Abstract
Ethinyl estradiol (EE), a synthetic, orally bio-available estrogen, is the most commonly prescribed form of estrogen in oral contraceptives, and is found in at least 30 different contraceptive formulations currently prescribed to women as well as hormone therapies prescribed to menopausal women. Thus, EE is prescribed clinically to women at ages ranging from puberty to reproductive senescence. Here, in two separate studies, the cognitive effects of cyclic or tonic EE administration following ovariectomy (Ovx) were evaluated in young female rats. Study I assessed the cognitive effects of low and high doses of EE, delivered tonically via a subcutaneous osmotic pump. Study II evaluated the cognitive effects of low, medium, and high doses of EE administered via a daily subcutaneous injection, modeling the daily rise and fall of serum EE levels with oral regimens. Study II also investigated the impact of low, medium and high doses of EE on the basal forebrain cholinergic system. The low and medium doses utilized here correspond to the range of doses currently used in clinical formulations, and the high dose corresponds to doses prescribed to a generation of women between 1960 and 1970, when oral contraceptives first became available. Here, we evaluate cognition using a battery of maze tasks tapping several domains of spatial learning and memory and basal forebrain cholinergic integrity by using immunohistochemistry and unbiased stereology to estimate the number of choline acetyltransferase (ChAT)-producing cells in the medial septum and vertical/diagonal bands. At the highest dose, EE treatment impaired multiple domains of spatial memory relative to vehicle treatment, regardless of administration method. When given cyclically at the low and medium doses, EE did not impact working memory, but transiently impaired reference memory during the learning phase of testing. Of the doses and regimens tested here, only EE at the highest dose impaired several domains of memory; tonic delivery of low EE, a dose that corresponds to the most popular doses used in the clinic today, did not impact cognition on any measure. Both medium and high doses of EE reduced the number of cholinergic cells in the basal forebrain and cell population estimates in the vertical/diagonal bands negatively correlate with working memory errors.
Keywords: Memory, Ethinyl Estradiol, Contraceptive, Hormone
1.0 Introduction
Ethinyl estradiol (EE), a synthetic form of 17β-estradiol (E2), is the most common estrogen in hormonal contraceptives (Shively, 1998), and is the only estrogen used in the contraceptive pill. National surveys estimate that, from 2006–2010, 10.6 million women between 2006–2010 (Jones et al., 2012), and 17.3% of all women between 2006–2008 (Mosher and Jones, 2010), used oral contraceptives. Over 30 contraceptive formulations contain EE (Curtis et al., 2005), including both oral regimens and non-oral, tonic delivery regimens, such as the transdermal patch and vaginal ring. EE is also found in hormone therapies (HT) for menopausal women, such as Estinyl™ and Femhrt™ (Curtis et al., 2005). Understanding the cognitive impact of estrogens is critical, as exogenous exposure to estrogens occurs throughout the lifespan through contraceptives and HT. Of note, EE is a synthetic analogue to E2; however, these estrogens have different pharmacokinetic profiles (Coelingh Bennink et al., 2004). EE is more biologically active than E2 (Dickson and Eisenfeld, 1981) and cannot be converted to estrone or other weaker estrogens (Fotherby, 1996), whereas E2 can (Prokai-Tatrai, et al., 2005). These estrogens also have different binding profiles, which vary across species (Paradiso et al., 2001).
Although EE is among the most commonly prescribed estrogens for contraception, and is prescribed to women from puberty to post-menopause, most preclinical research on the cognitive impact of estrogens has focused on 17β-estradiol and other endogenous estrogens, and does not include EE (for reviews see: Bimonte-Nelson et al., 2010; Acosta et al., 2013). Methodically evaluating EE in a rodent model allows the opportunity to systematically control for many variables that could impact cognitive scores, including mode of administration, dosing, endogenous hormone variations, age, and diet.
There have been a few studies investigating the cognitive effects of EE as a contraceptive or HT, with effects that vary across memory domains. In human contraceptive users, no impact of EE-containing contraceptives was found on several tests measuring memory and concentration (Silber et al., 1987). Another study found enhanced verbal memory during the active compared to the inactive phase of oral contraceptives, although benefits were not seen on visuospatial measures (Mordecai et al., 2008). Importantly, although each of the contraceptive formulations used in these studies contained EE, other aspects of the formulations differed, including dose and the progestin component. Thus, it is difficult to decipher whether or to what extent EE was responsible for these effects. In studies investigating EE as a HT, cognitive effects depend on the domain as well. In aged, ovariectomized (Ovx) rhesus monkeys, EE improved spatial working memory (Lacreuse et al., 2002), but impaired face recognition (Lacreuse and Herndon, 2003), and had no impact on executive function (Lacreuse et al., 2004). An fMRI study of menopausal women found EE-containing HTs increased frontal cortex activation during a working memory task (Smith, et al., 2006).
In women taking hormonal contraceptives, serum level patterns differ between daily and tonic regimens. Oral contraceptives produce a cycle of serum levels throughout the day, with concentrations highest 1–2 hours after ingestion of the pill (Devineni et al., 2007). Tonic hormone delivery via transdermal patches produces steady serum levels, unlike oral administration (Devineni et al., 2007). There is evidence for cognitive benefits of tonic estrogen delivery; in randomized, placebo-controlled studies on women with mild-moderate probable Alzheimer’s disease, transdermal E2 positively affects multiple measures of cognition (Asthana et al., 1999; Wharton et al., 2011). A study evaluating transdermal versus oral contraceptives found no difference in quality of life, side effects or regularity of the menses; however, more patch users reported a favorable impact on “daily activities” (Sucato et al., 2011). Additionally, metabolism by the liver following oral estrogen administration is linked to increases in markers of thromboembolic side effects, whereas transdermal administration is not (Scarabin et al., 1997; Decensi et al., 2002; Post et al., 2003). In the current series of experiments, we assess both modes of administration.
Study I evaluated the cognitive effects of tonic EE, administered via subcutaneous pumps that deliver at a steady rate, resembling the tonic pattern of EE delivery from a transdermal patch or vaginal insert (Theeuwes and Yum, 1976; Curtis et al., 2005). We evaluated a low dose of EE, corresponding to the most popular doses currently prescribed to women, along with a high dose that is outside of the range of current formulations, but is representative of the high doses of EE that were previously present in contraceptives (Dhont, 2010). The high EE dose is also roughly one-tenth of a dose of E2 previously shown to enhance performance on spatial tasks (Talboom et al., 2008), to account for the difference in biological activity between EE and E2 (Dickson and Eisenfeld, 1981).
Study II was performed to test the cognitive and neurobiological effects of cyclically administered EE, given via a daily injection, to model oral contraceptive use. In order to encompass the entire range of clinically used doses, an additional medium dose of EE was assessed, equivalent to the highest dose of EE currently available in contraceptives, (Curtis et al., 2005).
Estrogen Receptors (ERs) are highly expressed in several cognitive brain regions, including the basal forebrain (BF) (Shughrue et al., 1999), which contains cholinergic cell bodies that project to the hippocampus (McEwen, 2001; Gibbs, 2010). These projections are known to be intimately involved in spatial learning and memory (Luine et al., 1986) and are required for E2 to benefit rodent performance on a spatial delay-match-to-position (Gibbs, 2002, 2007). E2 and EE may differentially affect the basal forebrain cholinergic system due to differing receptor binding abilities in the hippocampus (Paradiso et al., 2001); however, no studies have evaluated the impact of EE on these projections, or how this impact relates to spatial learning and memory. In Study II, cholinergic cells in the BF were quantified using unbiased stereology and relations between cell populations and performance on cognitive tasks were evaluated. The current studies aim to isolate the cognitive and neurobiological effects of several clinically relevant administration regimens of EE, using the Ovx rodent model.
