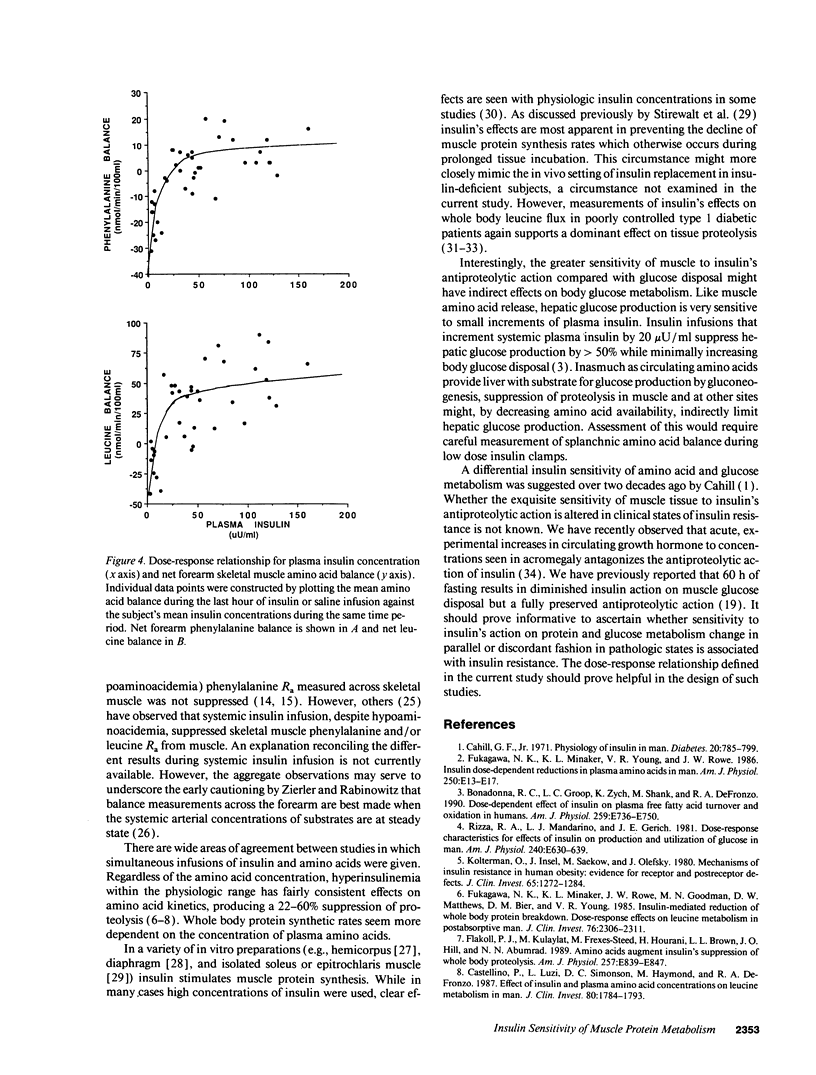
Abstract
