Abstract
Activation of cyclin E1, a key regulator of the G1/S cell-cycle transition, has been implicated in many cancers including hepatocellular carcinoma (HCC). Although much is known about the regulation of cyclin E1 expression and stability, its post-transcriptional regulation mechanism remains incompletely understood. Here, we report that nuclear factor 90 (NF90), a double-stranded RNA (dsRNA) binding protein, regulates cyclin E1 in HCC. We demonstrate that NF90 is upregulated in HCC specimens and that suppression of NF90 decreases HCC cell growth and delays G1/S transition. We identified cyclin E1 as a new target of NF90 and found a significant correlation between NF90 and cyclin E1 expression in HCC. The mRNA and protein levels of cyclin E1 were downregulated upon NF90 knockdown. Suppression of NF90 caused a decrease in the half-life of cyclin E1 mRNA, which was rescued by ectopic expression of NF90. Furthermore, NF90 bound to the 3′ untranslated regions (3′UTRs) of cyclin E1 mRNA in vitro and in vivo. Knockdown of NF90 also inhibited tumor growth of HCC cell lines in mouse xenograft model. Moreover, we showed that inhibition of NF90 sensitized HCC cells to the cyclin-dependent kinase 2 (CDK2) inhibitor, roscovitine. Taken together, downregulation of NF90 in HCC cell lines can delay cell-cycle progression, inhibit cell proliferation, and reduce tumorigenic capacity in vivo. These results suggest that NF90 has an important role in HCC pathogenesis and that it can serve as a novel therapeutic target for HCC.
INTRODUCTION
NF90, also known as nuclear factors associated with double-stranded RNA (dsRNA) (NFAR1), is one of the major products of alternative splicing of the interleukin enhancer binding factor 3 gene.1 NF90 was originally purified as a DNA-binding complex regulating the interleukin-2 (IL-2) promoter.2 Further work revealed that NF90 regulates IL-2 mRNA stability.3–5 In addition to its role in T cells, NF90 has also been shown to be involved in several other biological processes, including RNA processing, protein translation, DNA repair, host resistance to viral infections and mitosis.6–10 NF90 contains two conserved dsRNA binding motifs and an RGG domain responsible for its association with AU-rich elements (AREs), and post-transcriptionally regulate genes such as IL-2,Tau, VEGF, bcl-2, MKR-1, MyoD, p21 and CDC2.3–5,11–16 Microarray analysis showed that NF110, an alternative splice form of NF90, was overexpressed in malignant nasopharyngeal carcinoma cells.17 NF90 was found to bind to 3′ untranslated region (3′UTR) AREs of VEGF mRNA to enhance mRNA stability and increase its effect on breast cancer angiogenesis.12 The NF90/NF45 complex also mediates E6 oncogene expression in human papilloma virus-transformed cervical carcinoma cells.18 Although the regulation and function of NF90 have been extensively investigated, its role in tumorigenesis and tumor progression remains poorly understood.
Hepatocellular carcinoma (HCC), one of the most common solid tumors, has a high incidence and mortality rate in many countries including China.19 Although many signaling pathways have been implicated in HCC, the mechanism of liver carcinogenesis remains unknown. It is crucial to determine the pathogenic genes and molecular mechanisms of HCC formation to improve diagnosis of multiple stages of HCC and to facilitate drug development with specific liver cancer targets.
Loss of growth control is a hallmark of cancer, including HCC. Major regulatory events leading to cell proliferation occur in the G1 phase, including the uncontrolled expression of cyclins and cyclin-dependent kinases (CDKs). Cyclin E1, one of the most important cyclins, specifically drives the G1/S-phase transition in cell-cycle progression. In a normal cell cycle, cyclin E1 expression is restricted only to a very short period of time during the G1-to-S transition. Cyclin E1 binds to and activates CDK2, leading to phosphorylation of downstream substrates controlling the initiation of DNA replication and other S-phase events. The activity of the cyclin E1/ CDK2 complex reaches the highest when the cyclin E1 protein level is at its peak.20 Because cyclin E1 is a crucial regulator of the G1/S transition, dysregulation of its expression leads to uncontrolled cell growth and is often associated with malignant transformation. Upregulation of cyclin E1 is found in a variety of human cancers including breast, ovarian, colorectal and HCC.21–24 In addition, sustained high-level expression of cyclin E1, but not cyclin D1 or cyclin A, can cause genome instability and centrosome amplification, which is conducive to tumor formation.25
Most studies on the regulation of cyclin E1 have focused on gene amplification and proteolysis.26,27 Recently, it was reported that HuR, an AU-binding protein (AUBP), can post-transcriptionally regulate cyclin E1 in breast cancer by associating with cyclin E1 mRNA and regulating its stability.28 In this study, we found that NF90 is upregulated in HCC tissues. NF90 regulates cyclin E1 mRNA stability in HCC through binding to its 3′UTR and there is a significant correlation between NF90 and cyclin E1 expression in human HCC specimens. NF90 suppression in HCC cell lines delays the cell cycle in G1/S transition, inhibits cell proliferation and reduces the tumourigenic capacity in vivo. These results suggest an important new role of NF90 in HCC tumorigenesis and raise the possibility that NF90 may be a new therapeutic target for HCC.
RESULTS
NF90 is upregulated in HCC
We determined the expression of NF90 in HCC specimens paired with the corresponding neighboring normal tissues. Using quantitative real-time PCR (qRT-PCR), we found that NF90 was significantly upregulated in >60% of the HCC specimens (27 of 45 cases increased more than twofold) (Figure 1a). To confirm the qRT-PCR results, we also assessed NF90 protein levels in 27 paired HCC specimens by western blot analysis. More than 70% (21 of 27) cases exhibited higher protein levels of NF90 in the tumors compared with their corresponding controls (Figure 1b). Thus, NF90 is upregulated in the majority of HCC in comparison with neighboring normal tissues.