2.0 Methods
2.1 Study I
2.1.1 Subjects
Subjects were 29 female Fischer-344 rats raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). Animals were three months old at the beginning of the study, four months old at maze testing, and five months old at euthanasia. After arrival, animals were pair-housed, had food and water ad-lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee and adhered to National Institutes of Health standards.
2.1.2 Experimental Design and Hormone Treatments
At three months old, all animals received Ovx surgery. Rats were anesthetized via isoflurane inhalation, received bilateral dorsolateral incisions in the skin and peritoneum, and ovaries and tips of the uterine horn were ligatured and removed. Muscle and skin were then sutured closed. During surgery, rats received an injection of Rimadyl™ (5mg/ml/kg) for pain, and saline (2ml) to prevent dehydration.
Eighteen days after Ovx, all animals received a subcutaneous Alzet osmotic pump to deliver vehicle or hormone treatment; the Alzet 2006 model was used, which held a total of 200µl of solution, released for 6 weeks, at a rate of 0.15µl per hour. Rats were anesthetized via isoflurane inhalation, a small incision was made, and a subcutaneous pocket was created in the dorsal scruff of the neck. A pump filled with vehicle, or one of two EE doses was inserted below the skin, and the skin was closed with surgical staples. Rats were randomly assigned to one of three treatments: vehicle (propylene glycol) (n=10), low EE (0.125µg per day) (n=9), or high EE (0.3µg per day) (n=10). EE (Sigma, St. Louis, MO) was dissolved in polyethylene glycol and released at 2.5µl per hour throughout the remainder of the study. The pumps use increasing osmotic pressure to dispense solution from an internal bladder and flow regulator, ensuring a constant, steady rate of administration across time (Theeuwes and Yum, 1976). The low EE dose was based on the 30–35µg daily regimen that an average 60–70kg woman would be prescribed in an oral contraceptive (Curtis et al., 2005), adjusted to the weight of a rat (about 0.25kg). Since EE is 10 times as potent as E2 (Fotherby, 1996), the high EE dose was one-tenth of a similar dose of E2 previously shown to enhance performance on spatial tasks (Talboom et al., 2008). The high dose of EE also corresponds to a 75–80µg/day dose of EE (in a 60–70kg woman), within the range of previously available contraceptives for women in the 1960’s, before the benefits of lower-dose formulations were known (Chadwick et al., 2012). Doses exceeding this are also currently used for emergency contraceptives (Curtis et al., 2005).
2.1.3 Water Radial-Arm Maze
Eighteen days later, subjects were tested for 13 days on the eight-arm, win-shift Water Radial-Arm Maze (WRAM) to evaluate spatial working and reference memory, as previously described (Bimonte and Denenberg, 1999; Bimonte et al., 2000, 2002, 2003; Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004). The maze was an eight-arm apparatus (each arm 38.1 × 12.7cm) filled with opaque, room temperature water. Water temperature was measured at the beginning of each day of testing, and was between 18–20°C for testing. Four of the eight maze arms contained hidden platforms (10cm diameter) just beneath the surface of the water and spatial cues were present to aid the animals in spatial navigation. Each subject was assigned different platform locations that remained fixed across all days of testing. A subject was released from the start arm and given 3 minutes (min) to locate a platform. Once a platform was found, the animal remained on it for 15 seconds (s), then was returned to its heated testing cage for a 30s inter-trial interval (ITI). During the ITI, the just-found platform was removed from the maze and the water was cleaned to remove any debris and obscure olfactory cues. The animal was then placed back into the start arm and given another 3min to locate a platform. Each animal received four trials per day for 13 days, with the number of remaining platforms reduced by one on each subsequent trial. Thus, the working memory system was increasingly taxed as trials progressed within a day, allowing working memory load to be assessed. On the 13th day of testing, a six-hour delay was given between trials 2 and 3 to test delayed memory retention.
Errors were quantified using orthogonal measures of working and reference memory, as done previously (Jarrard et al., 1984; Bimonte et al., 2000; Hyde et al., 2000; Bimonte et al., 2002; Braden et al., 2010, 2011). Working Memory Correct (WMC) errors were defined as all entries into arms that previously contained a platform, Reference Memory (RM) errors were first entries into arms that never contained a platform and Working Memory Incorrect (WMI) errors were all subsequent entries into arms that never contained a platform. An arm entry was counted when the tip of a rat’s snout crossed a mark on the outside of the arm (not visible from inside the maze; 11cm into the arm).
2.1.4 Morris water maze
One day after the WRAM, spatial reference memory was evaluated using the Morris water maze (MM). The apparatus was a round tub (188cm diameter) filled with opaque, room temperature water (18–20°C) containing a submerged platform (10cm diameter) in the northeast quadrant. The platform location remained fixed across all days and trials, with spatial cues available to aid the animals in spatial navigation, testing spatial reference memory (Morris et al., 1982). Animals received six trials per day for three days. At the beginning of each trial, animals were dropped off from one of four starting points (north, south, east or west), varying semirandomly. Animals had 60s to locate the platform, where they remained for 15s before being placed back into a heated cage for an ITI of 5–8min. To evaluate whether animals utilized a spatial strategy, a seventh probe trial was given on the third day of testing, during which the platform was removed and animals were given 60s to swim freely in the maze. A video camera and tracking system tracked and measured each rat’s swim path (Ethovision; Noldus Instruments, Wageningen, The Netherlands).
2.1.5 Visible platform task
After completion of behavioral testing, motor and visual competence were evaluated using the visible platform task. This was a non-spatial adaptation of the spatial MM task, previously used to dissociate visual and motor acuity from place memory (Morris et al., 1982). This task is ideal for this purpose due to its similarity to other spatial water-maze tasks with respect to motor and visual requirements, differing only in that animals are not required to associate the location of the platform with distal cues. The apparatus was a rectangular tub (100 × 60cm) filled with clear room temperature water (18–20°C). A black platform (10cm wide) was positioned 4cm above the surface of the water, following previously published methods (Hunter et al., 2003). A ring of opaque curtains surrounded the maze, blocking all obvious spatial cues to prevent spatial navigation. Animals received six trials in one day. The drop off location remained the same across trials; however the platform location varied semi-randomly across three locations. Each rat had 90s to locate the platform, where it remained for 15s before being placed back into a heated cage for an ITI of 5–8min.
2.1.6 Markers of Peripheral Stimulation
To verify Ovx and subsequent hormone treatment, vaginal smears were taken at four months old for four days, after animals were given hormone treatment. Smears were classified as proestrus, estrus, metestrus, or diestrus (Goldman et al., 2007; Engler-Chiurazzi et al., 2012). At euthanasia, uteri of all subjects were removed and trimmed of visible fat, and wet uterine weight (grams) was measured, as done previously (Westerlind et al., 1998; Engler-Chiurazzi et al., 2012). Osmotic pumps were visually inspected at euthanasia for visible cracks or tops that had come off of the pumps.
