Abstract
PD-1H is a recently identified cell surface co-inhibitory molecule of the B7/CD28 immune modulatory gene family. We showed previously that single injection of a PD-1H agonistic monoclonal antibody (mAb) protected mice from graft versus host disease (GVHD). We report here two distinct mechanisms operate in PD-1H-induced T cell tolerance. First, signaling via PD-1H co-inhibitory receptor potently arrests allo-reactive donor T cells from activation and expansion in the initiation phase. Second, donor regulatory T cells are subsequently expanded to maintain long-term tolerance and GVHD suppression. Our study reveals the crucial function of PD-1H as a co-inhibitory receptor on allo-reactive T cells and its function in the regulation of T cell tolerance. Therefore, PD-1H may be a target for the modulation of allo-reactive T cells in GVHD and transplantation.
Keywords: PD-1H, coinhibitory, cosignaling, immune modulation, graft versus host disease, GVHD, alloantigen, allogeneic, agonistic, antibody, regulatory, T cell, Treg, tolerance, MH5A
Introduction
Targeting cell surface immune modulatory pathways including costimulatory and coinhibitory molecules have been widely investigated as a strategy for inducing T cell tolerance (1). Costimulatory and coinhibitory molecules on the surface of T cells could deliver positive and negative signals, respectively, following interaction with specific ligands or counter-receptors primarily expressed on APCs and stromal cells (2). Costimulatory receptors such as CD28 are expressed on naive T cells and function as a second signal in co-ordination with T cell receptor signaling to activate T cells in most physiological settings (3). Meanwhile, expression of coinhibitory receptors like PD-1 is characteristically upregulated on T cells following activation and suppresses T cell responses (2). Therefore, manipulating these pathways to maintain and actively induce tolerance has been investigated in detail (4–10). However, to date, strategies targeting known co-signaling molecules have proven less successful to fully inhibit T cell-mediated graft versus host disease (GVHD) (5, 6, 11–13).
GVHD is a reaction of donor derived T cells that is directed against host tissues. GVHD is a major complication of hematopoietic cell transplantation for the treatment of hematologic malignancies, but can also occur in the setting of solid organ transplantation (1, 6, 14). In GVHD, allogeneic, or major histocompatibility complex (MHC) mismatched T cells derived from the donor transplant are primed and activated in both lymphoid tissues and in peripheral sites, and are largely dependent upon the interaction of donor T cells with antigen presenting cells (APCs) displaying peptide-MHC complexes recognized as foreign by donor T cells (15–21). Activation of allogeneic T cells result in broad tissue damage and even death, and typically require immune suppression, which then increases the risk of opportunistic infection, malignancy and metabolic disorders (14). Therefore, developing methods of inducing hematopoietic chimerism, or the stable co-existence of host and donor blood cells, and long-term transplantation tolerance, while avoiding broad immunosuppression, remain necessary for transplantation success and improved quality of life for transplant recipients.
PD-1H (Programmed Death One Homolog, also called VISTA) is a recently identified and broadly expressed coinhibitory molecule with dual functions as both a receptor and a ligand (22–26). PD-1H functions as a coinhibitory receptor on T cells to limit naive T cell activation, while PD-1H expressed on APCs interacts with an unknown receptor on T cells to suppress T cell responses (23, 24). We have previously identified agonist PD-1H specific mAbs that potently inhibit T cell responses (22, 23), while another group has developed distinct PD-1H mAbs that enhance T cell immunity (24–26). Here we utilize both PD-1H KO mice and MH5A agonist mAb to demonstrate that PD-1H expression specifically on donor T cells is crucial for regulating alloantigen responses through initial arrest of donor T cell activation and selective expansion of donor regulatory T cells.
Materials and Methods
Animals
All mouse procedures were performed in accordance with institutional guidelines at Yale University. Mice were maintained according to National Institutes of Health Animal Care guidelines, and experimental protocols described in this study were approved by the Yale University Institutional Animal Care and Use Committee. WT C57BL/6, C57BL/6 × DBA/2 F1, and BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). PD-1H KO mice (gene symbol: 4632428N05Rik) were purchased from the mutant mouse regional resource center (MMRRC, University California-Davis) as described in (23). B6.albino β-actin luciferase mice (stock #10500) were purchased from Taconic Inc. (Hudson, NY).
GVHD models
For GVHD models, mice were irradiated with 12 Gy (BDF1) or 10 Gy (B6) twelve hours prior to adoptive transfer of MACS isolated splenic and LN pan T cells (Miltenyi Biotec) and TCD-BM (MACS thy1.2-microbeads as previously described (22). For the B6 to BDF1 GVHD/CTL mice were not irradiated and 2.5 × 107 total splenocytes/mouse were adoptively transferred. For CD25 depletion, pan T cells were isolated with CD25-biotin (BD biosciences) and anti-biotin microbeads (Miltenyi), and passed over LD depletion columns (Miltenyi). 200 ug/mouse of MH5A or control Ab was injected via tail vein, and mice were monitored daily for all experiments.
GVHD model for in vivo imaging analysis
Balb/c recipients were lethally irradiated (9 Gy) 12 hours prior to adoptive transfer. Donor T cells were isolated from B6.albino β-actin luciferase (B6.luc) mouse lymph nodes and purified with a mouse pan T cell negative selection kit (Miltenyi). Bone marrow was isolated as above from wt B6 mice and T cells were depleted as described above. 2 × 106 T cells and 5 × 106 TCD-BM were adoptively transferred with 200 ug of MH5A or control Ab via tail vein (T = 0 hrs) in a total volume of 300 ul PBS.
Prior to imaging, mice were ip injected with 300 ug of luciferin subsrate approximately 5 minutes prior to being anesthetized using the XRT-8 gas (isofluorane) anesthesia system according to protocol. Anasthesia was maintained while mice were imaged for bioluminescence in the right lateral and ventral positions using a Lumina XR in vivo imaging system (Caliper, Hopkington, MA, a PerkinElmer Company, Waltham, MA) according to protocol. Briefly, luminescent detection was set to auto with a minimum detection level of 3000 photons. Mice were imaged on stage D at 1.5 cm height from stage. Units were set to radiance (photons/sec). Imaging and analysis were performed using Living Image software (Caliper, a PerkinElmer company). For analysis, binning was set to 4, and minimum and maximum radiance levels were determined for optimal view and comparison between groups at each timepoint. Calculation of total flux was determined by selecting regions of interest (ROI) (i.e. specific gating whole mouse, cervical LN, etc.) and measuring the total intensity in the region calculated as total flux (the radiance (photons/sec) in each pixel summed or integrated over the ROI area ((cm2) × 4π) by Living Image software.
