. Incubate the plate at 37°C for 1–2 hours after adding 50 µl of caspase-3 colorimetric substrate into each well. Read the plate on a microplate reader using a wavelength of 405 nm and the OD values were used for further analyzing.
2.10. Western blotting analysis
Mouse kidney cortex or podocytes were homogenized in lysis buffer. After concentration being measured, protein was subjected to SDS-PAGE gel under reducing condition and transferred onto a nitrocellulose membrane. After blocking with 5% milk, the membrane was incubated with anti-GAPDH (Millipore), anti-TSP1 (Thermo Scientific), anti-WT1 (Santa Cruz), anti-Nephrin (Santa Cruz), anti-cleaved caspase-3 (Cell signaling), anti-caspase-3 (Cell signaling) and anti- BAD (Cell signaling) antibodies at 4°C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Jackson Labs). The reaction was visualized using an enhanced chemiluminescence system (Pierce). Immunoblots were analyzed by scanning densitometry and quantified by Quantity One gel Analysis software (Bio-Rad Laboratories).
2.11. Real-time PCR
Total RNA was isolated from glomeruli or podocytes using TRIzol reagent (Invitrogen) and treated with DNaseI (Roche). The treated RNA was cleaned up using an RNeasy kit (Qiagen). Two micrograms of total RNA was used for cDNA synthesis with a High Capacity cDNA Reverse Transcription kit (Invitrogen). Real-time PCR analyses were performed using a SYBR Green PCR Master Mix kit with a MyiQ Real-time PCR Thermal Cycler (Bio-Rad Laboratories). All reactions were performed in triplicate in a final volume of 25 µl. Dissociation curves were run to detect nonspecific amplification, and we confirmed that single products were amplified in each reaction. The quantities of each test gene and internal control 18S RNA were then determined from the standard curve using MyiQ system software, and mRNA expression levels of test genes were normalized to 18S RNA levels as described previously. The primer sequences were illustrated in Table 1.
Table 1.
Primer sequences used in the study
| Genes | Forward (5'–3') | Reverse (5'–3') |
|---|---|---|
| hWT1 | TACAGATGCATAGCCGGAAGCACA | TCACACCTGTGTGTCTCCTTTGGT |
| hCD36 | CCCTGTTACTACCACAGTTG | ATGTCGCAGTGACTTTCC |
| hNephrin | CTCGGACCAAACCAACAT | CCTCATACCTGATGCAGAAC |
| hSynaptopodin | AGGTGAGATGCAGCACACTCCTAA | ATGAGCAGGGAGCTGGACATGAAA |
| hTSP1 | TTCCGCCGATTCCAGATGATTCCT | ACGAGTTCTTTACCCTGATGGCGT |
| mCD36 | ACTGGTGGATGGTTTCCTAGCCTT | TTTCTCGCCAACTCCCAGGTACAA |
| mNephrin | ACAAGAAGCTCCACGGTTAGCACA | AGGCTTGGCGATATGACACCTCTT |
| mSynaptopodin | GTCTCCAGACATCTTCCCTTTC | GACTTCCCACCCAGGTTTATT |
2.12. Statistical analysis
Data are the mean ± SE. Differences between groups were determined by one-way ANOVA followed by Turkey's post hoc tests or Student's t-test as appropriate. The significance level was P <0.05.
3. RESULTS
3.1. Saturated free fatty acid (FFA) induced human podocyte apoptosis and increased podocyte permeability
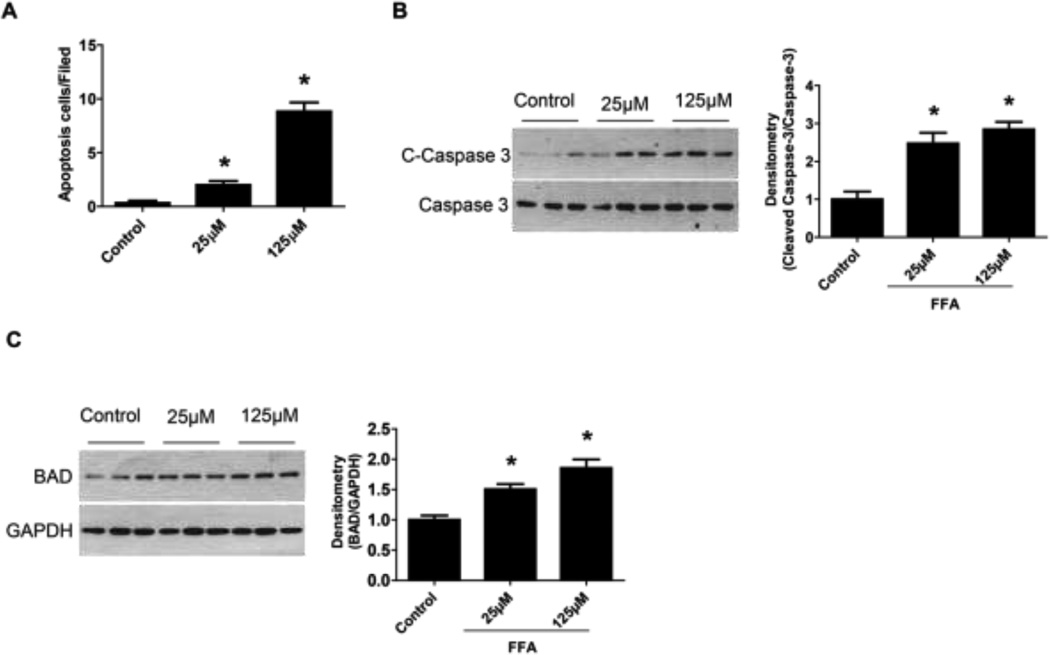
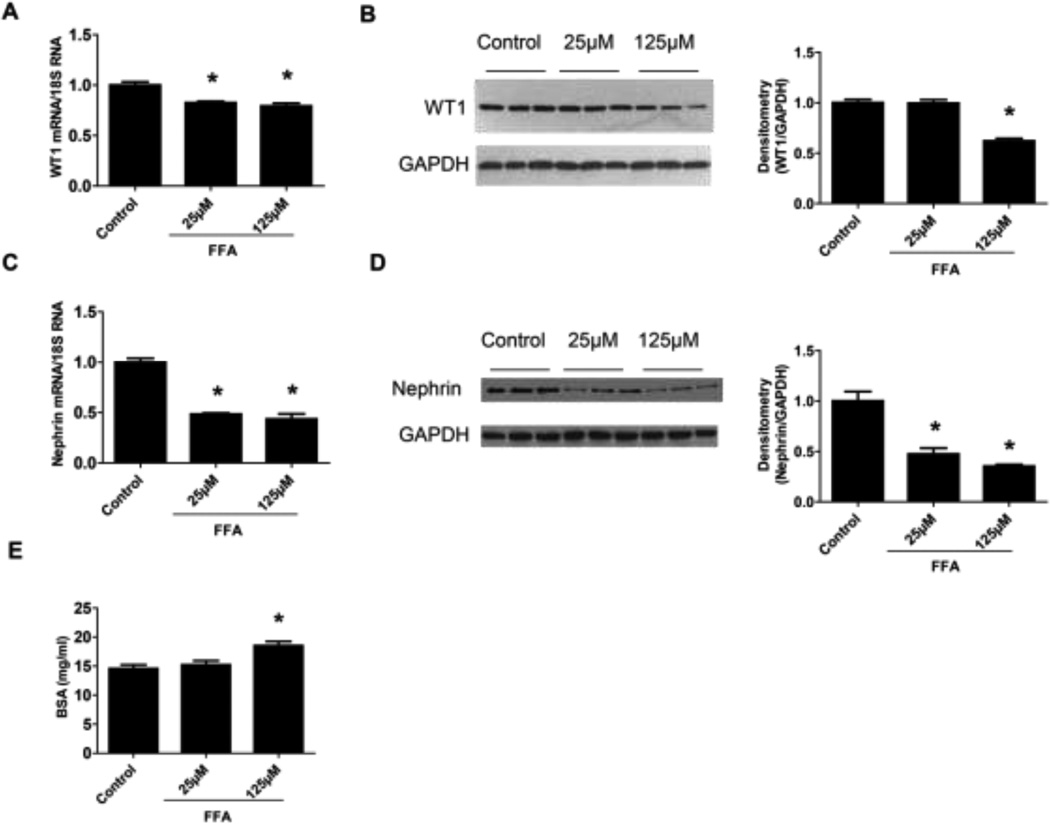