2.1.7 Statistical Analyses
We planned a priori to assess the differences in maze performance between each EE group and the Vehicle group to compare effects of each dose to a “blank” ovarian hormone background. After completion of the study, we performed additional post-hoc comparisons between the EE-low and EE-high groups. WRAM testing was blocked into learning (days 2–7) and asymptotic (days 8–12) phases, based on prior studies (e.g., Bimonte and Denenberg, 1999; Bimonte et al., 2000; Hyde et al., 2000; Bimonte et al., 2003). Data were analyzed separately for each type of error using repeated measures ANOVA, with treatment as the between-groups variable and number of errors on each trial as the dependent variable. Steroid treatment induced differences on the lattermost portion of WRAM testing have been observed previously, with most pronounced effects on trial 4, the highest working memory load trial (Bimonte and Denenberg, 1999; Bimonte-Nelson et al., 2003, 2004; Braden et al., 2010); therefore, interactions between treatment and working memory load (trials) were analyzed. Fisher PLSD post-hoc tests were used, alpha level was set at 0.05.
MM data were analyzed using repeated measures ANOVA, with treatment as the between-groups variable and distance to the platform as the dependent variable. Probe trial data were analyzed identically, except with percent distance in the northeast (platformed) and southwest (diagonally opposite of the platform) quadrants as the dependent variable.
Visible platform data were analyzed using repeated measures ANOVA, with treatment as the between-groups variable and latency to reach the platform on each trial as the dependent variable.
2.2 Study II
2.2.1 Subjects
Subjects were 36 female Fischer-344 rats raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). Similar to Study I, animals were three months old at the beginning of the study, four months old at maze testing initiation, and five months old at euthanasia. After arrival, animals were pair-housed, had food and water ad-lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee and adhered to National Institutes of Health standards.
2.2.2 Experimental Design and Hormone Treatments
Following arrival, experimental procedures were identical to those in Study I through Ovx surgeries. Eighteen days after Ovx, animals started receiving daily, subcutaneous injections at a volume of 0.1ml, continuing until euthanasia. Rats were randomly assigned to one of four treatment groups (n=9 per group): vehicle (sesame oil), low EE (0.125µg per day), medium EE (0.18µg per day), or high EE (0.3µg per day). EE (Sigma, St. Louis, MO) was dissolved in sesame oil at the appropriate dose at the beginning of the study, then aliquoted into daily quantities and stored in the refrigerator (2–4°C) until needed. The medium EE dose was based on a 45–50µg per day regimen that an average woman weighing 60–70kg would be prescribed in an oral contraceptive (Curtis et al., 2005), adjusted to the weight of a rat (about 0.25kg). Behavioral testing and statistical analyses were identical to those in Study I.
2.2.3 Euthanasia
Animals were euthanized one day after completion of the visible platform task by researchers blinded to treatment group. Animals were decapitated under isoflurane anesthesia; brains were rapidly removed and blocked just posterior to the BF. The anterior portion of each brain was fixed in 4% paraformaldehyde for 48 hours following removal, then transferred to 0.1 M phosphate-buffered solution (PB, pH 7.4). Brains were then soaked in 30% sucrose solution in PB for 72 hours, frozen, and sectioned at 40µm through the BF (plates 1–28 Paxinos and Watson, 2005) using a Microtome Cryostat (Microm HM 500 OM).
2.2.4 Serum Analyses
Serum levels of E2, Estrone (E1) and EE were obtained from a subset of the vehicle and EE-medium groups to verify Ovx status and to determine whether experimental treatments resulted in circulating serum EE levels similar to those found in women taking EE-containing hormonal contraceptives. Blood was obtained via cardiocentesis at the time of euthanasia, and estrogen levels were determined using mass spectrometry with a lower detection limit of 10pg/ml.
2.2.5 Immunohistochemistry
ChAT-immunoreactive (ChAT-IR) cells in the BF were labeled using immunohistochemistry, following similar previously published protocols from our laboratory following treatment with estrogens (Acosta et al., 2009). Briefly, four animals from each group were selected, and a series of every third section through the BF was selected from each brain for immunohistochemistry processing, yielding six sections per animal 120µm apart, corresponding to plates 23–28 (roughly 1.2mm– 0.6mm Bregma) from Paxinos and Watson (2005), similar to prior publications (Gibbs, 2002). See supplemental materials for more detailed methods.
2.2.6 Stereology
Unbiased stereology was used to quantify ChAT-IR cells within the medial septum (MS) and vertical/diagonal bands (VDB), regions that contain neurons known to innervate the hippocampus (Lewis and Shute, 1967; Dutar et al., 1995; Banuelos et al., 2013). One researcher blind to treatment groups used the optical fractionator method, where the number of cells counted in a known, uniformly random sample of a region of interest is used to estimate the total cell population in that region (Gundersen, 1986; West, 1999; Banuelos et al., 2013).
2.2.7 Statistical Analyses
Statistical analyses for behavior testing were identical to those used in Study I. One-way ANOVA was used to analyze treatment group differences in the number weighted mean section thickness population estimate (ChAT-IR cell counts) in each region, and correlations between region population estimates and behavioral measures were examined. Accuracy of stereological estimates was evaluated using Gundersen’s smoothness classification m=1 coefficients of error (CEs).
3.0 Results
3.1 Study I
3.1.1 Water Radial Arm Maze
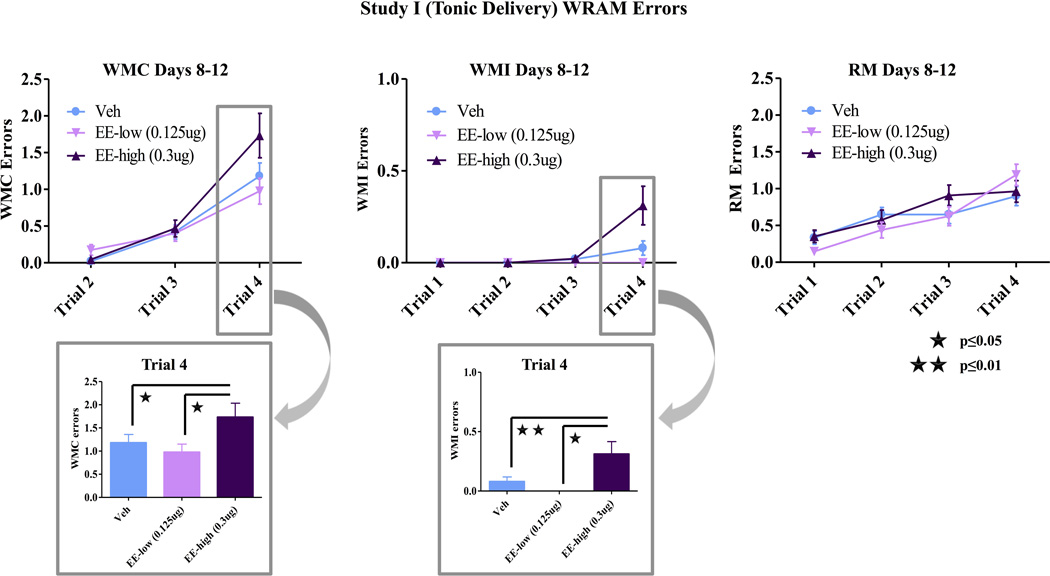
There were no effects of EE treatment on WMC, WMI, or RM errors during the learning phase of testing. During the asymptotic phase of testing, there was a Trial × Treatment interaction for both WMC [F(4,52)=4.14; p<0.01] and WMI [F(6,78)= 4.23; p<0.01] errors (fig 1). Two group planned comparisons showed that the high EE group committed more WMC [F(2,36)=3.38; p<0.05] and WMI [F(3,54)=5.06; p<0.01] errors than the vehicle group as working memory load increased (Trial × Treatment interaction). Post-hoc analyses showed that high EE animals made more WMC (Fisher, p<0.05) and WMI (Fisher, p<0.05) errors than low EE animals on trial 4, the trial with the highest working memory load (fig 1). There was also a main effect of Treatment for WMI errors across all trials [F(2,26)= 4.05; p<0.05] during the asymptotic portion of testing. Planned comparisons showed that the high EE group made more WMI errors than the vehicle group [F(1,18)= 4.38; p=0.05], and post-hoc analyses showed that the high EE group also made more errors than the low EE group (Fisher, p<0.05) across all trials. There were no differences between the low EE and vehicle groups, and there were no differences in number of RM errors for any group comparison (fig 1). There were no group differences on the post-delay trials on day 13.