Antibodies, cytokines, kits and reagents
MH5A was previously developed by immunizing Armenian hamsters with a mouse PD-1HIg fusion protein and Freund’s adjuvant as previously described in detail (22). Control hamster IgG was purchased from Rockland Immunochemicals (Gilbertsville, PA). Fluorescently labeled antibodies for flow cytometry including CD4, CD8, H-2Kd, IFN-γ, CD19, CD25, Gr-1, CD11b, DX5, and anti-hamster IgG-biotin were purchased from BD Biosciences (San Jose, CA). CD3 and CD28 mAbs for cell culture were also purchased from BD Biosciences. AnnexinV and 7-AAD were purchased from BD Biosciences. FoxP3-APC, FoxP3-FITC, and streptavidin-APC and PerCP, and TNF-α ELISA were purchased from eBioscience (San Diego, CA). Ki67-APC and ZombieNIR were purchased from Biolegend. CFSE (Vybrant CFDA cell tracer kit) was purchased from Life Technologies. TGF-β and rIL-2 were purchased from R&D Systems (Minneapolis, MN).
Flow cytometry
Splenocytes and/or lymph nodes were disaggregated with frosted class slides, passed through a 100 micron screen, red blood cells were lysed with ACK lysis buffer. Livers were disaggregated using a GentleMACS systems (Miltenyi Biotec) and lymphocytes were isolated in Percoll diluted to 35% in PBS (Sigma-Aldrich) and centrifugation at 1000g for 20 min at room temp. For flow cytometry analysis, donor cells were differentiated from recipient cells based on H-2Kd staining (B6 = H-2Kd−, BALB/c = H-2Kd+). FoxP3 and Ki67 staining was performed using eBioscience FoxP3 staining kit according to instructions. In some experiments cells were restimulated ex vivo with phorbol 12-myristate 13-acetate (PMA)(Sigma) at 1 ug/ml, ionomycin at 50 ng/ml (Sigma) and golgistop (BD Biosciences) for 5 hours prior to intracellular staining for IFN-γ using BD Biosciences intracellular staining kit according to protocol. AnnexinV and 7-AAD staining were performed according to BD Biosciences protocols. ZombieNIR staining was performed according to Biolegend protocol. Splenocytes for tolerance studies were labeled with 2 uM CFSE for 10 min in RPMI-complete in a 37 degree C water bath followed by two washed in excess RPMI-complete. CFSE labeled cells were then added to 48 well plates at a 1:1 ratio with 4 Gy irradiated B6 splenocytes and restimulated for 72 hours followed by staining of cells with H-2Kd, CD4, and CD8 for flow cytometry analysis of CFSE dilution. Flow cytometry was performed using a FACScalibur or LSRFortessa (BD Biosciences)
In vitro Treg induction, cell proliferation and Treg suppression in MLR assay
Treg cells were induced in 24 well plates with coated anti-CD3 (5 ug/ml coated overnight at 4 degrees in PBS), soluble anti-CD28 (1 ug/ml), IL-2 (5 ng/ml) and TGF-β at 5 ng/ml or concentrations indicated. Control antibody or MH5A was added at 10 ug/ml where indicated. For Treg proliferation, Treg cells culture for 5 days as above were MACS isolated by CD25 positive selection and plated in 96-well plates pre-coated with anti-CD3 at indicated concentrations and IL-2 (5 ng/ml). 3H-thymidine (Perkin-Elmer) was added to wells during final 8 hours of culture and cells were analyzed for DNA-incorporation (proliferation) using a Trilux Microbeta (Perkin-Elmer). Treg suppressive function in the presence of MH5A was analyzed in an MLR assay using induced Treg cells as above. Treg cells were incubated with either control Ab or MH5A for one hour, then washed and added to 96-well round bottom plates containing B6 T cells (responders) and lethally irradiated BDF1 target splenocytes. Ratio indicates the Treg to responder ratio, with responder cells remaining consistent in all wells, and Treg cells decreasing along the x-axis of graph. Cells were cultured for 5 days. 3-H-thymidine was added during final hours of culture and analyzed as above.
Graphs and statistical analysis
Graphs and statistical analyses were generated with Graphpad Prism 6 (Graphpad Software, Inc.) and Microsoft Excel. Statistical analyses of survival experiments were performed using Log Rank (Mantel-Cox) test; all other analyses were performed by one-tailed, equal variance T –test. *** P < 0.005; ** P < 0.025; * P < 0.05.
Results
PD-1H mAb prevents damage of allo-reactive T cells in GVHD target organs
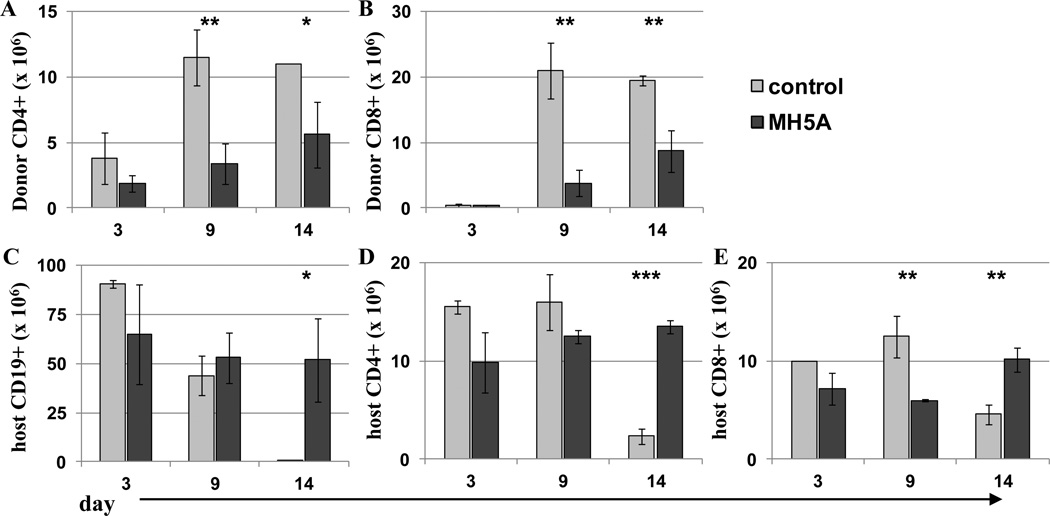
We previously showed in mouse models of GVHD that a single dose of anti-PD-1H mAb (clone MH5A) on day 0 protects nearly 100% of mice from lethality (22). MH5A was demonstrated to be effective at a range of doses (50–250 ug) when administered on day 0, without any observed toxicity at this dose range (Supplemental Fig. 1). To examine if MH5A functionally inhibited both donor T cell expansion and cytotoxic capacity, we used a GVHD-CTL model in which total splenocytes from C57BL/6 (B6; H-2b) wild type (WT) mice were adoptively transferred to partially MHC-mismatched, or semi-allogeneic C57BL/6 × DBA/2 F1 hybrid mice (BDF1; H-2bxd) and treated with MH5A or control IgG on day 0. In this model, donor cells recognize the recipient cells as foreign because of MHC H-2d expression, while BDF1 mice recognize B6 cells as self because of MHC H-2b expression. Therefore, only donor cells will attack recipient cells, but not vice-versa. Importantly, because recipients are not irradiated, we are able to monitor donor derived cytotoxic killing of recipient B cells and T cells, which are major targets in acute GVHD, while simultaneously monitoring the expansion of allo-reactive donor T cells. Mice treated with MH5A had significantly reduced expansion of donor CD4+ and CD8+ T cells, whereas there was no loss of recipient CD19+ B cells, CD4+ T cells, and CD8+ T cells in comparison with control IgG-treated mice, which had significant losses in all three cell populations (Fig. 1A–E). This finding indicates that MH5A directly inhibits allo-reactive T cell expansion and cytotoxic function.