Previous studies reported that immortalized murine podocytes are susceptible to the saturated FFA (palmitic acid) induced lipotoxicity and undergo apoptosis [27–29]. In the current study, similarly, we found that saturated FFA (palmitate) treatment induced immortalized human podocytes apoptosis. TUNEL positive cells were increased by FFA treatment (Figure 1A). The protein levels of apoptotic cell markers including cleaved caspase-3 and bcl-2-associated death promoter protein (BAD) were significantly increased in FFA treated human podocytes (Figure 1B–C). Conversely, podocyte functional markers including WT1 and Nephrin, were decreased by FFA treatment (Figure 2A–D). Moreover, we determined the effect of FFA on podocyte permeability by measuring the transepithelial passage of BSA across differentiated podocytes grown on Transwell chambers. As shown in Figure 2E, FFA treatment significantly increased albumin passage through podocytes, suggesting increased podocyte permeability.
Figure 1. Free fatty acid (FFA) induced human podocyte apoptosis.
Podocytes were treated with 25 µM or 125 µM of palmitate for 24 h and then tunnel staining for podocytes was undertaken. Semiquantitative analysis was performed by calculating the apoptosis cells/filed (A). In addition, cleaved-caspase 3 (B) and bcl-2-associated death promoter protein (BAD) (C) protein levels were analyzed by Western blot assay. Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 vs. control group.
Figure 2. FFA induced podocyte functional changes.
Podocyte functional markers, WT1 (A, B) and Nephrin (C, D), were examined by Real-time PCR and Western blot assay, respectively. In addition, podocyte BSA filter assay was performed (E). Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 vs. control group.
3.2. Saturated FFA treatment stimulated thrombospondin 1 (TSP1) expression in human podocytes at transcriptional level and MAPK pathway was involved
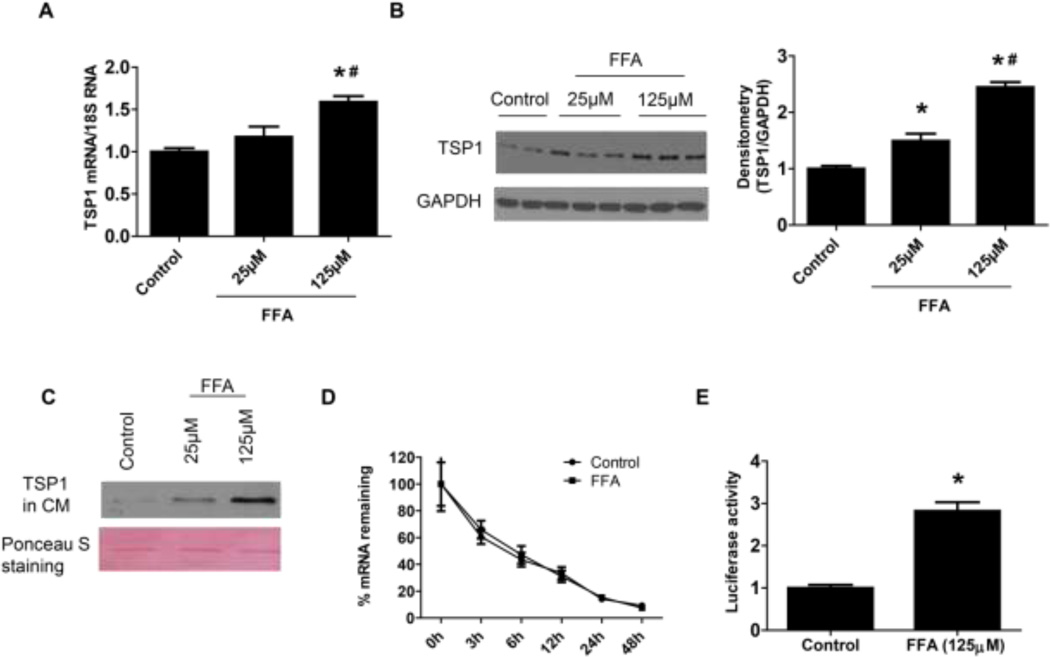
TSP1 is an important player in obesity and diabetes associated renal complications [8, 12, 26, 30–32]. Moreover, saturated FFA stimulated TSP1 expression in macrophages [33]. Therefore, we determined whether FFA could stimulate TSP1 expression in podocytes. As shown in Figure 3 A–B, FFA treatment stimulated TSP1 expression (mRNA and protein levels) in human podocytes. TSP1 protein levels in the conditioned media were also increased by FFA treatment (Figure 3 C). Moreover, to determine whether FFA induced TSP1 gene expression is at transcriptional level, TSP1 mRNA stability was tested. After 24 h of FFA treatment, actinomycin D was added to cell culture media to inhibit de novo RNA synthesis. After designated time points, cells were harvested for analyzing TSP1 mRNA levels. As shown in Figure 3D, TSP1 mRNA stability was unaffected by FFA treatment, suggesting that FFA-mediated increase in TSP1 mRNA levels is not due to the alteration in mRNA stability and further supporting the transcriptional mechanism. Thus, the effect of FFA treatment on the transcriptional activity of the TSP1 promoter was determined using the construct and method as previously described [26]. A full-length human TSP1 promoter-luciferase reporter construct was transiently transfected into podocytes. We found that 24-h FFA treatment significantly stimulated the transcriptional activity of the TSP1 promoter (Figure 3E). Taken together, these data suggest that FFA treatment stimulated TSP1 gene expression in podocytes at the transcriptional level.