Figure 1.
WRAM Performance Study I. During the asymptotic phase of testing, there was a Trial × Treatment interaction for both WMC [F(4,52)=4.14; p<0.01] and WMI [F(6,78)= 4.23; p<0.01] errors. Two group planned comparisons showed that the high EE group committed more WMC [F(2,36)=3.38; p<0.05] and WMI [F(3,54)=5.06; p<0.01] errors than the vehicle group as working memory load increased. High EE animals also made more WMI and WMC errors than low EE animals on trial 4, the trial with the highest working memory load (Fisher, p<0.05). There was also a main effect of Treatment for WMI errors across all trials [F(2,24)= 4.99; p<0.05] with the high EE group making more errors than the vehicle group [F(1,18)= 4.38; p=0.05], and the low EE group (Fisher, p<0.05). There were no differences between the low EE and vehicle groups, nor were there any group differences in RM errors.
3.1.2 Morris Water Maze
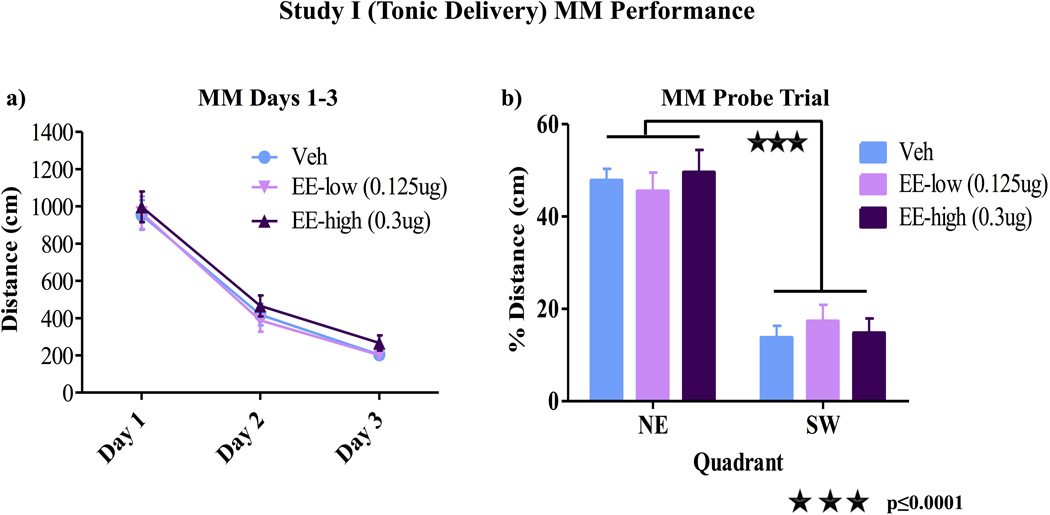
There were no Treatment main effects or interactions for days 1–3 of MM testing (fig 2a). For the probe trial, a higher percent distance was spent in the previously platformed quadrant versus the opposite quadrant [F(1, 26)=86.84, p<0.0001], with no Quadrant by Treatment interaction (p>0.05), indicating that all groups spatially localized the platform quadrant by the end of testing (fig 2b). Treatment did not impact number of crossings through the platform area during the probe trial (data not shown).
Figure 2.
MM Performance Study I. a) There were no group differences for days 1–3 of MM testing (p>0.05). b) For the probe trial, a higher percent distance was spent in the previously platformed quadrant vs. the opposite quadrant [F(1, 26)=86.84, p<0.0001] with no percent distance by treatment interaction, indicating that all groups spatially localized the platform by the end of testing.
3.1.3 Visible platform task
The escape time across all six trials was very short (average of 6.06s, standard deviation of 6.20s), indicating successful task completion. There were no Treatment effects for Latency (p>0.05), indicating that all treatment groups possessed a similar level of capability to solve a water maze task (data not shown).
3.1.4 Markers of Peripheral Stimulation
Thirty-one days after pump insertion surgeries, all vehicle-treated rats exhibited diestrus smears indicating a lack of uterine stimulation, while animals treated with any dose of EE alternated between estrus and metestrus smears, with each smear showing numerous cornified cells, indicating uterine stimulation (Goldman et al., 2007). For wet uterine weight, there was a significant effect of Treatment [F(2,26)= 75.53; p <0.0001], with planned comparisons showing that that uteri of vehicle treated rats weighed less than uteri of low EE [F(1,17)= 114.29; p <0.0001] and high EE [F(1,18)= 181.37; p <0.0001] treated rats (supplemental fig 1). Pump inspection at euthanasia revealed that all pumps were intact with no visible cracks.
3.2 Study II
3.2.1 Water Radial Arm Maze
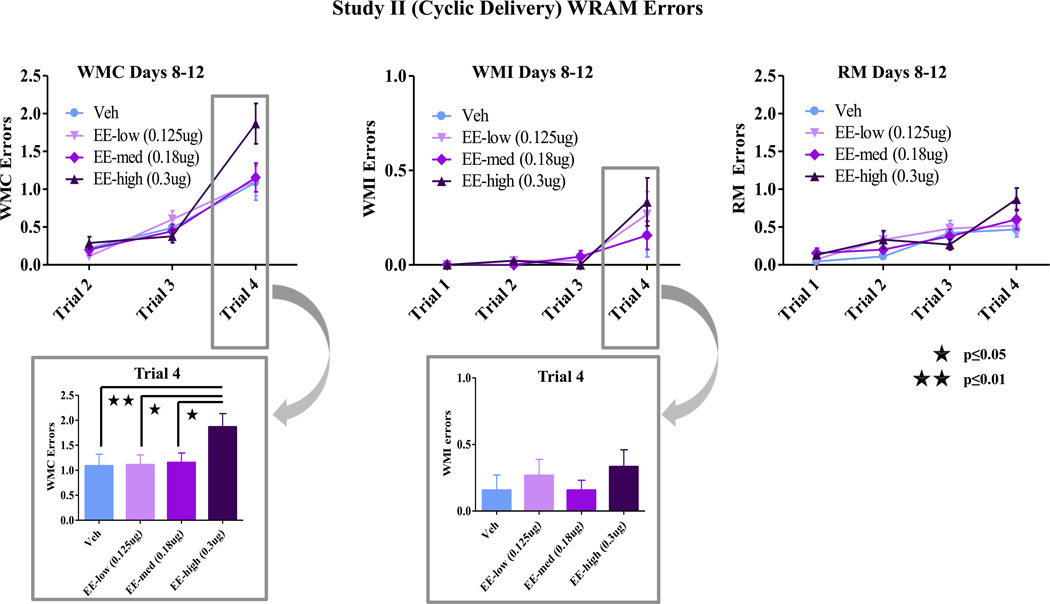
When delivered via daily subcutaneous injection, there were no effects of EE treatment on WMC, WMI, or RM during the learning portion of testing. During the asymptotic phase of testing, similar to effects with tonic EE treatment, there was a Trial × Treatment interaction for WMC errors [F(6,64)= 2.82; p<0.05] with a planned comparison showing that the high EE treated animals made more errors than vehicle treated animals as working memory load increased [F(2,32)= 5.78; p<0.01] (fig 3). Post-hoc analyses also showed that the high EE group committed more WMC errors than the low EE (Fisher, p<0.05) and medium EE (Fisher, p<0.05) animals at the highest working memory load (fig 3). There were no differences in WMC errors between the vehicle group and the low EE or medium EE group, and there were no group differences for WMI or RM errors during the asymptotic phase of testing (fig 3). There were no group differences on the post-delay trials on day 13.