Figure 1.
PD-1H mAb inhibits allo-reactive T cell expansion and cytotoxicity. 2.5 × 107 total splenocytes from B6 wt mice were adoptively transferred to non-irradiated BDF1 mice with MH5A or control Ab to monitor donor expansion and cytotoxic killing of recipient cells. Splenocytes were counted and analyzed by flow cytometry at indicated time points for determination of cell numbers of (A) donor CD4+ T cells, (B) donor CD8+ T cells, (C) recipient CD19+ T cells, (D) recipient CD4+ T cells, and (E) recipient CD8+ T cells.
Because recipient T cells numbers were unchanged in the in vivo GVHD/CTL assay, it was unlikely that MH5A was cytotoxic or depleting in vivo. However, we performed several studies to rule out these potential effects. Using a B6 to BDF1-GVHD model in which recipient BDF1 mice were lethally irradiated to mimic the setting of human GVHD, we analyzed splenocytes 2 to 72 hours after adoptive T cell transfer for CD4+ and CD8+ accumulation. Early accumulations of donor CD4+ and CD8+ T cell numbers were decreased in MH5A-treated mice compared to control Ig treated mice (Supplemental Fig. 2A), which confirmed our previous studies (22). However, when viable donor cells were assayed for PD-1H expression, we found that PD-1H was greatly enhanced in the presence of MH5A, suggesting that MH5A did not deplete PD-1H+ T cells (Supplemental Fig. 2B). Additionally, there was no difference in the percentage of apoptotic/dead donor CD4+ and CD8+ T cells, as measured by 7-AAD staining, in MH5A and control IgG treated mice, further suggesting MH5A was not cytotoxic in vivo (Supplemental Fig. 2C). To further validate that MH5A did not deplete PD-1H+ T cells, we determined that MH5A treatment of normal WT mice had no effect on T cell numbers (Supplemental Fig. 2D), and that MH5A did not induce complement-mediated lysis of PD-1H+ T cells in vitro (Supplemental Fig. 2E). Together, these data suggest that MH5A does not induce cytotoxic, depleting or apoptotic mechanism of donor T cell death.
MH5A mediates early and systemic control of allo-reactive T cells in vivo
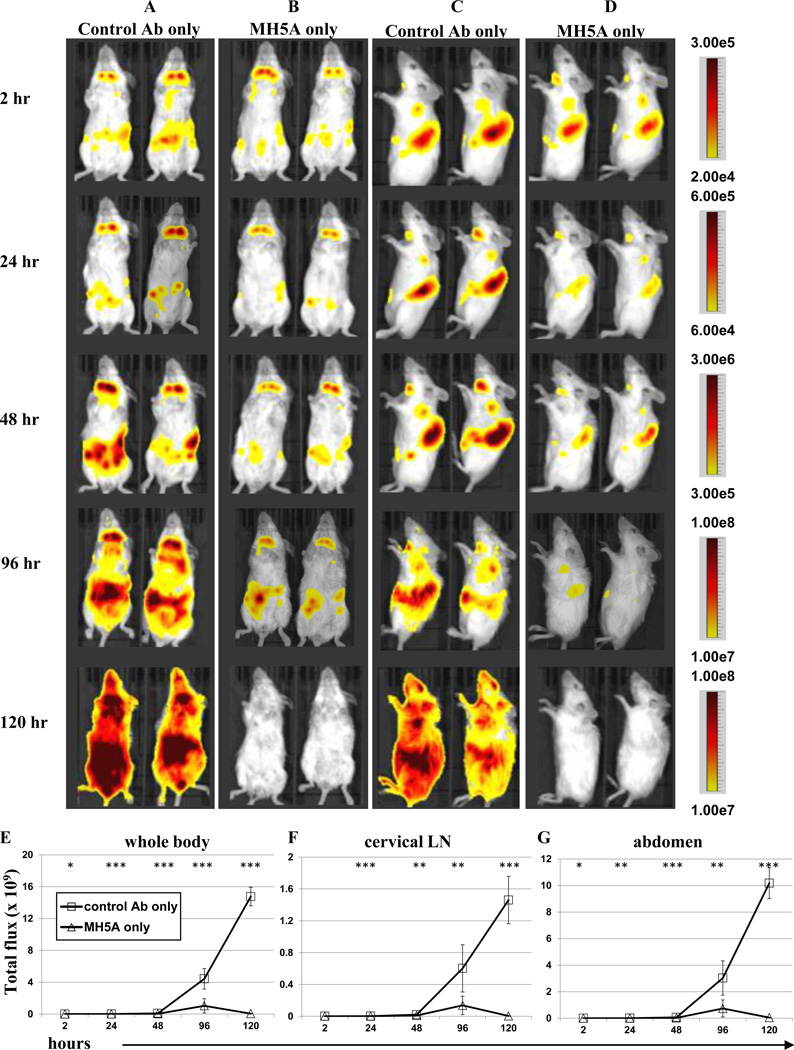
To visually examine MH5A-mediated modulation of allogeneic T cell responses in vivo we utilized a bioluminescent imaging (BLI) model of GVHD. BLI has proven very successful in GVHD studies for monitoring cellular responses (27). In this model, T cells from albino B6 transgenic mice expressing luciferase under the ubiquitous β-actin promoter (B6.luc) (28, 29) were purified and adoptively transferred together with WT B6 TCD-BM (to avoid bioluminescent interference from BM cells) to lethally irradiated BALB/c (H-2d) recipient mice. Therefore, in this model only adoptively transferred B6.luc donor T cells would express luciferase, allowing us to specifically investigate the effect of MH5A on the systemic accumulation and expansion of donor T cells in live mice over time. Mice treated with control Ab or MH5A were imaged in the ventral (Fig. 2A–B) and right lateral positions (Fig. 2C–D) at several time points for bioluminescence (measured as radiance) to compare relative T cell numbers and localization. We found that while overall radiance was relatively similar at 2 hours for all groups, the MH5A treated mice had little or no increase in radiance levels over time. This was in striking opposition to control mice which illustrated profoundly increased systemic radiance levels by 120 hours. Quantitation of radiance (calculated as total flux) in the whole body (Fig. 2E), cervical lymph node (Fig. 2F) and abdomen (Fig. 2G) clearly shows that MH5A remarkably inhibits donor T cells systemically.
Figure 2.
MH5A inhibits systemic expansion of allo-reactive donor T cells. B6.luc Tg donor T cells and WT B6 TCD-BM were adoptively transferred to lethally irradiated BALB/c recipients with (A and C) control Ab, or (B and D) MH5A. Mice were imaged for bioluminescence at 2, 24, 48, 96, and 120 hours for T cell accumulation and expansion measured as radiance for relative comparison between groups. Ventral (A and B) and right lateral (C and D) imaging of 2 mice per treatment group are shown as representatives of 5 mice/group. Minimum and maximum radiance levels and range are indicated at each time point for optimal view. Gating of (E) whole body, (F) cervical LN region and (G) abdominal region in the ventral position was performed for calculation of total flux.