Figure 3. FFA treatment stimulated TSP1 expression in podocyte at the transcriptional level.
FFA treated human podocytes were harvested for analyzing TSP1 expression in mRNA (A) and protein (B) levels by Real-time PCR and Western blot assay, respectively. TSP1 protein levels in the conditioned media (CM) were determined by immunoblotting. The ponceau S staining was shown as loading control (C). The effect of FFA on TSP1 mRNA stability was determined by adding actinomycin D to inhibit de novo RNA synthesis after 24-h FFA treatment (D) and the effect of FFA on TSP1 transcriptional activity was tested by transfection with a mouse full-length TSP1 promoter-luciferase before 24-h FFA treatment (E). Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 vs. control group; #, P <0.05 vs. 25 µM group.
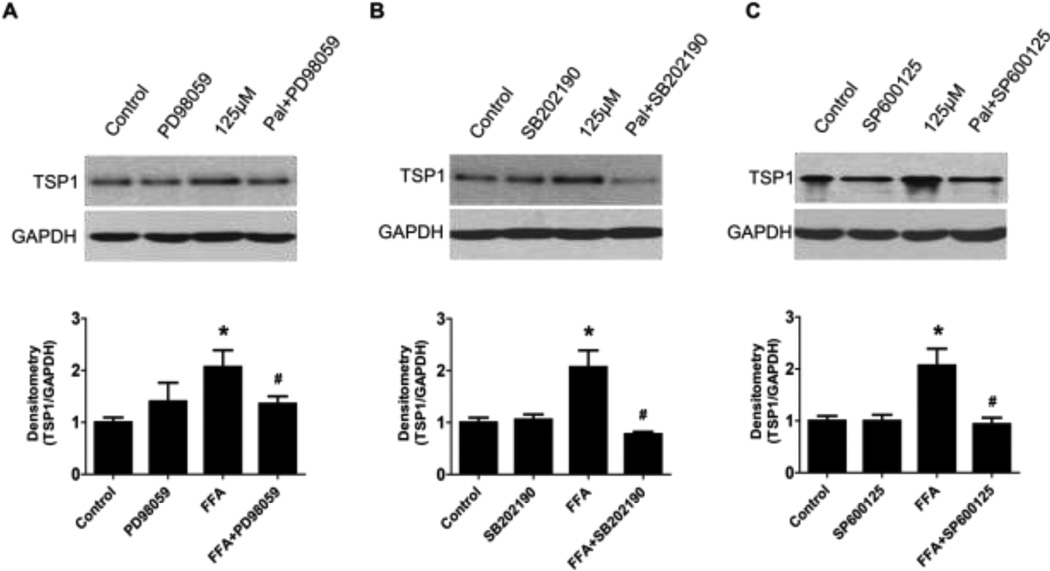
The full-length human TSP1 promoter contains binding sites for many transcription factors such as MAPK targets of c-jun, c-fos, CREB, and c-myc. Moreover, ERK/JNK signaling pathways have been shown to involve in leptin-induced TSP1 gene expression [34]. To determine which signaling pathway is involved in FFA-induced TSP1 expression in podocytes, pathway inhibitors were utilized. Human podocytes were pre-incubated with MAPKs inhibitors including PD98059 (ERK p42/44 inhibitor, at 10−5 mol/L), SB202190 (p38 inhibitor, at 10−5 mol/L) and SP600125 (JNK inhibitor, at 10−5 mol/L) for 2 hours and then treated with 125 µM of FFA for 24 h. As shown is Figure 4, increased TSP1 expression by FFA treatment were blocked by PD98059 (Figure 4A), SB202190 (Figure 4B) and SP600125 (Figure 4C), suggesting ERK and p38 MAPK dependent pathways.
Figure 4. FFA treatment stimulated TSP1 expression in podocyte through activation of MAPK pathways.
Podocytes were preincubated for 2 h with the following MAPKs inhibitors: PD98059 (ERK p42/44 inhibitor, at 10−5 mol/L), SB202190 (p38 inhibitor, at 10−5 mol/L) and SP600125 (JNK inhibitor, at 10−5 mol/L) and then treated with 125 µM of palmitate for 24 h. After that TSP1 protein levels were determined by Western blot assay. Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 vs. control group; #, P <0.05 vs. FFA group.
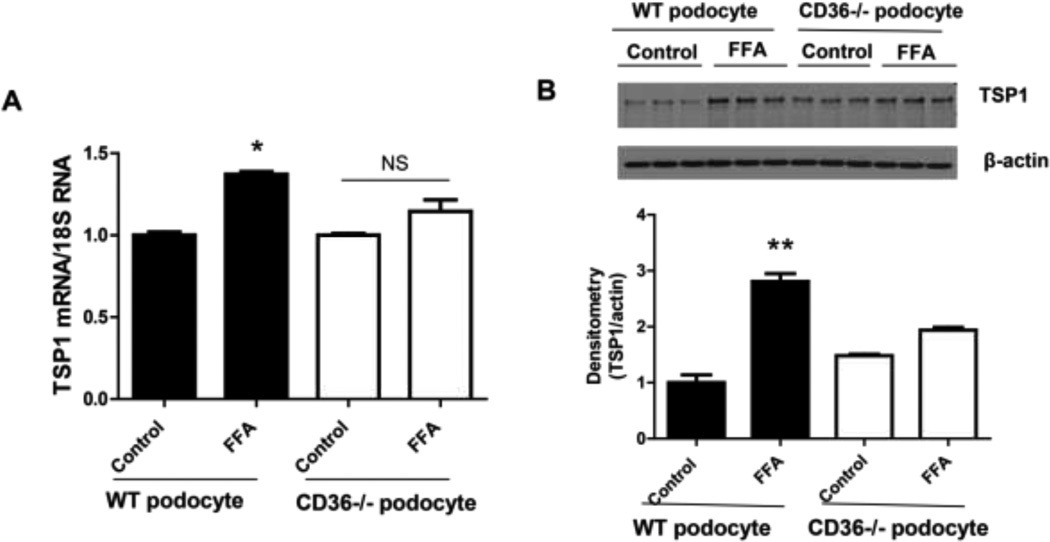
To further determine the mechanisms of FFA mediated MAPK activation and to determine whether FFA stimulates TSP1 gene expression after uptake into cells through CD36-the fatty acid translocase, we isolated podocytes from CD36 null mice and wild type mice. The effect of FFA on TSP1 expression in these cells was determined. As shown in Figure 5, TSP1 mRNA or protein levels were not significantly altered in these cells after FFA treatment. This data suggest that FFA stimulated TSP1 gene expression after uptake into podocyte through CD36. Based on these observations, we suggest that FFA is uptaken into podocyte through CD36 and activates MAPK pathway, leading to increased TSP1 gene transcription.