Figure 3.
WRAM Performance Study II. During the asymptotic phase of testing, there was a Trial × Treatment interaction for WMC errors [F(6,64)= 2.82; p<0.05] with the high EE treated animals making more errors than vehicle treated animals as working memory load increased [F(2,32)= 5.78; p<0.01]. Post-hoc analyses also showed that the high EE group committed more WMC errors than the low EE (Fisher, p<0.05) and medium EE (Fisher, p<0.05) animals at the highest working memory load. There were no differences in WMC errors between the vehicle group and the low or med EE group. There were no differences for WMI or RM errors.
3.2.2 Morris Water Maze
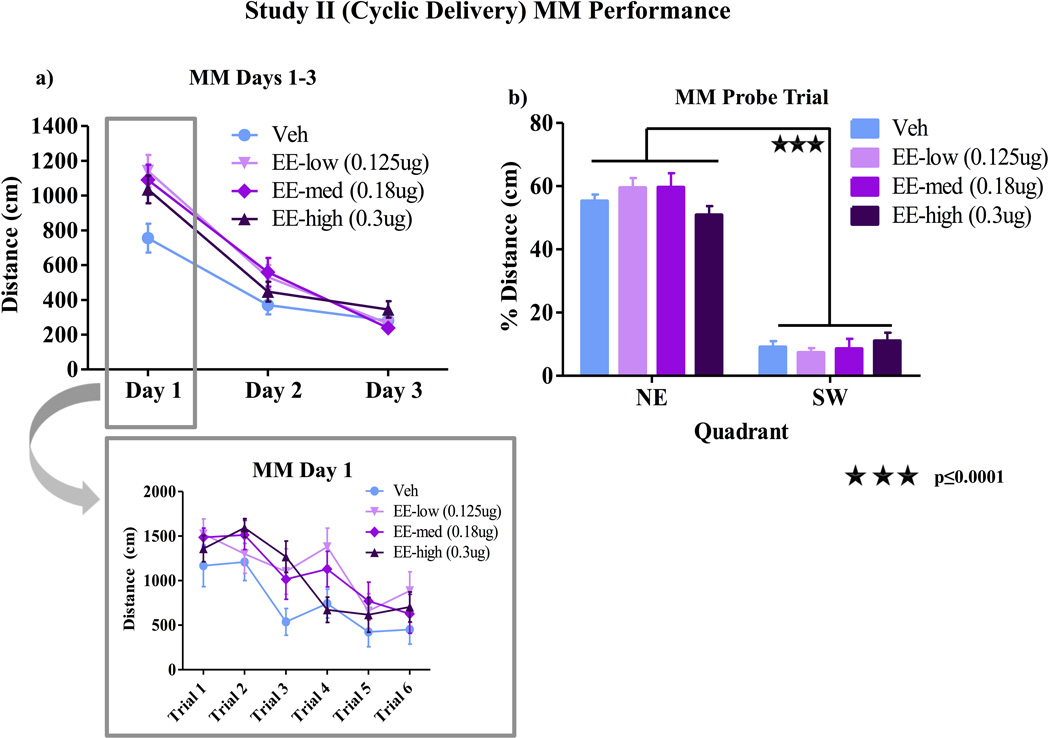
There was a marginal Treatment × Day interaction for MM testing [F(6,64)=2.21; p=0.05]. Further analyses revealed a main effect of Treatment [F(3,32)= 3.22; p<0.05] for Day 1 of MM, whereby the vehicle group performed better than the low EE [F(1,16)= 6.84; p<0.05], medium EE [F(1,16)= 8.51; p<0.05], and high EE [F(1,16)= 9.47; p<0.01] groups. There was no Treatment × Trial interaction for Day 1, indicating that this effect was present across all trials and was not carried by the initial exposure to the task on trial 1. There were no effects of Treatment for Days 2 or 3 of MM testing (fig 4a). A higher percent distance was spent in the previously platformed quadrant versus the opposite quadrant [F(1,32) =374.33; p<0.0001] for the probe trial, with no quadrant by Treatment interaction, indicating that all groups spatially localized the platform quadrant by the end of testing (fig 4b). Treatment did not impact number of crossings through the platform area during the probe trial (data not shown).
Figure 4.
MM Performance Study II. a) There was a Treatment × Day interaction for MM testing [F(6,64)=2.21; p=0.05]. Further analyses revealed a main effect of Treatment [F(3,32)= 3.22; p<0.05] for Day 1 of MM, whereby the vehicle group performed better than each of the low EE [F(1,16)= 6.84; p<0.05], med EE [F(1,16)= 8.51; p<0.05], and high EE [F(1,16)= 9.47; p<0.01] groups. There was no Treatment × Trial interaction for Day 1, indicating that this effect was present across all trials and was not carried by the initial exposure to the task. There were no effects of Treatment during days 2–3 of MM testing. b) A higher percent distance was spent in the previously platformed quadrant vs. the opposite quadrant [F(1,32)= 374.33; p<0.0001] for the probe trial, with no percent distance by treatment interaction, indicating that all groups spatially localized the platform equally by the end of testing.
3.2.3 Visible platform task
The average escape time across all 6 trials was a rapid 7.71 seconds with a standard deviation of 7.47 seconds. There were no treatment effects for latency (data not shown), indicating that all animals possessed similar procedural capabilities to solve a water maze task (p>0.05).
3.2.4 Markers of Peripheral Stimulation
Fourteen days after the start of injections, all vehicle-treated rats exhibited diestrus smears indicating a lack of uterine stimulation, while animals treated with any dose of EE alternated between estrus and metestrus smears, with each smear showing numerous cornified cells, indicating uterine stimulation (Goldman et al., 2007). One uterine weight score was lost due to experimental error and was not included in these analyses. For wet uterine weight, there was a significant effect of Treatment [F(3,31)=29.88; p<0.0001], with uteri of vehicle-treated rats weighing less than low EE- [F(1,15)= 62.17; p <0.0001], medium EE- [F(1,16)= 117.36; p <0.0001], and high EE- [F(1,16)= 109.10; p <0.0001] treated rats (supplemental fig 2).
3.2.5 Serum Analyses
Circulating serum E1 and E2 levels were below the lower limit for detection (10pg/ml) in all animals, verifying Ovx status. The mean circulating serum EE concentration in the medium EE treatment group was 23.17 pg/ml with a standard deviation of 12.50 pg/ml, which is remarkably similar to the range of serum levels found in women taking an oral contraceptive containing 35ug of EE near the beginning of their monthly cycle (Devineni et al., 2007).
3.2.6 ChAT Cell Counts
Mean measured tissue thickness was 27µm, CEs ranged from 0.05 to 0.10 and were less than half of the observed variation across subjects (coefficients of variation ranging from 0.21 to 0.34), indicating that the sampling and counting parameters utilized here were adequate to detect differences in cell populations among treatment groups (Gundersen and Osterby, 1981; Gundersen and Jensen, 1987; West, 1999; Dorph-Petersen et al., 2001).