PD-1H deficient T cells exacerbate GVHD
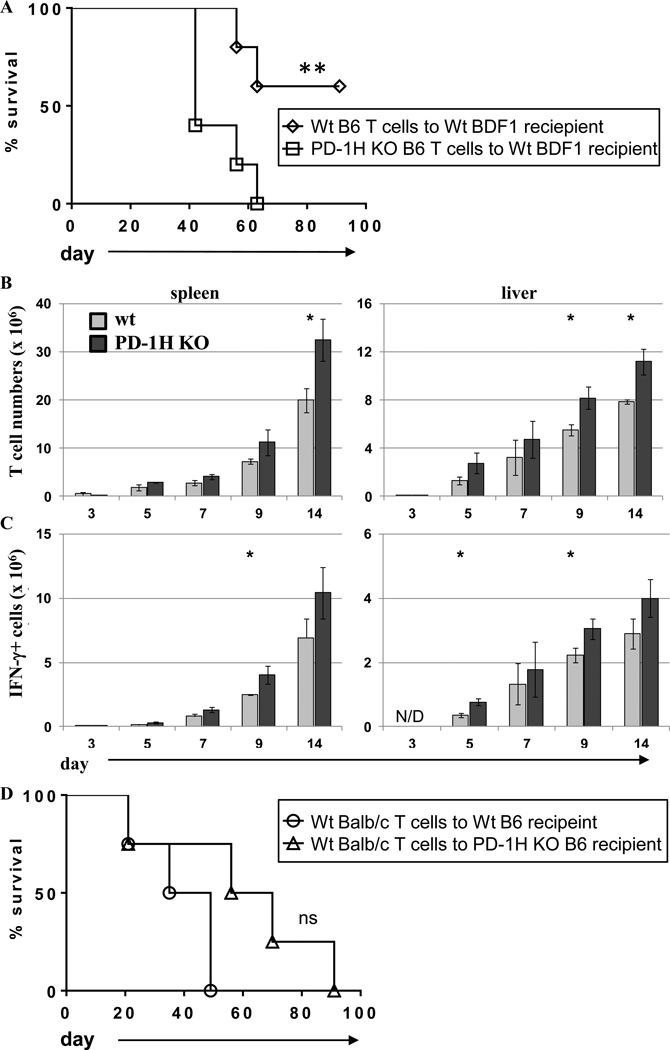
In order to clarify the direct function of PD-1H on donor T cells, we utilized PD-1H KO T cells in a B6 to BDF1 GVHD model (23). In this model T cells and TCD-BM from PD-1H KO or B6 WT littermate donor mice were adoptively transferred to lethally irradiated BDF1 mice. We found that mice adoptively transferred with PD-1H KO T cells had exacerbated GVHD and decreased survival compared to mice receiving WT T cells (Fig. 3A). Concordantly, PD-1H KO T cell numbers were significantly increased in the spleen and liver by day 14 compared to WT T cells (Fig. 3B). There was also a significant increase in the number of IFN-γ producing T cells in the spleen and liver in PD-1H KO donor T cells by nine days after adoptive T cell transfer (Fig. 3C).
Figure 3.
Adoptive transfer of PD-1H deficient donor T cells exacerbates GVHD. (A) PD-1H KO donor T cells + TCD-BM or wt littermate donor T cells + TCD-BM from B6 background were adoptively transferred to lethally irradiated wt BDF1 recipients and monitored for survival or (B) splenocytes and liver lymphocytes were isolated at indicated time points and absolute number of T cells was determined. (C) Total splenocytes or liver lymphocytes were restimulated ex vivo for 5 hours with PMA, ionomycin, and golgistop and intracellularly stained for IFN-γ production. (D) Wt BALB/c LN cells and BM were adoptively transferred to lethally irradiated B6 wt or PD-1H KO recipients and monitored for survival. 5 mice per group were used for survival experiments and 3 mice per group were used for time point analyses. Experiments were repeated 2–3 times.
Figure 3A indicated that PD-1H expression on donor T cells regulates allo-reactive responses. In order to examine whether the absence of PD-1H expression on recipient cells (non-T cells) would also exacerbate GVHD, we adoptively transferred BALB/c WT T cells (PD-1H+) and TCD-BM to lethally irradiated PD-1H KO or WT B6 recipients. Interestingly, we observed a slightly increased rather than decreased survival time in PD-1H KO recipients, thus demonstrating that PD-1H expression on recipient cells had little effect on the regulation of allogeneic T cell responses (Fig. 3D). Together, these results show that PD-1H expression on T cells, but not other cell subsets, such as APCs, in which PD-1H could potentially function as an inhibitory ligand (24–26), is crucial for modulating T cell responses in GVHD.
PD-1H expression on T cells is essential for MH5A-directed suppression of murine GVHD
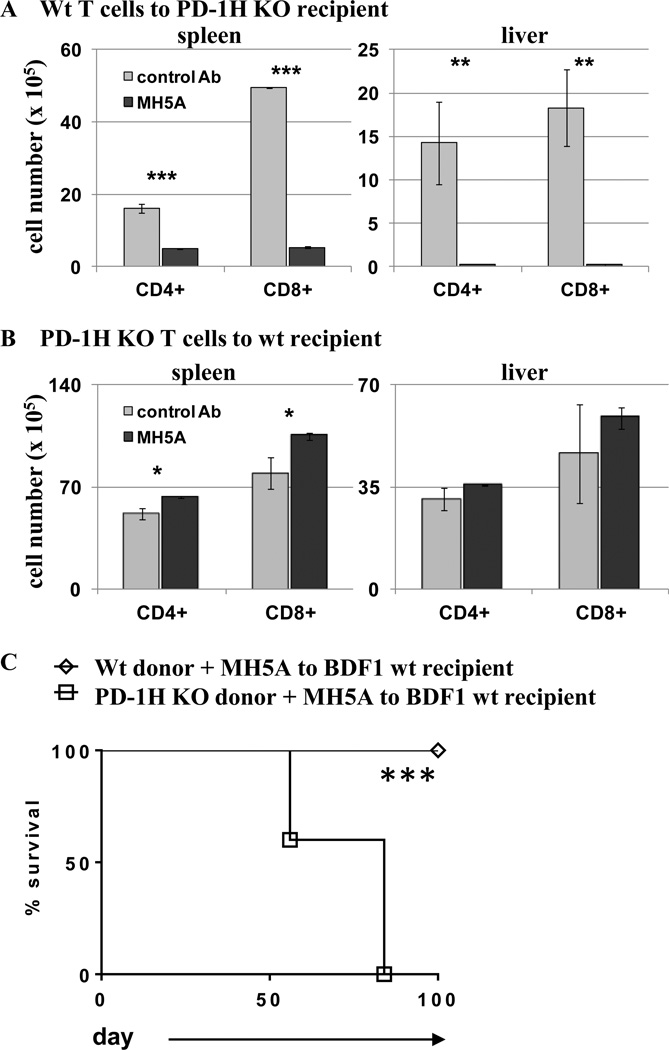
Our results indicate that PD-1H expression on T cells is crucial for controlling donor T cell responses in GVHD. However, it was unknown if MH5A-mediated inhibition required PD-1H expression on donor T cells. To investigate this, we utilized both WT (PD-1H+) and PD-1H KO T cells in combination with MH5A treatment. First, PD-1H+ T cells from BALB/c mice were adoptively transferred to lethally irradiated PD-1H KO recipients on a B6 background with either MH5A treatment or control IgG. Spleens and livers were then analyzed six days later. In this setting, in which PD-1H was expressed on donor T cells but not recipient cells, MH5A potently inhibited WT (PD-1H+) donor T cell accumulation in the spleen and liver compared to control Ab, showing that PD-1H expressed specifically on donor T cells was sufficient for MH5A mediated T cell inhibition (Fig. 4A). Secondly, we adoptively transferred PD-1H KO T cells from B6 mice to PD-1H sufficient BDF1 recipients with either MH5A or control IgG treatment. In this setting MH5A did not inhibit, but actually slightly enhanced T cell accumulation in the spleen or liver compared to control treated mice (Fig. 4B). We next performed a GVHD survival study in which either purified PD-1H KO or WT PD-1H+ T cells from a B6 background were transferred to BDF1 recipients with MH5A treatment. We found that MH5A treatment was only protective in mice that were transferred WT, but not PD-1H KO T cells (Fig. 4C), thus confirming the requirement for PD-1H expression and signaling on donor T cells for MH5A-directed T cell inhibition.