Figure 1. Upregulated NF90 expression in HCC specimens.
(a) NF90 mRNA expression levels were analyzed in 45 paired HCC specimens by qRT-PCR and normalized to β2-microglobulin (β2 MG). A twofold change threshold was set for identifying significant changes in gene expression. (b) The protein level of NF90 in 27 paired HCC specimens. GAPDH was used as the loading control. The commercial NF90 antibody could recognize both the NF90 and NF110 isoforms. The quantification of immunoblots is shown in the left bottom panel. T, tumor; N, non-tumor.
Knockdown of NF90 delays cell-cycle progression
To assess its potential role in HCC proliferation, we first knocked down NF90 expression using RNA interference. As NF90 and the larger alternative splice variant NF110 might have distinct functions, we selectively depleted either isoform. Western blot analysis revealed that the expression patterns of the NF90 or NF110 proteins were similar in the majority of the HCC cell lines (Supplementary Figure 1). We selected two HCC cell lines, SMMC-7721 and QGY-7703, to perform the knockdown experiments. The targeted small interfering RNA (siRNA) oligonucleotides were able to selectively repress either the NF90 or NF110 isoforms in SMMC-7721 and QGY-7703 HCC cell lines (Figure 2a). Both HCC cell lines with decreased NF90 expression had slower growth rates as judged by MTS (3-(4,5-dimethyl-thiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. In contrast, we did not observe any significant effect on cell growth when NF110 was knocked down in both HCC cell lines (Figure 2b).
Figure 2. Suppression of NF90 induces G1/S-transition arrest.
(a) Transient knockdown of NF90 in HCC cell lines. SMMC-7721 (left) and QGY-7703 (right) cells were transfected with siRNA against NF90 or NF110. (b) A cell growth assay was carried out over a 7-day culture period. Points, mean (n=6); bars, s.d. ***P < 0.001. (c) The fractions of viable cells in the G0-G1, S and G2-M phases were quantified by flow cytometry. Columns, mean (n=3); bars, s.d. *P < 0.05, **P < 0.01, ***P < 0.001. The stable NF90 knockdown SMMC-7721 cells (d) and QGY-7703 cells (e) were constructed and confirmed by western blotting. Cell growth and cell-cycle assays were analyzed as described above. Positive clones: 7721-2, 7721-3, QGY-4 and QGY-6; control: 7721-C and QGY-C.
Next, we carried out the flow cytometry and an apoptosis assay to determine whether the decrease in cell proliferation upon NF90 knockdown was due to inhibition of cell-cycle progression or cell death. Knockdown of NF90 resulted in an increase in the G1 population, and a decrease in S-phase cells (Figure 2c). No obvious difference was observed in the sub-G1 phase cells, indicating that NF90 knockdown affected cell-cycle progression without inducing apoptosis (data not shown). Consistent with these results, HCC cell lines with stable knockdown of NF90 also showed G1/S-transition arrest and a slower growth rate than the control cells (Figures 2d and e). These findings were further confirmed in colony formation assays (Supplementary Figure 2). Together, these results suggested that NF90, but not NF110, is required for HCC cell proliferation and cell-cycle progression.
NF90 regulates cyclin E1 expression
To search for putative NF90 target genes involved in cell-cycle regulation, we analyzed the genes from the microarray data (Supplementary Table 2).16 We compared the levels of these potential target genes and their family members between control cells and cells with transiently decreased NF90 levels. As shown in Figure 3a, cyclin D1, cyclin E1 and MAPK6 mRNAs were consistently decreased by more than twofold in the two HCC cell lines upon NF90 knockdown. Cyclin E1 protein levels were also significantly downregulated in the NF90-knockdown cells (Figures 3b and c). Surprisingly, the level of cyclin D1 did not change appreciably. These results suggested that cyclin E1, but not cyclin D1, is likely to be the target of NF90 that mediates its effect on cell-cycle regulation.
Figure 3. Expression of cyclin E1 is associated with NF90.
(a) The relative mRNA abundance of cell cycle-related genes was analyzed by qRT-PCR. SMMC-7721 or QGY-7703 cells were transiently transfected with siRNA against NF90. Columns, mean (n=3); bars, s.d. ▲, the expression level changed >30% in the two HCC cell lines treated with si90-1 and si90-2 separately. (b) Cyclin E1 and cyclin D1 protein levels were detected by western blotting after cells were transiently transfected with siRNA. (c) Cyclin E1 and cyclin D1 protein levels were detected in the stable cell lines. (d) Cyclin E1 mRNA level in 45 paired HCC specimens was examined by qRT-PCR. The scatter plot shows the correlation of NF90 and cyclin E1 expression. The data were analyzed using a paired f-test, and correlation analysis was performed using Pearson’s correlation test. (e) Protein levels of NF90 and cyclin E1 were detected by western blotting in seven randomly selected paired samples.
To further confirm the relationship between NF90 and cyclin E1 expression in HCC in vivo, we analyzed cyclin E1 mRNA expression in the identical 45 paired human HCC tissues as mentioned above. A statistically significant correlation (r = 0.683, P < 0.001) was observed between the NF90 and cyclin E1 genes (Figure 3d). We also investigated NF90 and cyclin E1 protein levels in seven paired HCC specimens selected randomly, which also displayed a significant correlation (Figure 3e).