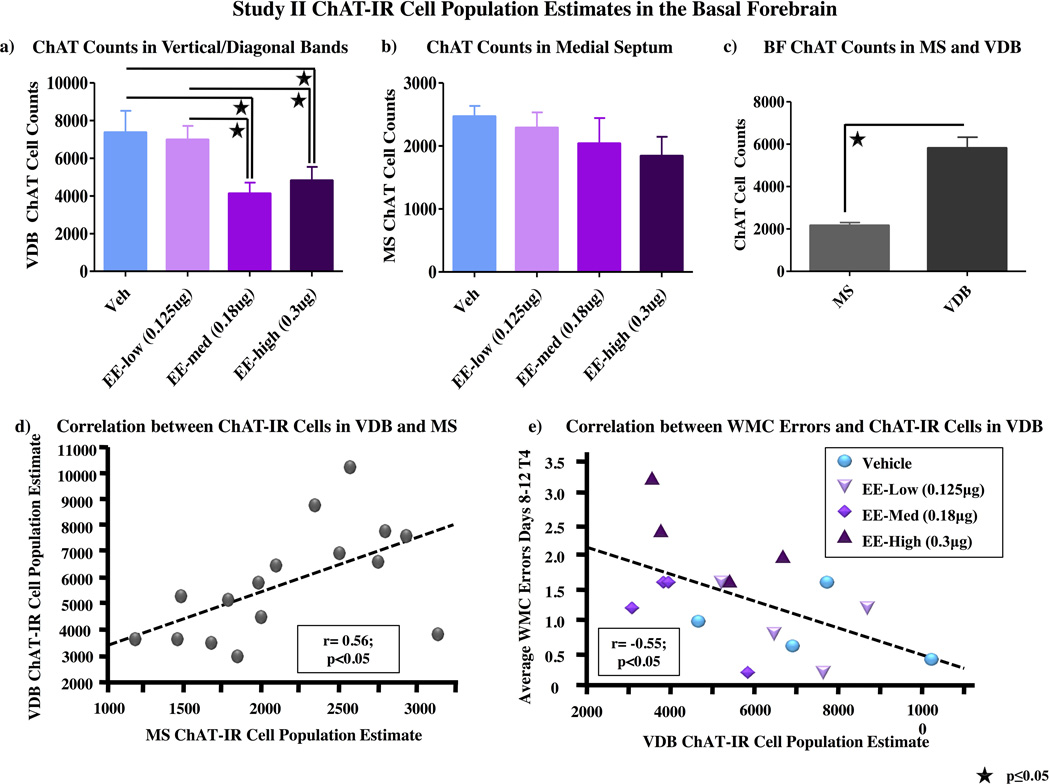
There was a main effect of Treatment [F(3,12)=3.66; p<0.05] in the VDB, whereby ChAT-IR cell counts were lower in the medium EE group (Fisher, p<0.05), and lower in the high EE group (Fisher, p=0.05), than those in the vehicle group (fig 5a). ChAT-IR cell counts in the low EE group did not differ from the vehicle group. Post-hoc tests indicate that the ChAT-IR cell counts were lower in the medium EE group (Fisher, p<0.05) and marginally lower in the high EE group (Fisher, p=0.09), than the low EE group (fig 5a). There were no effects of Treatment on ChAT-IR cell counts in the MS (fig 5b).
Figure 5.
ChAT Cell Population Estimates Study II. a) There was a main effect of Treatment [F(3,12)=3.66; p<0.05] in the VDB with a larger ChAT-IR cell population estimate in the vehicle-treated animals than both the med EE- (Fisher, p<0.05) and high EE-treated animals (Fisher, p=0.05). ChAT-IR cell population estimates in the low EE group did not differ from those in the vehicle group. ChAT-IR cell population estimates in the low EE group did not differ from those in the vehicle group, however the ChAT-IR cell population estimate in the low EE group were higher than in the med EE group (Fisher, p<0.05), and marginally higher than in the high EE group (Fisher, p=0.09). b) There were no effects of Treatment on ChAT-IR cell population estimates in the MS. c) There was a main effect of Region, whereby there were fewer cells overall in the MS relative to the VDB [F(1,12)=96.49, p<0.001]. d) There was a positive correlation between ChAT-IR cell counts in the VDB and MS of the basal forebrain (r=0.56, p<0.05). e) There was a negative correlation between ChAT-IR cell population estimates in the VDB and the average number of WMC errors on trial 4 during the asymptotic portion of WRAM testing [r= −0.55; p<0.05].
The MS had a lower ChAT-IR cell count than the VDB (F(1,12)=96.49, p<0.001) (fig 5c). There was also a positive correlation between ChAT-IR cell counts in the VDB and MS of the basal forebrain (r=0.56, p<0.05), indicating that animals with higher ChAT-IR cell counts in the MS tended to also have higher cell counts in the VDB (fig 5d).
3.2.7 Relationship between cholinergic cell population estimates and maze performance
Correlations between behavioral data and BF cell counts were assessed to determine whether group differences in behavior data relate to changes in BF cholinergic cell populations. There was a negative correlation between ChAT-IR cell counts in the VDB and number of WMC errors on the highest load trial (trial 4) during the asymptotic portion of WRAM testing [r= −0.55; p<0.05], such that animals with lower ChAT-IR cell counts committed more WMC errors (fig 5e). Both intra- and inter- class correlations were assessed to ensure that the directionality of intra-class correlations agreed with that of the overall correlation across groups; all intra-class correlations were also negative, and thus in accordance with the overall correlation, but not significant (data not shown). VDB ChAT-IR cell counts did not correlate with any other types of errors and there were no correlations between MS ChAT-IR cell counts and behavior data.
4.0 Discussion
The present studies are the first to investigate the cognitive effects of tonically or cyclically administered EE, and the impact of several doses of EE on the cholinergic system, in rodents. Study I investigated the cognitive effects of tonically administered low and high doses of EE. Study II expanded on these research questions by utilizing a cyclic administration regimen, broadening the dose range and evaluating a potential mechanism underlying the cognitive impact of EE. Overall, we found that: 1) EE impacted cognition in a dose- and administration- dependent manner, with high tonic and cyclic EE treatments impairing high demand spatial working memory, and low cyclic treatment producing only modest transient impairments in a different memory domain, spatial reference memory, and 2) cyclic EE decreased the number of ChAT-positive neurons in the BF at medium and high doses. Analysis of brain and behavior measures revealed a relationship between ChAT-IR cell counts in the VDB and working memory performance on the WRAM. Specifically, animals with higher VDB ChAT-IR cell counts tended to make fewer working memory errors.
In Study I, high tonic EE treatment impaired performance on multiple domains of a spatial working memory task when working memory demand was highest. Tonic treatment with low EE did not impact memory at any dose, as measured by both the WRAM and the MM. Similar to results seen in Study I, Study II found that cyclic EE treatment at the highest dose impaired spatial working memory, relative to vehicle treatment. Spatial working memory impairments were limited to one domain of working memory with cyclic delivery, while tonic delivery produced impairments on two orthogonal measures of working memory. When given cyclically, low and medium doses of EE did not impact spatial working memory, and all doses of EE produced a marginal transitory impairment in spatial reference memory. This impairment was only present during the first day of MM testing and was not evident by the second or third days of the task. Collectively, these results suggest that dose and method of delivery modify the cognitive impact of EE; while the high dose of EE produced working memory impairments regardless of administration method, tonic low EE treatment did not affect performance on any task and cyclic low and medium doses of EE produced a transient impairment on only one task. These findings are clinically important, as the low EE treatment corresponds to the low end of available doses currently prescribed to women in contraceptive formulations. These doses were chosen to model the exact formulations currently prescribed to women, adjusted to the weight of a rat (Curtis et al., 2005). The current results also indicate that tonic administration of the lowest dose of EE, corresponding to the most popular range of doses utilized clinically, does not impact spatial working or reference memory in a rodent model.