Figure 4.
MH5A-directed inhibition of allo-reactive T cells requires PD-1H expression on T cells. (A) BALB/c wt T cells were adoptively transferred to lethally irradiated PD-1H KO recipients on a B6 background with MH5A or control Ab. Splenocytes and liver lymphocytes were isolated 6 days later for determination of absolute number of donor CD4+ and CD8+ T cells. (B) B6 PD-1H KO T cells were adoptively transferred to lethally irradiated BDF1 recipients with MH5A or control Ab. Splenocytes and liver lymphocytes were isolated on day 7 for determination of absolute number of donor CD4+ and CD8+ T cells. (C) PD-1H KO T cells + TCD-BM or wt littermate T cells + TCD-BM from B6 mice were adoptively transferred to lethally irradiated BDF1 recipients with MH5A or control Ab and mice were monitored for survival. 3 mice per group were analyzed for T cell numbers and 5 mice/group were used for survival studies. Experiments were repeated 2–3 times.
MH5A mAb promotes tolerance in donor T cells
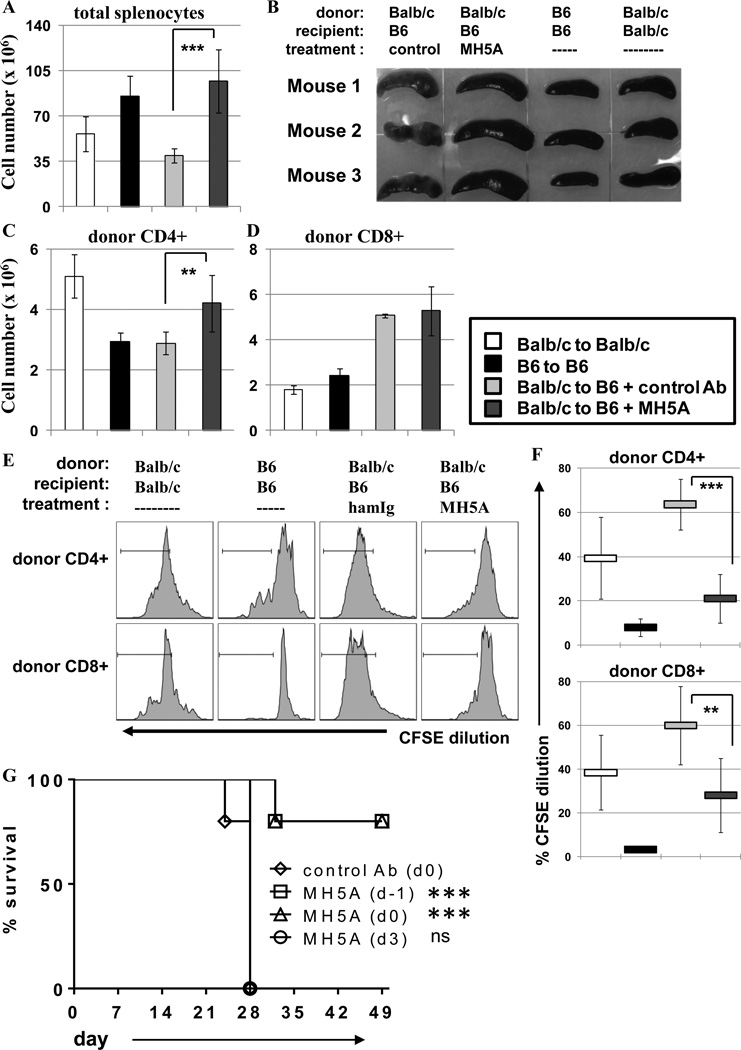
Our results clearly demonstrated that MH5A treatment inhibited PD-1H+ allo-reactive T cell expansion and function. However, mechanisms of donor T cell tolerance remained undefined. In order to investigate donor T cell tolerance induction, we used the fully MHC-mismatched model of GVHD in which BALB/c donor T cells were adoptively transferred to lethally-irradiated B6 recipient mice with MH5A or control IgG treatment. As positive and negative controls for tolerance induction experiments, syngeneic adoptive transfers of BALB/c to lethally irradiated BALB/c, and B6 to lethally irradiated B6 were included. In the BALB/c to B6 GHVD model, we have previously shown that MH5A treatment inhibits early donor T cell accumulation and function, and promotes long-term survival (22). Paradoxically, we noticed that by day 14 total splenocyte numbers were increased in MH5A-treated mice compared to controls (Fig. 5A). This was also evident by visual observation of enlarged spleens (Fig. 5B). Initial examination of T cell numbers on day 14 showed that MH5A-treated mice had increased numbers of donor CD4+ T cells but no change in CD8+ T cells compared to control IgG treated mice (Fig. 5C–D). Interestingly, despite the splenomegaly in MH5A-treated mice, the spleen appeared healthy, similar to syngeneic controls. Meanwhile, the control treated spleens were fibrotic and discolored; damage we have routinely observed in the spleens of mice with acute GVHD. The spleen appearance correlated with the overall health of MH5A- vs. control IgG-treated mice.
Figure 5.