NF90 regulates the half-life of cyclin E1 mRNA
Previously, we showed that IL-2 mRNA is regulated by NF90 at the mRNA level through its AREs. The cyclin E1 3′UTR also contains several ARE sequences,28 raising the possibility that its mRNA stability could also be regulated by AUBP. To determine whether cyclin E1 mRNA stability is affected upon NF90 knockdown, we blocked transcription and then analyzed cyclin E1 mRNA decay. The t1/2 of cyclin E1 mRNA in SMMC-7721 cells decreased from 2.03 ± 0.38 h to 0.69 ± 0.17 h or 0.65 ± 0.10 h when NF90 was knocked down. Similarly, the t1/2 of cyclin E1 mRNA declined from 3.77 ±0.16 h to 1.17 ± 0.23 h or 2.50 ±0.31 h upon NF90 knockdown in the QGY-7703 cell line (Figure 4a). To ensure the specificity of knockdown of NF90, we expressed an RNA interference-resistant version of Myc-tagged NF90 in cells stably suppressing NF90 and observed restoration of cyclin E1 expression in a dose-dependent manner (Figure 4b, lanes 3 and 4). The half-life of cyclin E1 mRNA was increased when NF90 was restored (Figure 4c). The distribution of the G1/S-phase populations was also partially recovered (Supplementary Figure 3). These data suggested that NF90 is required for stabilizing cyclin E1 mRNA.
Figure 4. The half-life of cyclin E1 mRNA is regulated by NF90.
(a) Suppression of NF90 shortened the half-life of cyclin E1 mRNA. SMMC-7721 (left) and QGY-7703 (right) cells were treated with siRNA against NF90, and the half-life of cyclin E1 mRNA was determined by qRT-PCR after treatment with actinomycin D for the indicated time. Data were normalized to RPLPO mRNA and plotted on semilogarithmic scales. Points, mean (n=3); bars, s.d. *P < 0.05, **P < 0.01, ***P < 0.001. Ectopic expression of NF90-Myc recovered the amount of the cyclin E1 protein (b) and increased the half-life of cyclin E1 mRNA (c) in HCC cells stably downregulating NF90. Points, mean (n=3); bars, s.d. *P < 0.05, **P < 0.01, ***P < 0.001.
NF90 binds to the 3′UTR of cyclin E1 mRNA
The finding that cyclin E1 mRNA stability was regulated by NF90 prompted us to determine whether NF90 is bound to the 3′UTR of cyclin E1 mRNA. To assess the association between NF90 and the cyclin E1 3′UTR, we conducted an in vitro RNA pull-down assay and a ribonucleoprotein immunoprecipitation assay. First, we made constructs with the 3′UTR of IL-2, cyclin D1, cyclin E1 and three 3′UTR truncations of cyclin E1 mRNA (Figure 5a). qRT-PCR was performed to examine RNA enrichment in the protein-RNA complex pulled down by GST-NF90Δ, containing only two dsRNA-binding domains recombined with a GST tag (Supplementary Figure 4). As shown in Figure 5b, the in vitro transcribed cyclin E1 3′UTR was greatly enriched in the GST-NF90Δ pull-down complex, even more than the positive control—the IL-2 mRNA 3′UTR. Moreover, three 3′UTR truncation fragments of cyclin E1 mRNA could be bound by GST-NF90Δ, but significantly less than the wild-type sequence. In contrast, no apparent enrichment was observed for the cyclin D1 3′UTR.
Figure 5. NF90 combines with cyclin E1 mRNA in vitro and in vivo.
(a) Schematic view of the constructs containing the 3′UTR of IL-2, cyclin D1, cyclin E1 wild-type and three 3′UTR truncates of cyclin E1 mRNA. (b) The relative abundance of transcripts obtained after the RNA pull-down assay using GST or GST-NF90Δ protein as measured by qRT-PCR, the fold differences were plotted on a log scale. Columns, mean (n = 3); bars, s.d. ***P < 0.001. (c) Cyclin E1 mRNA is present in the NF90 IP complex. Left, The NF90-Myc protein complex was precipitated with Myc antibody and detected by an antibody against endogenous NF90. Right, the 3′UTRs of cyclin E1 and cyclin D 1 mRNA isolated from each IP complex and cell lysates (input) were identified by PCR.
Commercial antibodies of NF90 recognize not only NF90 but also NF110 isoforms, which can also bind mRNA via the same dsRNA-binding domain. To avoid NF110 immunoprecipitation artifacts, we established a Hep3B cell line that stably expressed exogenous Myc-tagged NF90 (terminal) protein and used it for ribonucleoprotein immunoprecipitation assays with anti-Myc antibodies (Figure 5c). Cyclin E1 mRNA was seen in the samples pulled down with Myc antibodies but not with the IgG control. And we could not detect any PCR product of the cyclin D1 mRNA 3′UTR. Moreover, overexpression of NF90 increased cyclin E1 protein expression, proliferation rate and G1/S progression compared with the control (Supplementary Figure 5). These results supported the notion that NF90 specifically binds to the cyclin E1 mRNA 3′UTR both in vitro and under physiological conditions.