In Study II, while the cognitive profiles of the low and medium EE doses did not differ, the medium EE dose decreased the number of ChAT-positive cells in the VDB of the BF, while treatment with low EE did not alter this cell population, relative to vehicle treatment. Of note, while the present studies did not detect overt maze learning or memory differences following treatment with the medium dose of EE, this dose was sufficient to alter our brain measure of cholinergic cell counts. Additionally, cell populations in the VDB of the BF negatively correlated with maze errors. This effect size is large, and it can be alternately stated that as cell populations decreased, number of working memory errors in the maze increased. This information can be used to design and implement future studies investigating the mechanisms responsible for the cognitive impact of EE.
These findings, combined with results showing that our rat serum hormone levels correspond with serum levels in women using hormonal contraceptives, raise concerns about the impact EE has on the brain and its function, as clinically prescribed for women. It is still unknown how extended exposure to these hormones may modulate their impact or whether cessation of hormone treatment would attenuate these effects. It also remains to be determined whether exposure to EE early in life, such as for contraception, may impact the cognitive impact of hormone loss or estrogen-containing HT later in life.
The mechanisms by which EE modulates the BF cholinergic system are still unknown, although there are multiple points at which estrogens can influence this system. E2 is well known to interact with this system, however it has been reliably shown to produce an increase in BF cholinergic cell counts and there is strong evidence that E2 produces cognitive benefit through the BF cholinergic system (For review see Gibbs and Aggarwal, 1998, and Gibbs, 2010), the opposite of the impact of EE seen here. There is ample evidence that dose and duration of E2 administration alter its impact on the cholinergic system and, in fact, E2 delivered for a comparable duration, and at an equivalent dose, to the regimen used here has been shown to decrease cell populations in the MS (Gibbs, 2010). Although we do not see treatment group differences in the MS, the medium EE and high EE groups tended to have fewer ChAT-IR cells in the MS, relative to the vehicle group. This evidence, in conjunction with the clear behavioral deficits produced by EE treatment here, seems to indicate that EE and E2 are working on similar targets, but in different manners.
Differences in the structure and function of EE and E2 may account for these opposing effects. For example, Paradiso et al., 2001 found that a small structural difference between human and rat α4β2 Nicotinic Acetylcholine Receptors (NAchRs) in the binding domain results in an inability of E2, but not EE, to potentiate this receptor in rats. Interestingly, the human α4β2 NAchR can be stimulated by both E2 and EE. These receptors, along with the α7 subtype, are the primary type of NAchRs present in the rodent brain (Flores et al., 1992), and are closely related to many cognitive processes, including hippocampal-dependent learning and memory (for review, see Hogg et al., 2002). Direct potentiation of hippocampal α4β2 NAchRs from exogenous EE may contribute to a downregulation in the production of endogenous acetylcholine. While this distinction has the potential to contribute to the opposing cognitive impacts of EE and E2, the cholinergic system is extensively complex and there are many factors yet to be investigated that will likely modulate the impact of different estrogens.
It is crucial to mention that clinically used EE-containing contraceptives and HTs require a progestin component to prevent the increased risk of endometrial cancer associated with unopposed estrogen. There are currently several clinically available progestins, each of which has a distinct pharmacological profile (Curtis et al., 2005). One commonly prescribed progestin, medroxyprogesterone acetate (MPA) (Curtis et al., 2005), when delivered alone, has been shown to impair spatial memory during treatment as well as several months later, when MPA levels are no longer detectable in serum (Braden et al., 2010, 2011). EE has yet to be methodically tested for cognition along with specific progestins, and it is unknown how the inclusion of a progestin may influence the behavioral or brain impact of EE.
Results from the present studies suggest that the contraceptive regimen which may produce the most favorable cognitive impact includes a low dose of EE (30–35µg EE/day or less) delivered tonically, such as with a transdermal patch or a vaginal ring, rather than delivered in a cyclic pattern such as a daily pill. These findings also offer insight into how small differences in hormone structure and function can produce large differences in behavioral and brain profiles. Further studies are necessary to outline the many mechanisms by which estrogens can alter cognitive brain regions and how changes produced by exogenously delivered and endogenously circulating estrogens relate to cognitive function.
The popularity that contraceptives have gained since their introduction in the 1960’s has effectively changed the lifetime hormone profile of the average woman. The current aging generation is the first to have had long-term exposure to synthetic hormones and it is now crucial to understand how a lifetime of different endogenous and exogenous hormone exposures can influence cognitive aging. The broad goal of this research is to elucidate the impact that clinically prescribed hormones have on cognitive function and, ultimately, to optimize contraceptive and HT use for healthy cognitive aging, beginning in young adulthood. We hope the results of the current studies will set the stage for a series of future methodical investigations into the effects these clinically-prescribed hormones have on the brain and related memory processes.
Supplementary Material
Highlights.
-
▪
Study of Ethinyl Estradiol’s (EE) impact on learning, memory and cholinergic cells
-
▪
Utilized low, med and high doses, as well as tonic and cyclic administration of EE
-
▪
High, but not low or med, EE impaired working memory, regardless of administration
-
▪
Cyclic, but not tonic, EE impaired reference memory at all doses
-
▪
Cyclic med and high EE reduced cholinergic cells, which correlated with maze errors
Acknowledgements
This research was funded by grants awarded to HAB-N from the National Institute on Aging (AG028084), the state of Arizona, ADHS, the NIH Initiative for Maximizing Student Development (IMSD) program (R25GM099650), the More Graduate Education at Mountain States Alliance (NSF), and the Western Alliance to Expand Student Opportunities Louis Stokes Alliance for Minority Participation Bridge to the Doctorate (WAESO-LSAMP-BD) National Science Foundation Cooperative Agreement HRD-1025879. The authors would like to acknowledge the intellectual contributions of Itamar S. Grunfeld during the conception of these studies, the assistance of Bryan W. Camp and Jazmin I. Acosta for data collection, and Julie Simpson for her valuable technical contributions to the Stereology protocol development and implementation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Mennenga, Sarah E.: Oversaw both studies and participated in all aspects of the study, including study design, treatment administration, behavior testing, immunohistochemistry, and data analyses. Also performed all of the stereology and oversaw the writing of the manuscript.
Gerson, Julia E.: Assisted in overseeing study I, participated in all aspects of the study, including study design, treatment administration, behavior testing and data analyses. Also assisted heavily in writing the manuscript.
Koebele, Stephanie V.: Performed behavior testing for study I and assisted with data processing and analyses.
Kingston, Melissa L.: Performed behavior testing for studies I and II, performed treatment administration for Study II, and assisted with data processing and analyses.
Tsang, Candy W.S.: Performed brain sectioning and immunohistochemistry for study II.
Engler-Chiurazzi, Elizabeth B.: Assisted with study design for both studies and stereology for study II.
Baxter, Leslie: Assisted with study design, interpretation of the data and manuscript preparation.