Donor T cell tolerance is induced by prophylactic MH5A treatment. BALB/c T cells + TCD-BM were adoptively transferred to lethally irradiated B6 recipients with MH5A or control Ab. Syngeneic adoptive transfers of BALB/c to lethally irradiated BALB/c and B6 to lethally irradiated B6 were also performed for controls in T cell tolerance studies. (A) Spleens were isolated on day 14 and analyzed for total splenocytes. (B) Pictures of spleens isolated from indicated mice on day 14. (C and D) Donor CD4+ and CD8+ T cell numbers on day 14. (E) Day 14 total splenocytes were labeled with CFSE and restimulated ex vivo with irradiated B6 splenocytes for 72 hours. Donor CD4+ and CD8+ T cells were analyzed by flow cytometry for cell division as determined by CFSE dilution. Syngeneic BALB/c to BALB/c splenocytes were used as positive controls while syngeneic B6 to B6 splenocytes were used as negative controls. (F) Average percentage of CFSE dilution was determined for donor CD4+ and CD8+ T cells. (G) Using the same BALB/c to lethally irradiated B6 GVHD model as above, mice were treated with MH5A on day -1, on day 0 at the time of adoptive T cell transfer, or 3 days after adoptive T cell transfer and monitored for survival. Day 14 tolerance experiments used 3 mice per group and survival experiments used 5 mice/group. Experiments repeated 2–3 times.
To investigate if donor T cells were tolerized in MH5A treated mice, we performed a modified mixed lymphocyte response (MLR) assay in vitro. In this experimental setting, total splenocytes from day 14 GVHD mice (as described above) were labeled with CFSE and cultured with irradiated B6 splenocytes for 72 hours. For controls, T cells isolated from the BALB/c to BALB/c adoptive transfer mice would serve as a positive control for responsiveness to irradiated allogeneic B6 splenocytes, while T cells from the B6 to B6 adoptive transfer mice should have no response against syngeneic B6 cells (negative control). Division of donor CD4+ and CD8+ T cells was then determined at 72 hours by CFSE dilution (Fig. 5E–F). While donor CD4+ and CD8+ T cells isolated from control IgG-treated mice proliferated vigorously, donor T cells from MH5A-treated mice had significantly reduced proliferation.
This finding suggested that early restraint of allo-reactive T cell activation by MH5A resulted in the T cell tolerance or suppression. In support of this notion, we found that the time of MH5A administration was crucial for GVHD inhibition. In the BALB/c to lethally irradiated B6 GVHD survival model, MH5A was administered one day before (d-1), the same day (d0) or three days after adoptive transfer of BALB/c T cells + TCD-BM. We found that treatment with MH5A on day -1 or day 0 effectively prevented GVHD lethality, while treatment on day 3 was not protective (Fig. 5G). These findings supported the notion that regulation of allo-reactive T cells through PD-1H signaling on T cells during T cell priming and activation was required for subsequent tolerance. However, mechanisms through which MH5A treatment maintained long-term tolerance despite the expansion of donor T cells required additional study.
Regulatory T cells expansion maintains tolerance in MH5A treated mice
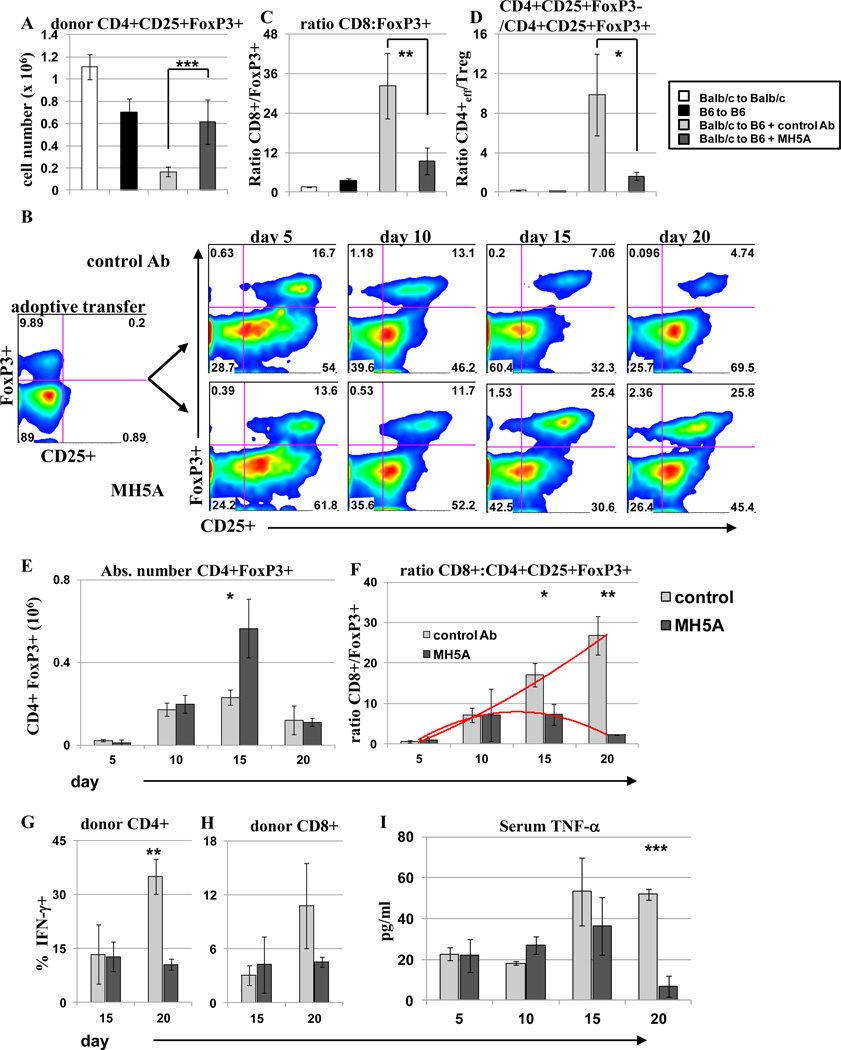
Because donor CD4+ T cells were significantly increased in MH5A treated mice on day 14 (Fig. 5C) along with the induction of long-term tolerance and survival, we posited that CD4+ regulatory T (Treg) cells may be increased as a mechanism of allo-reactive T cell suppression. Indeed, we found significantly increased FoxP3+ Treg cells in MH5A treated mice on day 14 (Fig. 6A). Moreover, CD4+ FoxP3+ Treg cell percentages increased in MH5A treated mice, while CD4+FoxP3+ Treg cell percentages actually decreased over time in control IgG-treated mice (Fig. 6B). The ratio of both CD8+ T cells and CD4+ T cells to Treg cells was significantly reduced in MH5A-treated mice on day 14 (Fig. 6C–D). However, the absolute number of Treg cells in the spleen was only transiently increased in MH5A treated mice compared to controls (Fig. 6E), while a time course analysis of the ratio of donor CD8+ cells to donor Treg cells clearly illustrated an altered cell composition MH5A-treated mice compared to controls (Fig. 6F). These findings supported the direct suppression of effector T cells by PD-1H signaling, rather than direct effects on Treg cells. Concomitantly, IFN-γ production was reduced in CD4+ and CD8+ T cells on day 20 in MH5A treated mice (Fig. 6F–G), while serum TNF-α levels were also significantly reduced by day 20 in MH5A treated mice (Fig. 6I). These findings support a model in which donor effector T cells are directly suppressed by PD-1H signaling, thus allowing for the selective expansion of donor Treg cells, which further suppress allo-reactive donor T cells to maintain tolerance.
Figure 6.