Knockdown of NF90 decreases tumor growth in vivo
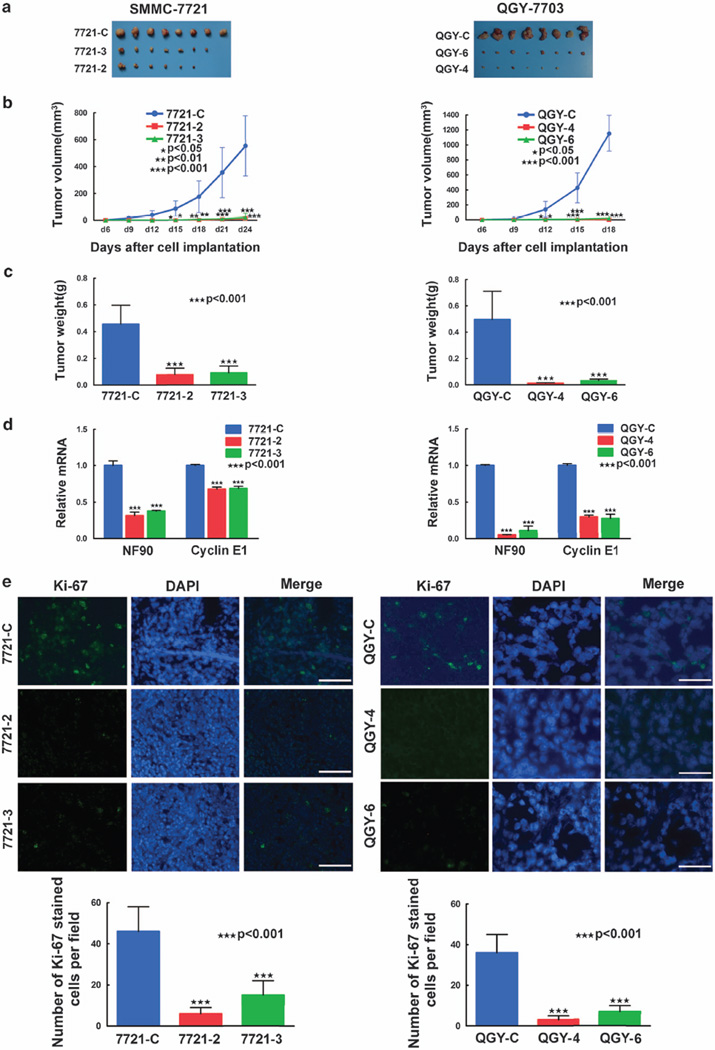
The finding that NF90 promoted proliferation of HCC cells in vitro prompted us to determine whether it exerts a similar effect in vivo. Control or NF90-knockdown cell lines were subcutaneously injected into nude mice. NF90 knockdown resulted in a significant decrease in the volume and weight of tumors (Figures 6a–c). NF90 and cyclin E1 mRNA levels were decreased in the tumor samples from the NF90-knockdown cell line xenografts (Figure 6d). Ki-67, a cell proliferation marker, was almost undetectable in NF90-knockdown xenografts (Figure 6e). These results indicated that downregulation of NF90 inhibits the growth of HCC tumors by repressing in vivo cell proliferation.
Figure 6. Suppression of NF90 decreases tumorigenicity.
(a) The resected tumors from individual nude mice. Eight mice were used in each group. Top, SMMC-7721 cells; bottom, QGY-7703 cells. (b) The tumor volume of the xenografts. Points, mean (n=8); bars, s.d. *P < 0.05, **P < 0.01, ***P < 0.001. (c) The tumor weight of the xenografts after dissection. Columns, mean (n=8); bars, s.d. ***P < 0.001. (d) The relative mRNA abundance of the resected tumors. Columns, mean (n = 3); bars, s.d. ***P < 0.001. (e) Immunohistochemical analysis of Ki-67 in the tumor tissues (top: immunostaining; bottom: the quantified result). Columns, mean (n=4); bars, s.d. ***P < 0.001.
NF90 knockdown sensitizes HCC cells to the CDK inhibitor roscovitine
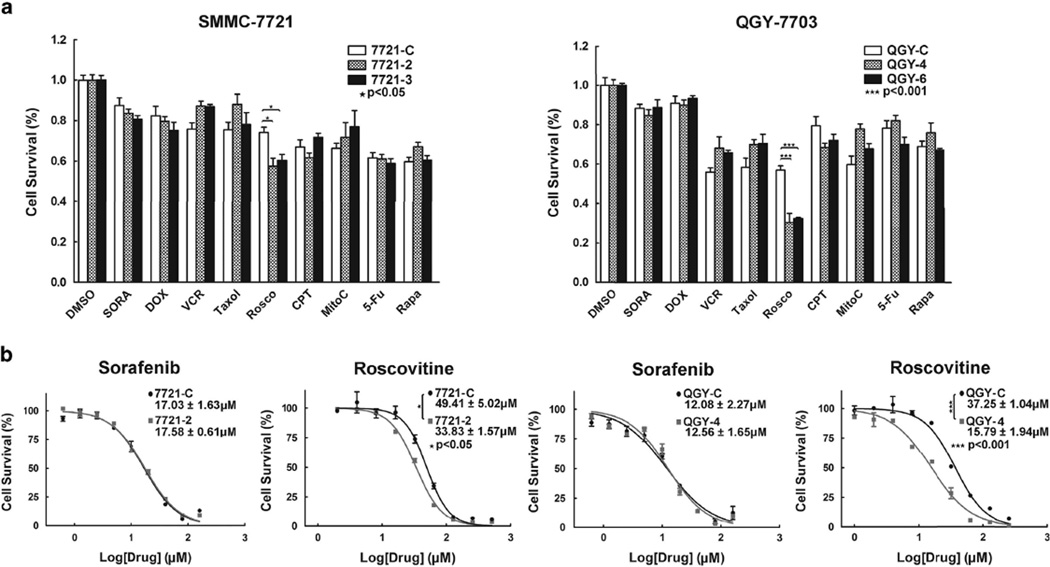
There are currently effective treatments for HCC, due in large part to a high level of resistance to various chemotherapeutic drugs. We thus determined whether the upregulation of NF90 contributed to the resistance of HCC cells to chemotherapy. We tested eight commonly used antineoplastic drugs as well as the CDKs inhibitor roscovitine (Selleck, Houston, TX, USA). No obvious difference was observed between cells with decreased NF90 and controls after treatment with any of the agents except for roscovitine (Figure 7a). Roscovitine, a potent and selective inhibitor targeting cyclin E/CDK2, cyclin B/ CDK1 and cyclin A/CDK2, has undergone phase II clinical trials.29–31 We also examined whether NF90 knockdown sensitized HCC cells to roscovitine-induced cell death. As shown in Figure 7b, the IC50 of 7721-2 was 33.8 ± 1.57 µm and therefore was more sensitive than the control 7721-C (IC50 = 49.4 ± 5.02 µm) after roscovitine treatment. This difference was more pronounced in QGY-7703 stable cell lines (IC50 of QGY-4 = 15.8 ± 1.94 µm and IC50 of QGY-C = 37.3 ± 1.04 µm). The IC50 for sorafenib did not significantly change. These results suggested that NF90 suppression also decreases the resistance of HCC cell lines to roscovitine.