Bimonte-Nelson, Heather A.: Principal investigator, oversaw both studies and the entire lab personnel and facilities, including animal husbandry areas, behavior testing rooms, surgical suites, wet lab space for tissue processing and microscopy equipment for stereology. Also participated in every aspect of the study, including study design, treatment administration, behavior testing, data analyses, immunohistochemistry and stereology.
The authors have no actual or potential conflict of interest to disclose that could inappropriately influence, or be perceived to influence, this work.
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: models, mazes, and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta J, Mayer L, Talboom J, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm behav. 2009;55(3):454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker L, Raskind M, Birnbaum R, Lofgreen C, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer's disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24(6):657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Bañuelos C, LaSarge CL, McQuail JA, Hartman JJ, Gilbert RJ, Ormerod BK, Bizon JL. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol Aging. 2013;34(3):845–862. doi: 10.1016/j.neurobiolaging.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte H, Denenberg V. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Granholm A-CE, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328(1):50–54. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Hyde L, Hoplight B, Denenberg V. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(3–4):311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm A-CEC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H, Acosta J, Talboom J. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15(9):6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson H, Singleton R, Hunter C, Price K, Moore A, Granholm A-CE. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117(6):1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson H, Singleton R, Williams B, Granholm A-CE. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118(4):707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Braden B, Garcia A, Mennenga S, Prokai L, Villa S, Acosta J, Lefort N, Simard AR, Bimonte-Nelson HA. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology. 2011;218(2):405–418. doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden B, Talboom J, Crain I, Simard A, Lukas R, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93(3):444–453. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick K, Burkman R, Tornesi B, Mahadevan B. Fifty years of "the pill": risk reduction and discovery of benefits beyond contraception, reflections, and forecast. Toxicol Sci. 2012;125(1):2–9. doi: 10.1093/toxsci/kfr242. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJT. Are all estrogens the same? Maturitas. 2004;4:269–275. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Curtis MG, Overholt S, Hopkins M, DelConte A. Contraception. Lippincott Williams & Wilkins; 2005. Glass’ Office Gynecology; pp. 347–383. [Google Scholar]
- Decensi A, Omodei U, Robertson C, Bonanni B, Guerrieri-Gonzaga A, Ramazzotto F, Johansson H, Mora S, Sandri MT, Cazzaniga M, Franchi M, Pecorelli S. Effect of transdermal estradiol and oral conjugated estrogen on C-reactive protein in retinoid-placebo trial in healthy women. Circulation. 2002;106:1224–1228. doi: 10.1161/01.cir.0000028463.74880.ea. [DOI] [PubMed] [Google Scholar]
- Devineni D, Skee D, Vaccaro N, Massarella J, Janssens L, LaGuardia KD, Leung AT. Pharmacokinetics and pharmacodynamics of a transdermal contraceptive patch and an oral contraceptive. J Clin Pharmacol. 2007;47:497–509. doi: 10.1177/0091270006297919. [DOI] [PubMed] [Google Scholar]
- Dhont M. History of oral contraception. Eur J Contracept Repr. 2010;15:S12–S18. doi: 10.3109/13625187.2010.513071. [DOI] [PubMed] [Google Scholar]
- Dickson R, Eisenfeld A. 17 Alpha-ethinyl estradiol is more potent than estradiol in receptor interactions with isolated hepatic parenchymal cells. Endocrinology. 1981;108(4):1511–1518. doi: 10.1210/endo-108-4-1511. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant M, Senut M, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Talboom J, Braden B, Tsang C, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson HA. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm Behav. 2012;62(1):1–9. doi: 10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Rogers S, Pabreza L, Wolfe B, Kellar K. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41(1):31–37. [PubMed] [Google Scholar]
- Fotherby K. Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy. Contraception. 1996;54(2):59–69. doi: 10.1016/0010-7824(96)00136-9. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42(3):245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52(3):352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31(2):224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm Behav. 1998;4(2):98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Goldman J, Murr A, Cooper R. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Bendtsen T, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hogg R, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Bioch P. 2002;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Hunter C, Bimonte H, Granholm A. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138(2):121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Hyde L, Sherman G, Hoplight B, Denenberg V. Working memory deficits in BXSB mice with neocortical ectopias. Phys Behav. 2000;70(1–2):1–5. doi: 10.1016/s0031-9384(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Vital Stat Rep. 2012:60. [PubMed] [Google Scholar]
- Lacreuse A, Chhabra R, Hall M, Herndon J. Executive function is less sensitive to estradiol than spatial memory: performance on an analog of the card sorting test in ovariectomized aged rhesus monkeys. Behav Process. 2004;67(2):313–319. doi: 10.1016/j.beproc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG. Estradiol selectively affects processing of conspecifics' faces in female rhesus monkeys. Psychoneuroendocrinology. 2003;28(7):885–905. doi: 10.1016/s0306-4530(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Lacreuse AS, Wilson M, Herndon J. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23(4):589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lewis P, Shute C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Luine V, Renner K, Heady S, Jones K. Age and sex-dependent decreases in ChAT in basal forebrain nuclei. Neurobiol Aging. 1986;7(3):193–198. doi: 10.1016/0197-4580(86)90042-4. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2001;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54(2):286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Morris R, Garrud P, Rawlins J, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat. 2010;23:1–44. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic press. 2005 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach J. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21(17):6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post MS, Christella M, Thomassen LG, Mooren MJ, van der, Baal WM, van, Rosing J, Kenemans P, Stehouwer CD. Effect of oral and transdermal estrogen replacement therapy on hemostatic variables associated with venous thrombosis: a randomized, placebo-controlled study in postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1116–1121. doi: 10.1161/01.ATV.0000074146.36646.C8. [DOI] [PubMed] [Google Scholar]
- Prokai-Tatrai K, Prokai L. Impact of metabolism on the safety of estrogen therapy. Ann N Y Acad Sci. 2005;1052:243–257. doi: 10.1196/annals.1347.018. [DOI] [PubMed] [Google Scholar]
- Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071–3078. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- Shively CA. Behavioral and neurobiological effects of estrogen replacement therapy and a history of triphasic oral contraceptive exposure. Psychoneuroendocrinology. 1998;23:713–732. doi: 10.1016/s0306-4530(98)00039-0. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 1999;96(1):41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Silber M, Almkvist O, Larsson B, Stock S, Uvnäs-Moberg K. The effect of oral contraceptive pills on levels of oxytocin in plasma and on cognitive functions. Contraception. 1987;36(6):641–650. doi: 10.1016/0010-7824(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta J-KK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91:4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucato GS, Bhatt SK, Murray PJ, Ott MA. Transdermal contraception as a model for adolescent use of new methods. J Adolesc Health. 2011;49:357–362. doi: 10.1016/j.jadohealth.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Talboom J, Williams B, Baxley E, West S, Bimonte-Nelson H. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90(1):155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes F, Yum SI. Principles of the Design and Operation of Generic Osmotic Pumps for the Delivery of Semisolid or Liquid Drug Formulations. Ann Biomed Eng. 1976;4(4):343–353. doi: 10.1007/BF02584524. [DOI] [PubMed] [Google Scholar]
- West M. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Westerlind K, Gibson K, Malone P, Evans G, Turner R. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Miner Res. 1998;13(6):1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- Wharton W, Baker L, Gleason C, Dowling M, Barnet J, Johnson S, Carlsson C, Craft S, Asthana S. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer's disease: results of a randomized controlled trial. J Alzheimers Dis. 2011;26(3):495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.