Figure 5. FFA induced TSP1 gene expression was abolished in CD36 deficient podocytes.
Podocytes were isolated from wild type or CD36 deficient mice and treated with 125 µM of palmitate for 24 h. After treatment, TSP1 mRNA (A) and protein levels (B) were determined by real-time PCR and western blotting, respectively. Data are presented as mean ± SE (n = 3 individual experiments). *P <0.05 and ** P<0.01 vs. control of WT podocyte
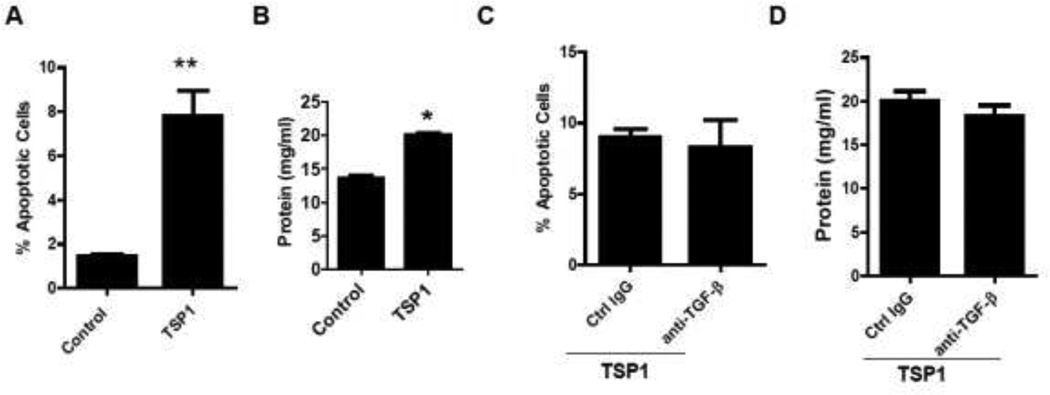
3.3. Recombinant TSP1 treatment induced human podocyte apoptosis and increased podocyte permeability through a TGF-β independent mechanism
We determined whether TSP1 treatment could induce human podocytes apoptosis and functional changes as shown in FFA treatment. Recombinant human TSP 1 was added to the differentiated human podocytes for 24 hours. Annexin V/PI staining and podocyte BSA filter assay were performed. As shown in Figure 6A, Percent of apoptotic cells (Annexin V positive staining and PI negative staining) was increased after TSP1 treatment, indicating that TSP1 treatment induced human podocyte apoptosis. In addition, TSP1 treatment significantly increased albumin passage through podocytes, suggesting increased podocyte permeability (Figure 6B). The mechanisms of TSP1 induced podocyte injury were further determined. TSP1 is an important regulator for latent TGF-β activation [35] and TGF-β induces podocyte apoptosis [36, 37]. Therefore, we determined whether TSP1-induced podocyte apoptosis is through activation of TGF-β dependent pathway. Functional blocking anti-TGF-β antibody (15 µg/ml, R&D system) was used to pretreat human podocyte. The effect of TSP1 on podocyte apoptosis and function was examined. As shown in Figure 6C–D, anti-TGF-β antibody had no effect on TSP1-induced podocyte apoptosis or functional changes, suggesting that TSP1 induces podocyte apoptosis or dysfunction through a TGF-β independent pathway.
Figure 6. TSP1 treatment induced podocyte apoptosis and functional change through a TGF-β independent pathway.
Human podocytes were treated with TSP1 (1µg/ml) for 24 h. Then Annexin V/PI staining for podocytes (A) and podocyte BSA filter assay (B) were performed. Human podocytes were pretreated with anti-TGF-β 1−3 antibody (15µg/ml) or control IgG for 30 minutes and then treated with TSP1 for 24 hours. After treatment, apoptotic cells (C) and BSA filter assay (D) were analyzed. Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 and **, P<0.01 vs. control group
3.4. TSP1 mediated saturated FFA induced podocyte apoptosis through interaction with CD36
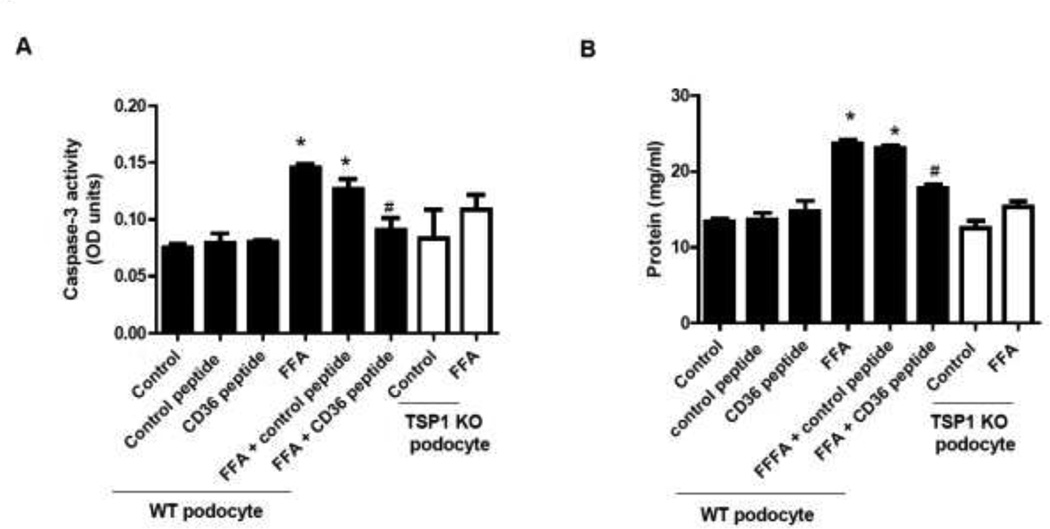
CD36 is an integral membrane protein existing on the surface of many cell types including podocytes. CD36 binds to many ligands including TSP1, FFA, and collagen et al [15, 38, 39]. It has been shown that CD36 mediates apoptosis signaling induced by TSP1 in endothelial cells [13], by ox-LDL in macrophages [40], and by advanced end product (AGE) or palmitate in tubular cell [38]. CD36 interacts with type 1 repeats domain of TSP1. The residues on CD36 molecule that specifically interact with TSP1 have been identified-called CD36 peptide (p93–110) [18, 19]. This CD 36 peptide has been shown to inhibit the effect of TSP1/CD36 interaction on TSP1 mediated macrophages activation in vitro [41, 42]. To determine whether TSP1 interaction with CD36 mediated FFA-induced podocyte apoptosis, podocytes were treated with FFA in the presence of CD36 peptide or scrambled control peptide for 24 hours. After treatment, caspase 3 activity and podocyte BSA filter assay were performed. In order to confirm the TSP1 mediated effect in the assay, TSP1 deficient podocytes were also included. As shown in Figure 7A–B, caspase 3 activity and BSA leakage from podocytes were increased by FFA treatment. This FFA mediated effect was abolished in TSP1 deficient podocytes or in wild type podocytes after exposed to CD36 peptide. In addition, CD36 peptide treatment had no effect on FFA-mediated increase in TSP1expression in podocytes (data not shown). Taken together, these data suggest that interaction between TSP1 and CD36 plays a role in FFA-induced podocyte apoptosis and dysfunction.