Figure 7. Suppression of NF90 increased the sensitivity of HCC cells to the CDK2 inhibitor roscovitine.
(a) The sensitivity of different drugs in SMMC-7721 (left) or QGY-7703 (right) cells upon knockdown of NF90. Columns, mean (n = 4); bars, s.d. *P < 0.05, ***P < 0.001. (b) Semi-logarithmic dose of sorafenib or roscovitine in SMMC-7721 or QGY-7703 upon stable knockdown of NF90, IC50 values were displayed respectively. Points, mean (n = 3); bars, s.d. *P < 0.05, ***P < 0.001.
DISCUSSION
NF90 was originally identified as a post-transcriptional regulator of IL-2 mRNA through binding to the ARE of 3′UTR. However, its function is not restricted to T cells, as it has also been found to regulate a number of other mRNAs and to be involved in the regulation of cell proliferation. In this study, we found that NF90 is upregulated in HCC and its expression increases HCC growth both in vitro and in vivo. Furthermore, we identified cyclin E1 as a novel target of NF90. NF90 stabilized cyclin E1 mRNA through binding to its 3′UTR, enhancing cyclin E1 expression and promoting cell proliferation.
The binding of NF90 to the 3′UTR of its target mRNA can have distinct consequences on gene expression. In the case of IL-2, NF90 binding to the AREs of IL-2 mRNA leads to its stabilization and an increase in IL-2 protein level.3–5 For a subset of NF90 target mRNAs, however, NF90 binding leads to the inhibition of translation, decreasing protein levels of the target mRNA.16 The effect of NF90 on cyclin E1 mRNA is similar to that on IL-2, as upregulation of NF90 was associated with increases in both RNA and protein levels of cyclin E1, while downregulation led to a decrease in cyclin E1 mRNA and protein levels.
It has been previously reported that repression of either NF90 or its binding partner NF45, but not NF110, leads to retardation of HeLa cell growth and accumulation of multinucleate giant cells.10 However, the underlying mechanism has remained undefined. Similar to that observation, we found that knockdown of NF90 decreased the growth of HCC cell lines and caused G1/S arrest (Figures 2b and c). Moreover, we identified cyclin E1, but not cyclin D1, as a target gene of NF90 after screening-related cell-cycle genes (Figures 3a–c). Upregulation of NF90 is closely correlated with elevation of cyclin E1 in human HCC specimens (Figures 3d and e). The association between NF90 and cyclin E1 might explain the G1/S arrest after the NF90 silencing in HCC cells and may explain the increased cyclin E1 levels in some HCC. Although cyclin D1 mRNA also decreased upon knockdown of NF90, cyclin D1 protein levels did not change appreciably. The decreased mRNA levels of cyclin D1 may have resulted from a secondary effect after downregulation of NF90. It remains to be determined how cyclin D1 protein, unlike its mRNA, remained unchanged.
A number of mRNA targets of NF90 have been reported to date, some of which are also known to be involved in the regulation of cell-cycle progression including p21 and CDC2.15,16 The decrease of p21 in NF90(−/−) mice resulted in an increase in apoptosis, which led to a remarkable decrease in mature skeletal muscle fibers.15 However, another report indicated that silencing the NF90/NF45 complex induced p21 and apoptosis by E6/E6AP-p53 pathway only in HeLa cells, not in non-HPV cervical carcinoma and non-cervical carcinoma cells.18 NF90 prevented the translation of CDC2, cyclin A and cyclin I, but had no obvious influence on the stability and concentration of their mRNAs. Silencing NF90 increased these proteins and moderately enhanced DNA replication.16 We also observed no change in the abundance of CDC2, cyclin A and cyclin I mRNAs in the NF90-knockdown cells (Supplementary Table 2).
Cyclin E1 has an important role in the proliferation of tumor cells, and cyclin E1 overexpression has been reported to have myriad effects on transcription and post-transcriptional events. Consistent with previous reports that NF90 functions as an AUBP to regulate IL-2 or VEGF,3–5,12 our results suggested that NF90 regulates cyclin E1 in the same manner. We showed that altering NF90 levels had a clear effect on the half-life of cyclin E1 mRNA (Figure 4). The regulation of mRNA stability is an important element of eukaryotic gene expression, particularly for acute phase genes with unstable mRNA. Cyclin E1 can quickly respond to growth factor stimulation and its 3′UTR contains multiple AREs, raising the possibility that its mRNA is regulated via these elements. In support of this, HuR, an AUBP, can stabilize cyclin E1 mRNA by binding to its 3′UTR AREs.28 Our studies revealed that NF90, another member of the AUBP family, also directly binds to the 3′UTR of cyclin E1 mRNA in its 3′UTR region in vitro and in vivo (Figures 5b and c), which enhances its stability in the HCC cell lines.
Through the xenograft assay, we found that the tumorigenic capacity of HCC cell lines in nude mice was decreased upon NF90 knockdown (Figure 6), suggesting that NF90 has an important role in HCC tumor formation in vivo. These results suggested that NF90 may serve as a new target for developing anti-HCC drugs. Furthermore, we searched for synergy between NF90 knockdown and existing anticancer drugs. Interestingly, among nine drugs or drug candidates, NF90 only enhanced the cytotoxic effect of roscovitine on HCC in vitro. In retrospect, this unique effect of NF90 knockdown can be explained by its effect on cyclin E1 mRNA. As roscovitine functions by inhibiting multiple CDKs including cyclin E/CDK2, it is not surprising that a concurrent decrease in cyclin E1 expression level as a consequence of NF90 knockdown would enhance the antitumor activity of roscovitine.