Regulatory T cells are transiently increased and promote tolerance in MH5A treated mice. BALB/c T cells + TCD-BM was adoptively transferred to lethally irradiated B6 mice with MH5A or control Ab. (A) Day 14 splenocytes were stained for surface CD4 and CD25, and for intracellular FoxP3 expression. (B) Representative dot plots of splenocyte Treg percentages in spleen. Plots are gated on CD4. On the far left is cells adoptively transferred and the right is analysis of FoxP3 and CD25 at indicated timepoints. (C and D) The ratio of the absolute number of donor CD8+ T cells to CD4+CD25+FoxP3+ T cells (C) and CD4+CD25+FoxP3− T cells to CD4+CD25+FoxP3+ T cells (D) was calculated on day 14. (E) Treg absolute cell numbers in the spleen on days 5, 10, 15 and 20. (F) The ratio of the absolute number donor CD8+ T cells to CD4+CD25+FoxP3+ T cells was calculated at the indicated time points. Red lines show trends over time. (G and H) Total splenocytes harvested on days 15 and 20 were restimulated ex vivo with PMA, ionomycin and golgistop for 5 hours and stained for intracellular levels of IFN-γ. Cells were gated on CD4+ or CD8+ T cells as indicated. (H) Serum from GVHD mice was isolated at the indicated time points and analyzed by ELISA for TNF-α levels.
Regulatory T cells are selectively promoted by MH5A in vivo
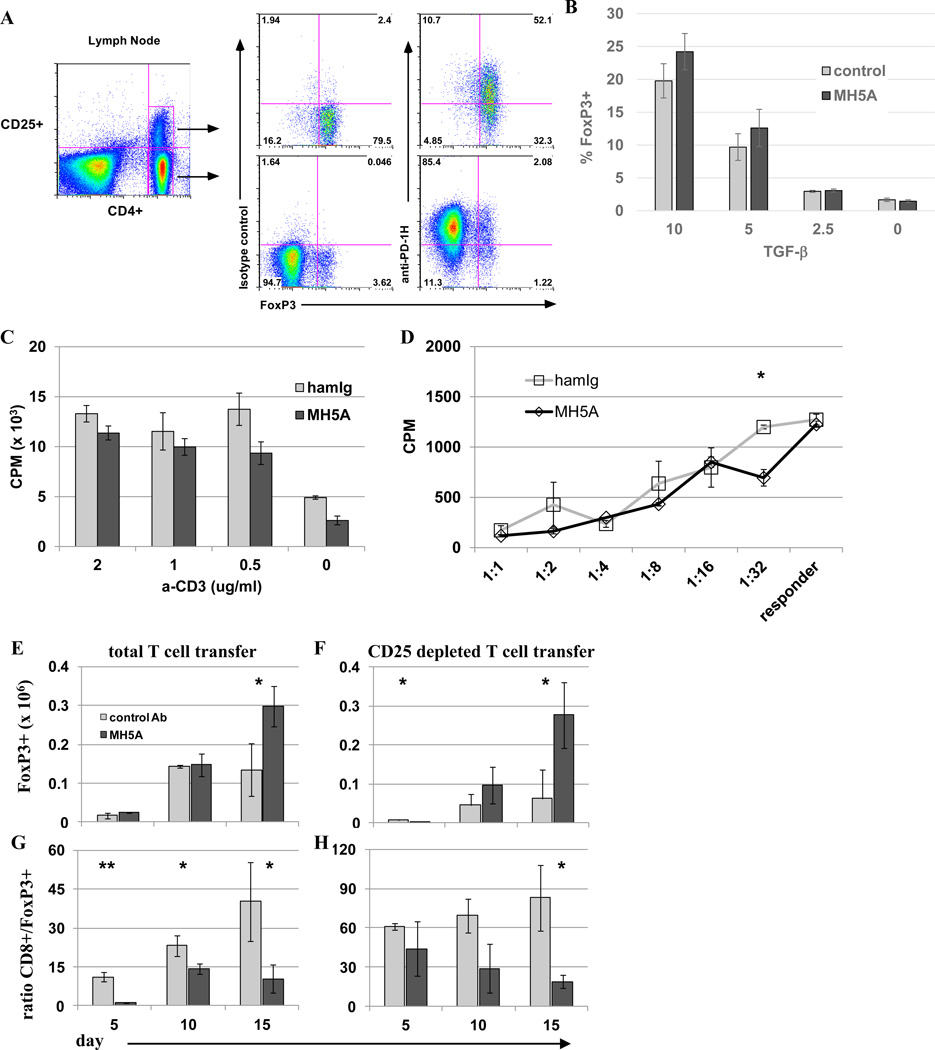
Because the majority of both CD4+FoxP3(−) naive T cells and CD4+FoxP3+ Treg cells express PD-1H (Fig. 7A), it is possible that MH5A directly regulates both naive and Treg cells. To examine the possibility that MH5A could induce Treg cells, we performed an in vitro Treg conversion assay. CFSE labeled naïve CD4+ T cells were cultured with IL-2 and titrated doses of TGF-β in the presence of MH5A or control IgG and monitored for proliferation and FoxP3 expression. We observed a slight but insignificant increase in FoxP3+ Treg cells in the presence of MH5A (Fig. 7B), thus suggesting that MH5A does not enhance Treg conversion in vitro. Next, we examined if the proliferative potential of induced Treg cells was changed in the presence of MH5A mAb. To do this, Tregs were induced for 5 days in the presence of TGF-β and were then added to 96-well plates coated anti-CD3 in the presence of 5 ng/ml IL-2, and with either control IgG or MH5A. No significant differences in proliferation were found 72 hours later (Fig. 7C). B6 Tregs induced in vitro were also used in a mixed lymphocyte response (MLR) assay in which 5 day induced Treg cells were pre-incubated with either control IgG or MH5A, then washed and added at various ratios to an MLR of B6 responder cells and irradiated BDF1 splenocytes as targets. Tregs in this assay were capable of suppressing equally well in the presence of control IgG or MH5A (Fig. 7D). These findings suggest that MH5A does not directly induce the conversion or expansion of Treg cells in vivo, or alter the suppressive function of Treg cells, although it remains unknown if the presence of additional factors in vivo that are not present in vitro may enhance MH5A effects on Treg cells in vivo.
Figure 7.