Figure 7. TSP1/CD36 interaction mediated FFA induced podocyte apoptosis and dysfunction.
Wild type podocytes were preincubated with 2 µg/ml CD36 peptide or control, followed by 24 h-treatment with FFA. TSP1 KO podocytes were treated with FFA for 24 hours. After treatment, caspase 3 activity (A) and podocyte BSA filter assay (B) were performed. Data are presented as mean ± SE (n = 3 individual experiments). *, P <0.05 vs. control group; #, P <0.05 vs. FFA group.
3.5. Obesity-induced podocyte apoptosis and dysfunction were attenuated in TSP1 −/− mice or CD36 −/− mice
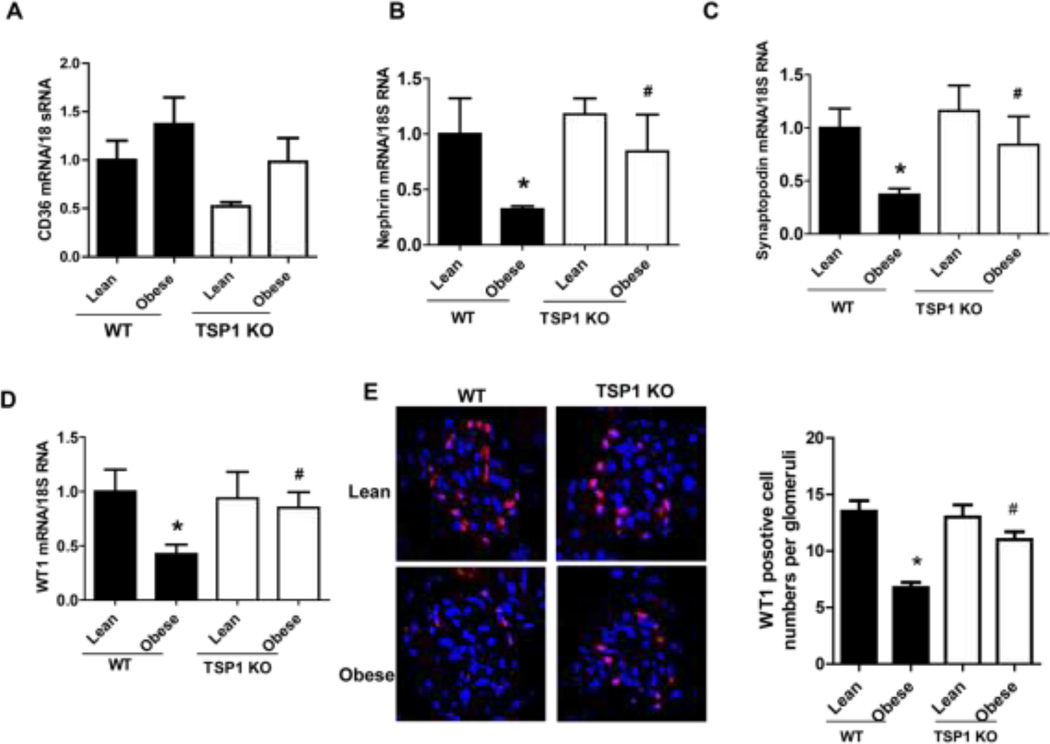
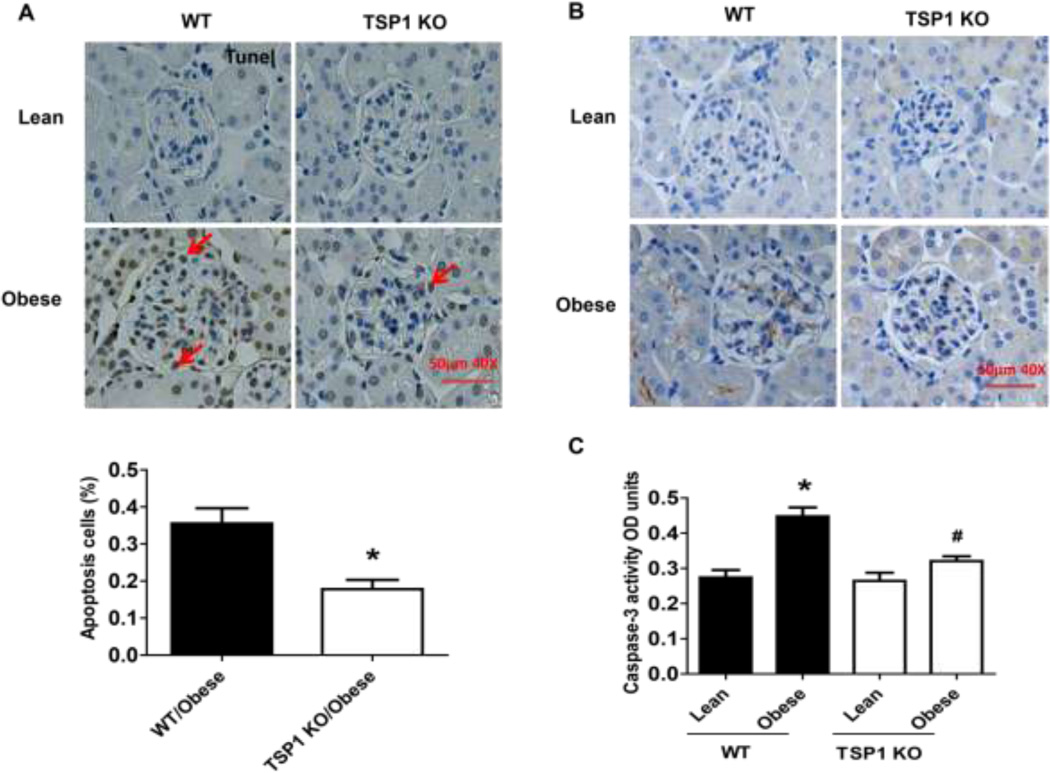
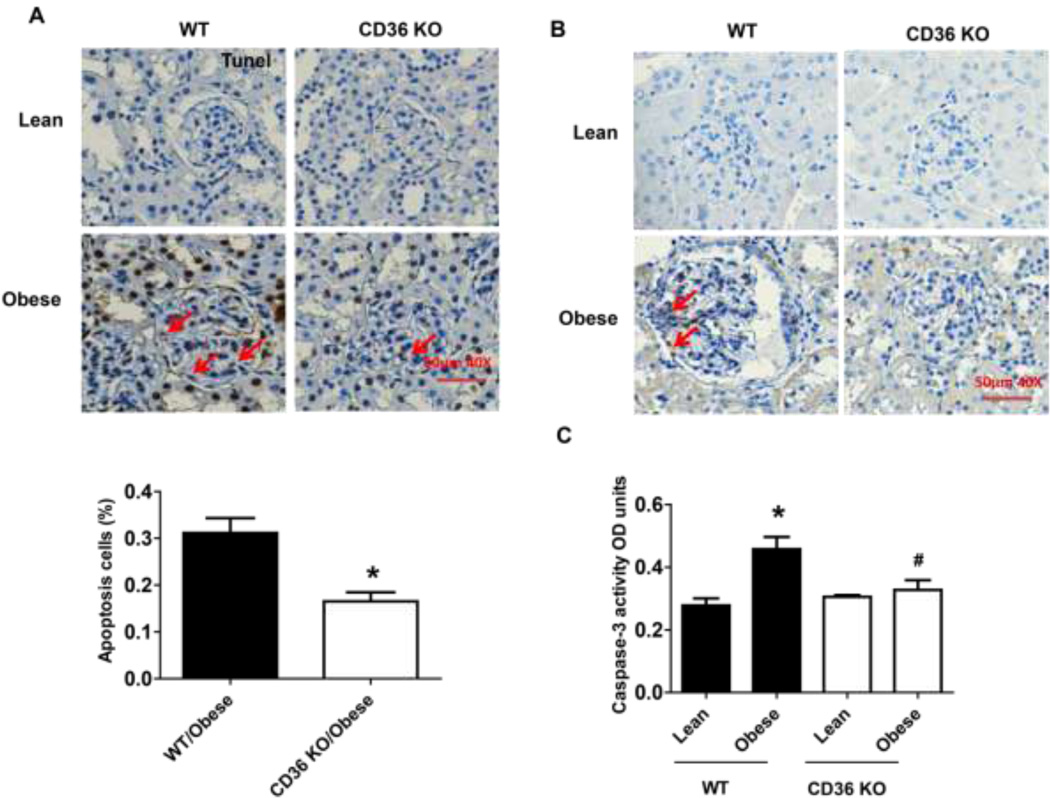
The in vivo importance of TSP1 and CD36 in obesity-associated podocyte injury was determined in the current study. We utilized the diet-induced obesity mouse model in TSP1 deficient mice as well as in CD36 deficient mice [11, 12, 22]. After 16 weeks of high fat diet feeding, male TSP1 −/− or CD36 −/− mice were sacrificed. Kidney tissues were collected for further analysis. Previously, we demonstrated that glomeruli TSP1 (including in mesangial cells and podocytes) was increased in high fat fed obese wild type mice [12]. In this study, glomeruli CD36 mRNA levels were comparable in four groups of mice (Figure 8A). However, podocyte functional markers, such as WT1, nephrin and synaptopodin were decreased in obese wide type mice. However, all these changes were attenuated in obese TSP1 −/− mice (Figure 8 B–D). Consistently, WT1 positive cells in glomerulus were significantly reduced in obese WT mice but not in obese TSP1−/− mice (Figure 8E). Apoptotic cell number, positive staining for active caspase 3 in kidney sections and caspase 3 activity assay in isolated glomeruli were increased in obese wide type mice, which were attenuated in either obese TSP1 −/− mice (Figure 9) or CD36 − /− mice (Figure 10). Taken together, these data provide in vivo evidence of the involvement of TSP1 and CD36 interaction in mediating obesity-associated podocytopathy.
Figure 8. Obesity induced podocyte dysfunction were attenuated in TSP1 KO mice.
Obesity was induced in male TSP1−/− mice or wild type (WT) controls by feeding with a high fat diet. Glomerular CD36 (A), nephrin (B), synaptopodin (C), and WT1 (D) mRNA levels were examined by Real-time PCR. Data are presented as mean ± SE (n = 10 mice/group). (E) Representative overlay images of immunofluorescence staining of frozen kidney sections with WT1 (red) and DAPI (purple) were shown and WT1 positive cells in glomeruli were counted. *, P <0.05 vs. WT/lean group; #, P <0.05 vs. WT/obese group.
Figure 9. Obesity induced podocyte apoptosis were attenuated in TSP1 KO mice.
Apoptosis cells were calculated after TUNEL staining (A). The scale bar represents 50 µm. In addition, glomerular caspase 3 protein expression (B) and caspase 3 activity (C) were determined by immunochemical staining and caspase-3 colorimetric assay kit, respectively. Data are presented as mean ± SE (n = 10 mice/group). *, P <0.05 vs. WT/lean group; #, P <0.05 vs. WT/obese group.