In summary, our findings suggest that regulation of cyclin E1 mRNA stability by NF90 is responsible for liver cancer progression. Clarification of the mechanism accounting for the synergy between NF90 knockdown and roscovitine might provide a new therapeutic approach for the treatment of HCC in the future.
MATERIALS AND METHODS
Tumor specimens
Fresh surgical specimens of HCC, including tumor tissues and the neighboring pathologically non-tumorous liver tissue, were obtained from liver cancer patients at the Qidong Liver Cancer Institute (Jiangsu province, China). All samples were immediately frozen in liquid nitrogen after surgery and then later stored at −80 °C before further analysis.
Western blot
Protein samples were separated by SDS–PAGE and then transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). After blocking, the membranes were incubated with specific antibodies against different proteins at room temperature for 2 h or 4 °C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was visualized by enhanced chemilumines-cence (GE Healthcare, Piscataway, NJ, USA). Antibodies were against NF90 (interleukin enhancer binding factor 3, BD Biosciences, Franklin Lakes, NJ, USA; Epitomics, Burlingame, CA, USA), cyclin E1 (Epitomics), cyclin D1 (Epitomics), Myc (Sigma, St Louis, MO, USA), h-actin (Sigma) and GAPDH (Sigma).
siRNA transient transfection
siRNA targeting NF90 or NF110 was synthesized by Genepharma (Shanghai, China). The siRNA oligo sequences for NF90 are si90-1: 5′-CAGCGUUGUUCGGCAUCAA-3′,12 si90-2: 5′-CAGACUGCUACGGCUA UCA-3′;10 the siRNA oligo sequences for NF110 are si110: 5′-GGAU GUUGUCACAGCUAGU-3′.12 The sequence of the negative control is non-silence: 5′-UUCUCCGAACGUGUCACGU-3′ (Genepharma). Human HCC cells were transfected with siRNA at a final concentration of 100nM using LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The knockdown efficiency was determined by western blotting.
Generation of stable cell line
For the generation of stable cell lines, we purchased lentiviral stocks from Geneparma, produced by cotransfection of 293T cells with recombinant lentiviral shRNA vector (pGLV-U6-Puro) containing the NF90 target sequence (si90-1) and the negative control, respectively. Stably transduced SMMC-7721 or QGY-7703 cells were selected using puromycin, adding the minimum concentration of puromycin required to kill untransduced cells. Individual colonies with NF90 knockdown were isolated and confirmed by green fluorescence and western blotting. Stable control colonies were also generated in parallel. Since surviving individual colonies with NF90 knockdown were very few, we randomly selected two colonies in each cell line (named 7721-2 and 7721-3, QGY-4 and QGY-6). We also randomly mixed 10 control colonies of each cell line (named 7721-C and QGY-C).
The NF90 cDNA was subcloned into the mammalian expression vector pcDNA3.1a (−) (Invitrogen) containing a Myc tag and neomycin resistance gene for establishment of stable transfectants. After selection with G418 and detection by western blotting, 10 randomly selected colonies expressing NF90-Myc were combined (named Hep3B-NF90). Control colonies were generated in parallel (named Hep3B-MV).
Cell proliferation assays
All cells were seeded on 96-well plates at a density of 1000 cells per well. During a 7-day culture period, cells were subjected to MTS (Promega, Madison, WI, USA) assay every 2 days. The absorbance of each well was measured at 2.5 h after incubation by a microtiter reader (Bio-Rad, Hercules, CA, USA) at 450 nm. Results are representative of three independent experiments with six samples for each cell.
Flow-cytometry analysis
For cell-cycle analysis, cells were harvested and resuspended in 70% ice-cold alcohol for fixation overnight. DNA was stained with propidium iodide (50 µg/ml) together with RNase treatment (100 µg/ml) before being analyzed by FACSCalibur (BD Biosciences). At least 10 000 cells were acquired for each sample. Results are representatives of three independent experiments with triplicate samples for each condition.
Quantitative real-time PCR
Total RNA was extracted from tissues or cultured cells using Trizol reagent (Invitrogen), and 1–2 µg of RNA was applied for reverse transcription using an oligo dT primer (Invitrogen) and reverse transcriptase (Invitrogen). qRT-PCR analysis was conducted using a SYBR Green Supermix kit (Toyobo, Osaka, Japan) with a Light Cycler 480 II (Roche, Basel, Switzerland). Properly diluted cDNA was used in a 10-µl qRT-PCR in triplicate for each gene. The cycle parameters were 95 °C for a 1-min hot start and 45 cycles of 95 °C for 10 s, 60 °C for 10 s and 720 °C for 20 s. Blank controls with no cDNA templates were performed to rule out contamination. The specificity of the PCR product was confirmed by melting curve analysis and gel electrophoresis. Gene expression levels were normalized to the β2MG housekeeping gene. The primers for each gene are shown in Supplementary Table 2.
Half-life of cyclin E1 mRNA
For mRNA stability measurements, samples were treated with actinomycin D (5 µg/ml). Total RNA was extracted at the indicated time points following addition of actinomycin D. Cyclin E1 mRNA expression levels were normalized to 18s RNA or RPLP0.
RNA pull-down assay using recombinant proteins
Plasmids were digested with BamH1 to linearize the vectors and purified. Biotin-labeled RNAs were in vitro transcribed with the Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Promega), treated with RNase-free DNase I (Promega) and purified with the RNeasy Mini Kit (QIAGEN). RNA was refolded by heating to 85–90 °C for 2–5 min and then slowly cooled to room temperature.
GST protein was incubated with 3 µg in vitro transcribed RNA in RIPA buffer (50 mm Tris-HCl (pH 7.9), 0.25 m NaCl, 1% Nonidet P-40 (NP-40), 10 mm EDTA, 1 mm EGTA and RNase inhibitor; Promega) for 18 h at 4 °C.