Selective expansion of regulatory T cells in vivo with MH5A treatment. (A) Peripheral lymph nodes were isolated from untreated wt B6 mice and analyzed by flow cytometry. Surface staining was performed for CD4, CD25, and control Ab or PD-1H, followed by intracellular staining for FoxP3. Top panel is gated on CD4+CD25+ T cells. Bottom panel is gated on CD4+CD25(−) T cells. (B) Naïve CD4+CD25 negative T cells were isolated by MACS bead negative selection of CD4+ T cells followed by depletion of CD25+ cells with biotin labeled CD25 followed by anti-biotin MACS bead magnetic depletion. Cells were then cultured with anti-CD3, anti-CD28, recombinant IL-2 and titrated concentrations of TGF-β in the presence of soluble MH5A or control Ab for 5 days and analyzed for percentages of CD4+FoxP3+. (C) Treg cells were induced as in (B) but without control Ig or MH5A treatment. These cells were then added to 96-well plates with 5 ng/ml IL-2 and pre-coated with anti-CD3. MH5A or hamIg was added to wells at a final concentration of 10 ug/ml. Treg cell proliferation was analyzed 72 hours later. Experiment was repeated three times. (D) Treg cells were induced as in (B) and used in a mixed lymphocyte response (MLR) assay. Treg cells were pre-incubated with control IgG or MH5A, then washed and added at various ratios to an MLR of B6 responder T cells and irradiated BDF1 splenocytes as targets. Responder proliferation was analyzed 5 days later. (E–H) Total T cells (CD25 replete) (E,G) or CD25-depleted T cells (F,H) and TCD-BM from B6 mice were adoptively transferred to lethally irradiated BDF1 mice with MH5A or control Ab. Splenocytes were isolated at the indicated time points and absolute numbers of CD4+FoxP3+ T cells (E,F) and the ratio of absolute numbers of donor CD8+ to donor CD4+FoxP3+ T cells (G,H) were calculated. 3–5 mice per group repeated a minimum of three times.
To investigate if MH5A promoted FoxP3+ Treg cell expansion and/or conversion in vivo, total T cells or CD25-depleted naïve T cells were adoptively transferred with TCD-BM from B6 donors to lethally irradiated BDF1 mice. Mice receiving total T cells or CD25-depleted T cells were treated with MH5A or control IgG on day 0. Spleens of these mice were examined on days 5, 10 and 15 for the number of CD4+FoxP3+ Treg cells and CD8+ T cells. We found that MH5A treatment resulted in enhanced expansion of donor Tregs in both adoptive transfer models (Fig. 7E, 7F). Concordantly, MH5A treatment led to a significant decrease in the ratio of CD8+ T cells to Treg cells in both settings (Fig. 7G, 7H). These in vivo data showed that MH5A selectively promotes Treg cell expansion, possibly through Treg cell conversion in vivo through direct or indirect mechanisms. In support of Treg cell conversion, we found little difference in proliferation or viability in Treg cells on days 10, 15 and 20, as measured by Ki67 and a fixable cell viability marker, respectively (Supplemental Fig. 3).
Discussion
We have previously shown that engagement of PD-1H coinhibitory receptor by agonistic mAb has profound effect in suppressing various types of T cell responses, including those to allo-reactive T cell responses and ameliorates GVHD in mouse models. The underlying mechanism, however, is yet to be elucidated. Our studies reveal two possible immunological mechanisms: prevention of early T cell priming upon engagement of allogeneic antigen and subsequent induction of regulatory T cells in vivo. In the GVHD models described here, cellular analysis and in vivo imaging demonstrate that engagement of PD-1H results in arrest of T cell expansion, an important prerequisite for the induction of T cell tolerance/anergy. Subsequently increased Treg in lymphoid organs provides another mechanism in the maintenance of long-term tolerance for allogeneic antigens. Overall, these findings support a two-stage model of PD-1H coinhibitory receptor-directed tolerance induction.
Although the nature of the PD-1H signaling pathways involved in suppressing T cell responses has yet to be elucidated, PD-1H engagement appears to “imprint” or program T cells with a tolerant status which results in allo-reactive T cells being unable to fully respond to allo-antigens. We noted that MH5A treated mice had similar radiance levels in the whole body and in lymphoid organs at 2 hours compared to control Ab treated mice, suggesting initial homing of allo-reactive T cells was similar in the presence of MH5A. However, radiance levels in MH5A treated mice remained low at later time points compared to control treated mice while vigorous proliferation of allo-reactive T cells occurs in control mice, illustrating the notion that MH5A restrains T cell activation and expansion during the T cell priming stage. It is noteworthy that PD-1H signaling seems to block naïve T cells from proliferating in the presence of allo-antigens, a condition that facilitates the induction of a tolerant status. This function is in sharp difference from its homolog, PD-1, that primarily functions in the peripheral environments to induce anergy or exhaustion of T cells (30).
The observation of T cell imprinting is consistent with the promotion of Treg cells as a major suppressive mechanism. As we have been unable to show that MH5A directly affects Treg cells in vitro, MH5A may indirectly promote Treg expansion through allo-reactive T cell modulation and reduction in IFN-γ and TNF-α levels (Figure 6). We have found that neutrophils largely outnumbered all other cell subsets following lethal irradiation and bone marrow reconstitution in both control and MH5A treated mice, suggesting rapid reconstitution of neutrophils (Flies et al, unpublished observation). We have shown previously that neutrophils constitutively express PD-1H. It is tempting to speculate that engagement of PD-1H on neutrophils may suppress their functions and indirectly affect Treg induction. However, because the majority of CD4+FoxP3+ Treg cells also express PD-1H, it remains possible that MH5A may directly regulate CD4+FoxP3+ Treg cells through PD-1H signaling. Interestingly, it has been shown that coinhibitory molecules often have differing functions on Treg cells and conventional T cells (31). Our results indicate that PD-1H signaling may utilize multiple pathways to influence T cell function.
In summary, our studies indicate that targeting of PD-1H on T cells imprints a tolerogenic status on allogeneic T cells and selectively promotes donor Treg cell expansion which together result in long-term T cell tolerance induction. Therefore, our findings implicate that prophylactic agonist targeting of PD-1H may be an effective therapeutic modality for promoting functional T cell tolerance to alloantigens in human transplantation.
Supplementary Material
Acknowledgements
We thank Beth Cadugan for editing the manuscript.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
This study was partially supported by the National Institutes of Health grants CA97085, CA16359, CA142779 and the United Technologies Corporation endowment.
References
- 1.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharpe AH. Mechanisms of costimulation. Immunol. Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 5.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- 6.Clarkson MR, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation. 2005;80:555–563. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard VM, Eng JM, Ramirez-Montagut T, Tjoe KH, Muriglan SJ, Kochman AA, Terwey TH, Willis LM, Schiro R, Heller G, Murphy GF, Liu C, Alpdogan O, van den Brink MR. Absence of inducible costimulator on alloreactive T cells reduces graft versus host disease and induces Th2 deviation. Blood. 2005;106:3285–3292. doi: 10.1182/blood-2005-01-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 9.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 10.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 14.Khaled Y, Reddy P, Krijanovski O. Emerging drugs for acute graft-versus-host disease. Expert Opin. Emerg. Drugs. 2009;14:219–232. doi: 10.1517/14728210903018891. [DOI] [PubMed] [Google Scholar]
- 15.Copelan EA. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 16.Shlomchik WD. Antigen presentation in graft-vs-host disease. Exp. Hematol. 2003;31:1187–1197. doi: 10.1016/j.exphem.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 18.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 19.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat. Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 22.Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, Chen L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J. Clin. Invest. 2014;124:1966–1975. doi: 10.1172/JCI74589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, Noelle R. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat. Rev. Immunol. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 28.Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, Reeves R, Taylor-Edwards C, Schulz S, Doyle TC, Fathman CG, Robbins RC, Herzenberg LA, Negrin RS, Contag CH. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- 29.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 31.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol. Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.