Figure 10. Obesity induced podocyte apoptosis were attenuated in CD36 KO mice.
Male CD36 −/− mice at eight weeks of age and age-matched wild type littermate controls were fed a low fat (10% kcal as fat) or a high fat diet (60% kcal as fat) for 16 weeks. After that, mice were harvested and kidney tissues were collected. Apoptotic cell number was calculated after tunel staining (A). The scale bar represents 50 µm. In addition, glomerular caspase 3 protein expression (B) and caspase 3 activity (C) were determined by using immunochemical staining and caspase-3 colorimetric assay kit, respectively. Data are presented as mean ± SE (n = 6 mice/group). *, P <0.05 vs. WT/lean group; #, P <0.05 vs. WT/obese group.
4. Discussion
In the current study, for the first time, we demonstrated that saturated FFA stimulated TSP1 gene expression in human podocytes at the transcriptional level. Moreover, increased TSP1 mediated FFA-induced human podocyte apoptosis and dysfunction partially through interaction with its receptor CD36. Importantly, using a diet-induced obese mouse model, our in vivo data demonstrated that obesity-associated podocyte apoptosis and dysfunction were attenuated in TSP1 −/− mice as well as in CD36 −/− mice. Taken together, these studies provide novel evidence that the interaction of TSP1 with its receptor CD36 contributes to obesity - associated podocytopathy.
Accumulating evidence have shown the strong correlation between obesity and the development of albuminuria/proteinuria [43]. Previously, clinical studies demonstrated that glomeruli enlargement and podocyte hypertrophy were found in extremely obese individuals (BMI>40 kg/m2) but not in age-matched nephrectomy patients without obesity. Additionally, this renal pathology change was accompanied by a significantly increased twenty four-hour protein excretion in extremely obese individuals [44], suggesting that podocyte dysfunction plays a key role in proteinuria formation in obese patients. Although there are some advances in studying the obesity-associated podocyte injury in recent years, the mechanisms are still not clear [45–48]. It is known that obesity is associated with increased plasma levels of FFA and increased FFA is an important cause of obesity-associated insulin resistance and metabolic syndrome [49]. Recently, studies have shown that podocytes are highly susceptible to the saturated FFA-induced lipotoxicity. FFA induces ER stress and causes podocyte death [27, 29, 50]. In the current study, consistently, podocyte apoptosis and dysfunction were induced either by FFA (palmitate) treatment in a dose dependent manner in vitro or in a high fat diet induced obese mouse model in vivo. These obese mice also developed albuminuria [12]. Using FFA-induced podocyte apoptosis as a model, we further determined the down-stream molecules that involved in FFA-induced podocyte injury in the current study.