Protein-RNA complexes bound to GST beads were washed five times with NT2 buffer (containing protease inhibitor cocktail and RNase inhibitor, 50 mm Tris-HCI (pH 7.4), 300 mm NaCl, 1 mm MgCl2, 0.05% NP-40, prepared in RNase-DNase-free H2O). NT2 buffer can also be supplemented with 30 µg of proteinase K to release the ribonucleoprotein components. The mixture was incubated for 30 min at 55 °C, flicking the tube occasionally.
Co-purified RNA was extracted by RNA purification kit (RNAeasy MiniElute kit, QIAGEN). Purified RNA was reverse transcribed to cDNA and detected by qRT-PCR. Primers for each fragment are shown in Supplementary Table 1.
Ribonucleoprotein immunoprecipitation
In all, 20 µl of protein A Sepharose beads was incubated with 50 µg of anti-Myc antibody or 50 µg of IgG in 500 µl IP buffer (0.5% Triton X-100, 200 mm NaCl, 10 mm Tris-HCI at pH 7.5, and 10 mm EDTA) at 4 °C overnight. Forty million cells were harvested and resuspended in 30–50 pellet volume of lysis buffer (0.5% Triton X-100, 10 mm NaCl, 10 mm Tris-HCI at pH7.5, 10 mm EDTA, 0.5 mm PMSF, 1 mm DTT, protease inhibitor cocktail (Calbiochem, Billerica, MA, USA), and 400 units/ml RNase inhibitor). The suspensions were incubated on ice for 20 min. After centrifugation, supernatant was precleared using 10µl of protein A sepharose (NaCl was added to a final concentration of 200 mm), followed by addition of yeast tRNA (Ambion, Foster City, CA, USA) to a final concentration of 40 µg/ml. The cleared supernatant was transferred to tubes containing antibody or IgG-coated beads, and IP was carried out by rotating the tubes at 4 °C for 4 h. Following IP, the beads were washed with IP buffer containing 10 µg/ml yeast tRNA.32 RNA extraction and reverse transcription were performed as mentioned above. Cyclin E1 3′UTR fragments were detected by two specific primers (Supplementary Table 1).
In vivo assays for tumor growth
Four- to six-week old BALB/c nu/nu mice obtained from the Institute of Materia Medica (CAS, Shanghai, China) were bred and maintained in our institutional pathogen-free mouse facilities. To determine the role of NF90 on HCC growth in vivo, 4×106 cells of SMMC-7721 or QGY-7703 stable cell lines suspended in 200 µl of PBS were implanted into the flank of nude mice. The tumor volumes were measured every 3 days from the sixth day after implantation.
Immunohistochemistry analysis
Frozen samples were cut into 4 µm slides and hydrated. Tissue sections were fixed with pre-cooled acetone (−20 °C) for 10 min. After acetone evaporation, slides were rinsed with PBS, and then incubated with 0.3% H2O2 solution in PBS at room temperature for 10 min to block endogenous peroxidase activity. After adding blocking buffer (10% fetal bovine serum in PBS) onto the sections, the slides were incubated in a humidified chamber at room temperature for 1 h. After PBS wash, slides were incubated with Ki-67 (Dako, Glostrup, Denmark) antibody overnight at 4 °C and then incubated with biotinylated secondary antibodies for 1 h both in a humidified condition. 4',6-diamidino-2-phenylindole (DAPI, Sigma) was used for nuclear staining.
Statistical analysis
For qRT-PCR, the relative gene expression levels normalized by β2-microglobulin (β2MG) were calculated by the formula 2−ΔCt, where the ΔCt (critical threshold) = Ct of genes of interest − Ct of β2MG. For the analysis of HCC specimens, fold changes of gene expression levels in the tumor specimens relative to the corresponding non-tumorous specimens (T/N) were calculated by the 2−ΔΔct method and transformed to log 2, where ΔΔCt = ΔCttumor − ΔCtnonturnorous. A twofold change threshold was set for identifying significant changes in gene expression. Statistical analysis was performed using the GraphPad Prism software (GraphPad Prism, La Jolla, CA, USA). A two-tailed Student’s t-test was used to evaluate the group-level differences. We considered P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***) to be statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Key Sci-Tech Special Project of China (2013ZX10002010), and the National Natural Science Foundation of China (31270830). We thank Dr Tilman Schneider-Poetsch, Sarah Head and Yang Xianmei for generously editing the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Duchange N, Pidoux J, Camus E, Sauvaget D. Alternative splicing in the human interleukin enhancer binding factor 3 (ILF3) gene. Gene. 2000;261:345–353. doi: 10.1016/s0378-1119(00)00495-9. [DOI] [PubMed] [Google Scholar]
- 2.Corthesy B, Kao PN. Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J Biol Chem. 1994;269:20682–20690. [PubMed] [Google Scholar]
- 3.Zhu P, Jiang W, Cao L, Yu W, Pei Y, Yang X, et al. IL-2 mRNA stabilization upon PMA stimulation is dependent on NF90-Ser647 phosphorylation by protein kinase Cbetal. J Immunol. 2010;185:5140–5149. doi: 10.4049/jimmunol.1000849. [DOI] [PubMed] [Google Scholar]
- 4.Shim J, Lim H, RY J, Karin M. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol Cell. 2002;10:1331–1344. doi: 10.1016/s1097-2765(02)00730-x. [DOI] [PubMed] [Google Scholar]
- 5.Pei Y, Zhu P, Dang Y, Wu J, Yang X, Wan B, et al. Nuclear export of NF90 to stabilize IL-2 mRNA is mediated by AKT-dependent phosphorylation at Ser647 in response to CD28 costimulation. J Immunol. 2008;180:222–229. doi: 10.4049/jimmunol.180.1.222. [DOI] [PubMed] [Google Scholar]