Data from this study demonstrated that TSP1 - a matricellular protein, was up-regulated by FFA in podocyte. Recently, TSP1 has been identified to be an adipokine, which is associated with obesity associated inflammation and insulin resistance [50]. TSP1 is also an important mediator for various kidney diseases including obesity and diabetes related kidney complications [8, 12, 26, 51]. Upon stimulation by various factors, kidney cells produce and secrete TSP1, which then activates the latent TGF-β and induces kidney fibrosis [52–54]. Our previous studies demonstrated that obesity-associated albuminuria was attenuated in obese TSP1 deficient mice [12]. Moreover, in TSP1 deficient mesangial cells, leptin’s stimulatory effect on TGF-β-dependent matrix protein production was abolished, suggesting a role of TSP1 in mesangial cell-mediated fibrosis and the development of albuminuria in the obese state. In addition to mesangial cells, podocytes are another major cellular sources of increased TSP1 expression in glomeruli under obese conditions [12]. Therefore, in this study, the contribution of TSP1 to obesity-associated podocyte injury was explored. In vitro data demonstrated that FFA treatment stimulated TSP1 expression in human podocyte at the transcription levels and involved in MAPK activation. It is known that CD36 is a fatty acid translocase and regulates the uptake of FFA into cells. The putative FFA binding site on CD36 molecule (amino acid residues 127–279) has been suggested by a study from Baillie et al [55]. Therefore, we determined whether FFA uptake is required for mediating TSP1 gene expression in podocytes. To do this, we isolated podocytes from CD36 deficient mice and treated these cells with FFA to look at TSP1 expression. We found that FFA induced TSP1 expression was abolished in CD36 deficient podocytes, suggesting that FFA uptake into cells via CD36 is required for stimulation of TSP1 gene expression in podocytes. In addition, studies from vascular cells showed that TSP1 binding to CD36 can inhibit myristic acid uptake [56]. Therefore, a potential negative feedback effect might exist on FFA induced TSP1 expression in podocytes. Through this mechanism, it is anticipated that FFA treatment only increases TSP1 expression in podocytes to a certain level.
Our data also showed that addition of purified TSP1 to culture media induced podocyte apoptosis and increased albumin leakage from cultured human podocytes. TSP1 is an important regulator for latent TGF-β activation [35] and TGF-β induces podocyte apoptosis [36, 37]. Therefore, we determined whether TSP1-induced podocyte apoptosis or dysfunction is through activation of TGF-β dependent pathway. Our results demonstrated that TSP1 induced podocyte apoptosis or dysfunction was not affected by anti-TGF-β antibody pretreatment, suggesting a TGF-β independent mechanism. Importantly, in vivo data showed that TSP1 deficiency attenuated obesity induced podocyte apoptosis. Together, these data provide evidence that TSP1 contributes to FFA/obesity associated podocytopathy.
TSP1 is a large homotrimer, with an NH2-terminal domain, procollagen homology domain, type I, type II and type III repeats, as well as a COOH-terminal domain in each monomer [57]. By interacting with a variety of different cell surface receptors, the diversity of domains in TSP1 molecular determines the multiple biological activities of TSP1. In addition to functioning as a fatty acid translocase and mediating fatty acid uptake into cells [58, 59], CD36 is a receptor for TSP1 and a membrane glycoprotein existing on many cell types including podocytes [15, 38, 39]. It has been shown that CD36 mediates apoptosis signaling induced by TSP1 in endothelial cells [13], by ox-LDL in macrophages [40], and by advanced end product (AGE) or palmitate in tubular cell [38]. CD36 interacts with type 1 repeats domain of TSP1. The residues on CD36 molecule that specifically interact with TSP1 have been identified-called CD36 peptide (p93–110) [18, 19], which is different from the FFA binding sites (p127–279) [55]. This CD 36 peptide has been shown to inhibit the effect of TSP1/CD36 interaction on TSP1 mediated macrophages activation in vitro [41, 42]. To determine whether TSP1/CD36 interaction mediates FFA-induced podocyte apoptosis, podocytes were treated with FFA in the presence of CD36 peptide or scrambled control peptide. We found that CD36 peptide treatment to block TSP1 binding to CD36 significantly reduced FFA-induced podocyte apoptosis. The in vivo importance of TSP1/CD36 interaction on obesity-associated podocyte injury was supported by using high fat diet induced obese CD36 deficient mouse model. CD36 deficiency attenuated obesity associated podocyte apoptosis. Based on our observation, we propose that under obese conditions, increased FFA enters into podocytes via binding to CD36, leading to activation of MAKP pathway and stimulation of TSP1 expression. The secreted TSP1 then binds to the different sites on CD36 and induces podocyte apoptosis and dysfunction.
5. Conclusion
Our data suggest that TSP1/CD36 interaction mediates FFA/obesity associated podocyte apoptosis and dysfunction, which may provide a new therapeutic strategy for treatment of obesity induced renal complications.
Highlights.
Saturated free fatty acid (FFA) stimulates thrombospondin1 (TSP1) expression in podocytes.
TSP1 mediates FFA induced podocyte apoptosis and dysfunction.
TSP1 stimulates podocyte apoptosis through a TGF-β independent mechanism.
Blocking TSP1/CD36 interaction attenuates FFA induced podocyte apoptosis.