- 6.Shiohama A, Sasaki T, Noda S, Minoshima S, Shimizu N. Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp Cell Res. 2007;313:4196–4207. doi: 10.1016/j.yexcr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Ting NS, Kao PN, Chan DW, Lintott LG, Lees-Miller SP. DNA-dependent protein kinase interacts with antigen receptor response element binding proteins NF90 and NF45. J Biol Chem. 1998;273:2136–2145. doi: 10.1074/jbc.273.4.2136. [DOI] [PubMed] [Google Scholar]
- 8.Xu YH, Grabowski GA. Molecular cloning and characterization of a translational inhibitory protein that binds to coding sequences of human acid beta-glucosidase and other mRNAs. Mol Genet Metab. 1999;68:441–454. doi: 10.1006/mgme.1999.2934. [DOI] [PubMed] [Google Scholar]
- 9.Nanda S, Havert MB, Calderon GM, Thomson M, Jacobson C, Kastner D, et al. Hepatic transcriptome analysis of hepatitis C virus infection in chimpanzees defines unique gene expression patterns associated with viral clearance. PLoS ONE. 2008;3:e3442. doi: 10.1371/journal.pone.0003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan D, Altan-Bonnet N, Parrott AM, Arrigo CJ, Li Q, Khaleduzzaman M, et al. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol Cell Biol. 2008;28:4629–4641. doi: 10.1128/MCB.00120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larcher JC, Gasmi L, Viranaicken W, Edde B, Bernard R, Ginzburg I, et al. Ilf3 and NF90 associate with the axonal targeting element of Tau mRNA. FASEB J. 2004;18:1761–1763. doi: 10.1096/fj.04-1763fje. [DOI] [PubMed] [Google Scholar]
- 12.Vumbaca F, Phoenix KN, Rodriguez-Pinto D, Han DK, Claffey KP. Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol Cell Biol. 2008;28:772–783. doi: 10.1128/MCB.02078-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bose SK, Sengupta TK, Bandyopadhyay S, Spicer EK. Identification of Ebp1 as a component of cytoplasmic bcl-2 mRNP (messenger ribonucleoprotein particle) complexes. Biochem J. 2006;396:99–107. doi: 10.1042/BJ20051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwano Y, Kim HH, Abdelmohsen K, Pullmann R, Jr., Martindale JL, Yang X, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Zhao G, Qiu D, Godfrey WR, Vogel H, Rando TA, et al. NF90 regulates cell cycle exit and terminal myogenic differentiation by direct binding to the 3′-untranslated region of MyoD and p21WAF1/CIP1 mRNAs. J Biol Chem. 2005;280:18981–18989. doi: 10.1074/jbc.M411034200. [DOI] [PubMed] [Google Scholar]
- 16.Kuwano Y, Pullmann R, Jr., Marasa BS, Abdelmohsen K, Lee EK, Yang X, et al. NF90 selectively represses the translation of target mRNAs bearing an AU-rich signature motif. Nucleic Acids Res. 2010;38:225–238. doi: 10.1093/nar/gkp861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung LF, Lo AK, Yuen PW, Liu Y, Wang XH, Tsao SW. Differential gene expression in nasopharyngeal carcinoma cells. Life Sci. 2000;67:923–936. doi: 10.1016/s0024-3205(00)00684-6. [DOI] [PubMed] [Google Scholar]
- 18.Shamanna RA, Hoque M, Pe'ery T, Mathews MB. Induction of p53, p21 and apoptosis by silencing the NF90/NF45 complex in human papilloma virus-transformed cervical carcinoma cells. Oncogene. 2013;32:5176–5185. doi: 10.1038/onc.2012.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, et al. Major causes of death among men and women in China. New Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 20.Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 21.Keyomarsi K, Pardee AB. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui L, Dong Y, Ohno M, Sugimoto K, Tai Y, Hando T, et al. Implication of malignancy and prognosis of p27(kip1), Cyclin E, and Cdk2 expression in epithelial ovarian tumors. Gynecol Oncol. 2001;83:56–63. doi: 10.1006/gyno.2001.6308. [DOI] [PubMed] [Google Scholar]
- 23.Cam WR, Masaki T, Shiratori TY, Kato N, Okamoto M, Yamaji Y, et al. Activation of cyclin E-dependent kinase activity in colorectal cancer. Dig Dis Sci. 2001;46:2187–2198. doi: 10.1023/a:1011962915280. [DOI] [PubMed] [Google Scholar]
- 24.Peng SY, Chou SP, Hsu HC. Association of downregulation of cyclin D1 and of overexpression of cyclin E with p53 mutation, high tumor grade and poor prognosis in hepatocellular carcinoma. J Hepatol. 1998;29:281–289. doi: 10.1016/s0168-8278(98)80014-7. [DOI] [PubMed] [Google Scholar]
- 25.Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 26.Akama Y, Yasui W, Yokozaki H, Kuniyasu H, Kitahara K, Ishikawa T, et al. Frequent amplification of the cyclin E gene in human gastric carcinomas. Jpn J Cancer Res. 1995;86:617–621. doi: 10.1111/j.1349-7006.1995.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–322. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 28.Guo X, Hartley RS. HuR contributes to cyclin E1 deregulation in MCF-7 breast cancer cells. Cancer Res. 2006;66:7948–7956. doi: 10.1158/0008-5472.CAN-05-4362. [DOI] [PubMed] [Google Scholar]
- 29.Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzi T. CYC-202 cyclacel. Curr Opin Invest Drugs. 2004;5:1311–1318. [PubMed] [Google Scholar]
- 31.Whittaker SR, Te Poele RH, Chan F, Linardopoulos S, Walton MI, Garrett MD, et al. The cyclin-dependent kinase inhibitor seliciclib (R-roscovitine; CYC202) decreases the expression of mitotic control genes and prevents entry into mitosis. Cell Cycle. 2007;6:3114–3131. doi: 10.4161/cc.6.24.5142. [DOI] [PubMed] [Google Scholar]
- 32.Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.