Acknowledgements
This study was supported in part by the Department of Veterans Affairs Merit Review Award (to S. Wang), the National Institutes of Health (NIH) Grant R01 DK081555 and DK098176 (to S. Wang), NIH Training Grant DK07778 (to H. Norman), and a COBRE grant P20GM103527-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14–22. doi: 10.1016/j.semnephrol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Amann K, Benz K. Structural renal changes in obesity and diabetes. Seminars in nephrology. 2013;33:23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ma R, Liu L, Liu X, Wang Y, Jiang W, Xu L. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Experimental and therapeutic medicine. 2013;6:649–656. doi: 10.3892/etm.2013.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi S, Tomioka M, Hiromura K, Sakairi T, Hamatani H, Watanabe M, Ikeuchi H, Kaneko Y, Maeshima A, Aoki T, Ohnishi H, Matozaki T, Nojima Y. SIRPalpha signaling regulates podocyte structure and function. American journal of physiology. Renal physiology. 2013;305:F861–F870. doi: 10.1152/ajprenal.00597.2012. [DOI] [PubMed] [Google Scholar]
- 6.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 7.Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of Constitutively Active cGMP-Dependent Protein Kinase Prevents Glucose Stimulation of Thrombospondin 1 Expression and TGF-beta Activity. Diabetes. 2003;52:2144–2150. doi: 10.2337/diabetes.52.8.2144. [DOI] [PubMed] [Google Scholar]
- 8.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers NM, Thomson AW, Isenberg JS. Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1538–1550. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bige N, Shweke N, Benhassine S, Jouanneau C, Vandermeersch S, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 2012;81:1226–1238. doi: 10.1038/ki.2012.21. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui W, Maimaitiyiming H, Qi X, Norman H, Wang S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol. 2013;305:F871–F880. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin LE. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. American journal of physiology. Heart and circulatory physiology. 2009;296:H1344–H1351. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayrhofer C, Krieger S, Huttary N, Chang MW, Grillari J, Allmaier G, Kerjaschki D. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol. 2009;174:1191–1202. doi: 10.2353/ajpath.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2011;21:79–85. doi: 10.1016/j.numecd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 18.Leung LL, Li WX, McGregor JL, Albrecht G, Howard RJ. CD36 peptides enhance or inhibit CD36-thrombospondin binding. A two-step process of ligand-receptor interaction. J Biol Chem. 1992;267:18244–18250. [PubMed] [Google Scholar]
- 19.Frieda S, Pearce A, Wu J, Silverstein RL. Recombinant GST/CD36 fusion proteins define a thrombospondin binding domain. Evidence for a single calcium-dependent binding site on CD36. J Biol Chem. 1995;270:2981–2986. doi: 10.1074/jbc.270.7.2981. [DOI] [PubMed] [Google Scholar]
- 20.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, Lee Y, Moeckel G, Calderwood DA, Holzman LB, Critchley DR, Zent R, Reiser J, Ishibe S. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest. 2014;124:1098–1113. doi: 10.1172/JCI69778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. 2012;7:e36785. doi: 10.1371/journal.pone.0036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awad AS, Rouse M, Liu L, Vergis AL, Rosin DL, Linden J, Sedor JR, Okusa MD. Activation of adenosine 2A receptors preserves structure and function of podocytes. J Am Soc Nephrol. 2008;19:59–68. doi: 10.1681/ASN.2007030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rico M, Mukherjee A, Konieczkowski M, Bruggeman LA, Miller RT, Khan S, Schelling JR, Sedor JR. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. American journal of physiology. Renal physiology. 2005;289:F431–F441. doi: 10.1152/ajprenal.00389.2004. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang S. Glycated albumin upregulates upstream stimulatory factor 2 gene transcription in mesangial cells. Am J Physiol Renal Physiol. 2010;299:F121–127. doi: 10.1152/ajprenal.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279:34311–34322. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 27.Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 2010;299:F821–F829. doi: 10.1152/ajprenal.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD, Orellana JM, Mundel P, Jehle AW. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol. 2013;183:735–744. doi: 10.1016/j.ajpath.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampe K, Sieber J, Orellana JM, Mundel P, Jehle AW. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am J Physiol Renal Physiol. 2014;306:F401–F409. doi: 10.1152/ajprenal.00454.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugo C. The thrombospondin 1-TGF-beta axis in fibrotic renal disease. Nephrol Dial Transplant. 2003;18:1241–1245. doi: 10.1093/ndt/gfg159. [DOI] [PubMed] [Google Scholar]
- 31.Yevdokimova N, Wahab NA, Mason RM. Thrombospondin-1 is the key activator of TGF-beta1 in human mesangial cells exposed to high glucose. J Am Soc Nephrol. 2001;12:703–712. doi: 10.1681/ASN.V124703. [DOI] [PubMed] [Google Scholar]
- 32.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;18:18. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 33.Finlin BS, Zhu B, Starnes CP, McGehee RE, Jr, Peterson CA, Kern PA. Regulation of thrombospondin-1 expression in alternatively activated macrophages and adipocytes: role of cellular cross talk and omega-3 fatty acids. J Nutr Biochem. 2013;22:00031–00034. doi: 10.1016/j.jnutbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez RJ, Haney RM, Cuadra RH, Ganguly R, Adapala RK, Thodeti CK, Raman P. Upregulation of thrombospondin-1 expression by leptin in vascular smooth muscle cells via JAK2- and MAPK-dependent pathways. Am J Physiol Cell Physiol. 2012;303:C179–C191. doi: 10.1152/ajpcell.00008.2012. [DOI] [PubMed] [Google Scholar]
- 35.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 36.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, Yu L, D'Agati V, Schlondorff D, Kriz W, Haraldsson B, Bottinger EP. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS medicine. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science signaling. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wintergerst ES, Jelk J, Rahner C, Asmis R. Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. European journal of biochemistry / FEBS. 2000;267:6050–6059. doi: 10.1046/j.1432-1327.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 41.Yehualaeshet T, O'Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol. 2013;19:32. doi: 10.1038/cmi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyvis K, Verrijken A, Wouters K, Van Gaal L. Plasma adiponectin level is inversely correlated with albuminuria in overweight and obese nondiabetic individuals. Metabolism: clinical and experimental. 2013;62:1570–1576. doi: 10.1016/j.metabol.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A. Renal injury in the extremely obese patients with normal renal function. Kidney international. 2008;73:947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 45.de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, D'Agati VD, Lamb HJ, Pongrac Barlovic D, Hojs R, Abbate M, Rodriquez R, Mogensen CE, Porrini E. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. The lancet. Diabetes & endocrinology. 2014;2:417–426. doi: 10.1016/S2213-8587(14)70065-8. [DOI] [PubMed] [Google Scholar]
- 46.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1629–F1636. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 47.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boden G. Obesity, insulin resistance and free fatty acids. Current opinion in endocrinology, diabetes, and obesity. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, 3rd, Lee MJ, Fried SK, McGehee RE, Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 52.Hugo C, Daniel C. Thrombospondin in renal disease. Nephron Exp Nephrol. 2009;111:e61–e66. doi: 10.1159/000198235. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Yan L, Chen W, Xu L, Zhang X. The renal protective effects of cilostazol on suppressing pathogenic thrombospondin-1 and transforming growth factor-beta expression in streptozotocin-induced diabetic rats. J Int Med Res. 2009;37:145–153. doi: 10.1177/147323000903700117. [DOI] [PubMed] [Google Scholar]
- 54.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 55.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 56.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 58.Kiyan Y, Tkachuk S, Hilfiker-Kleiner D, Haller H, Fuhrman B, Dumler I. oxLDL induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with CD36 and TLR4. Journal of molecular and cellular cardiology. 2013;66C:72–82. doi: 10.1016/j.yjmcc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]