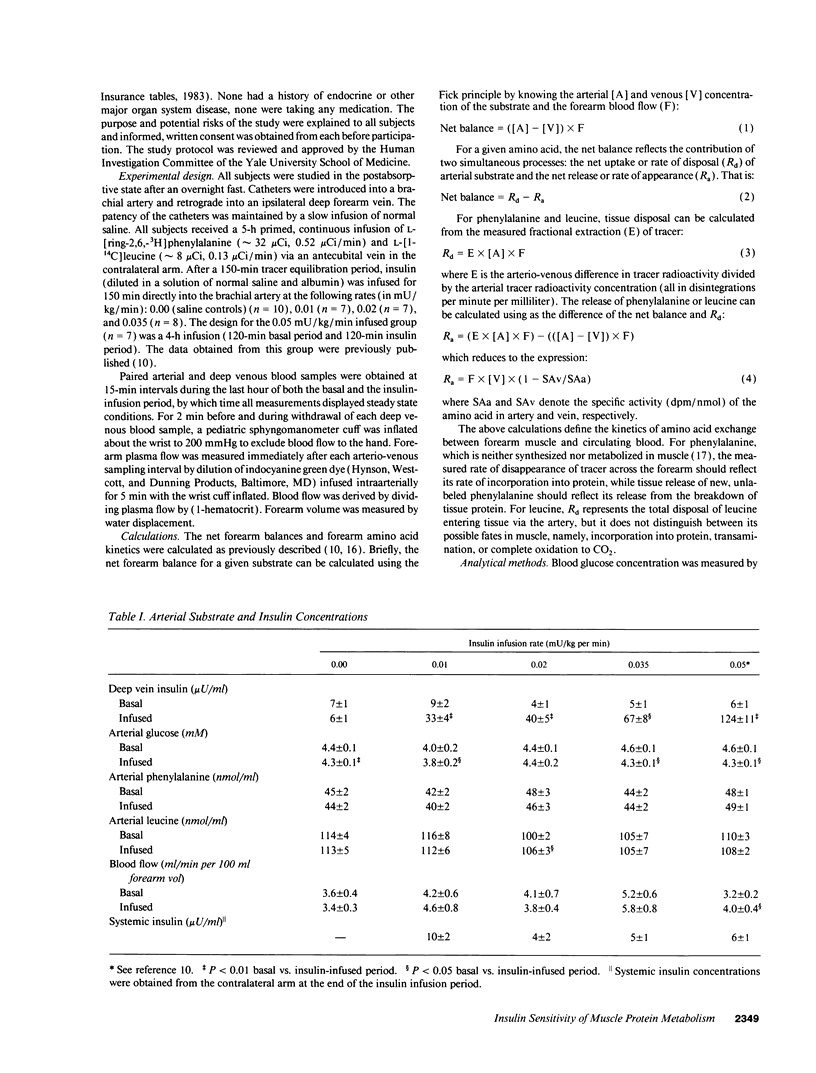
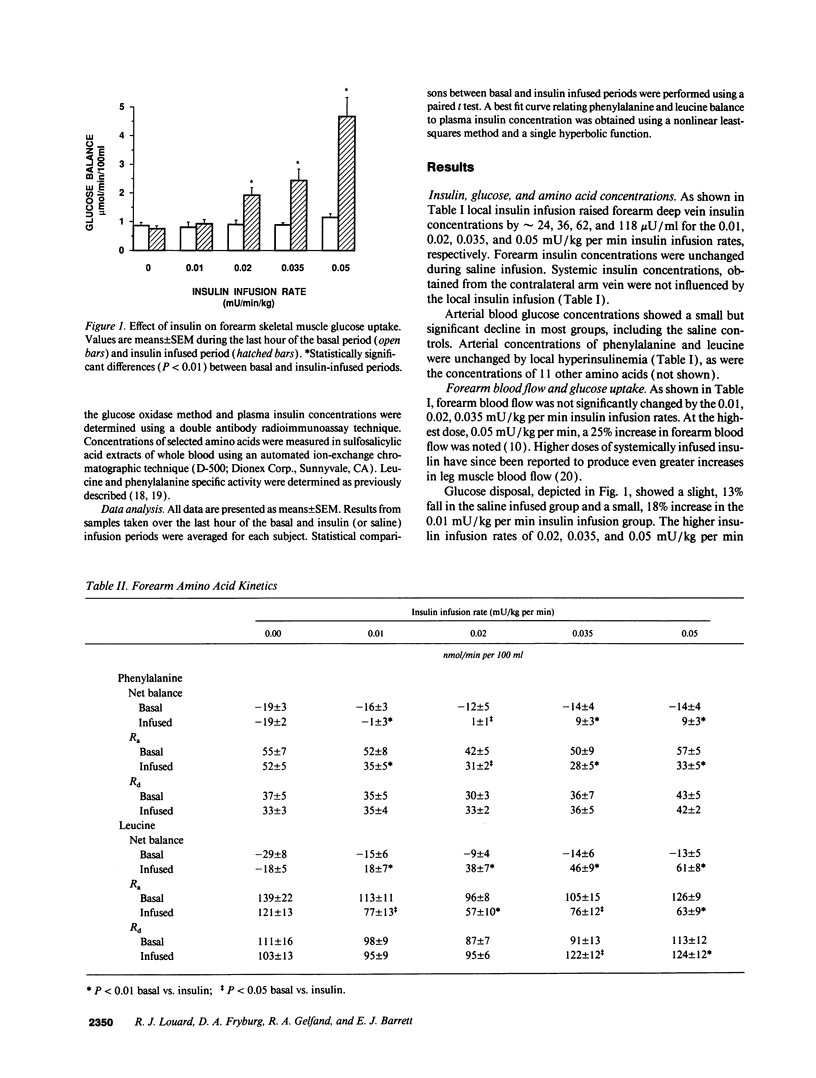
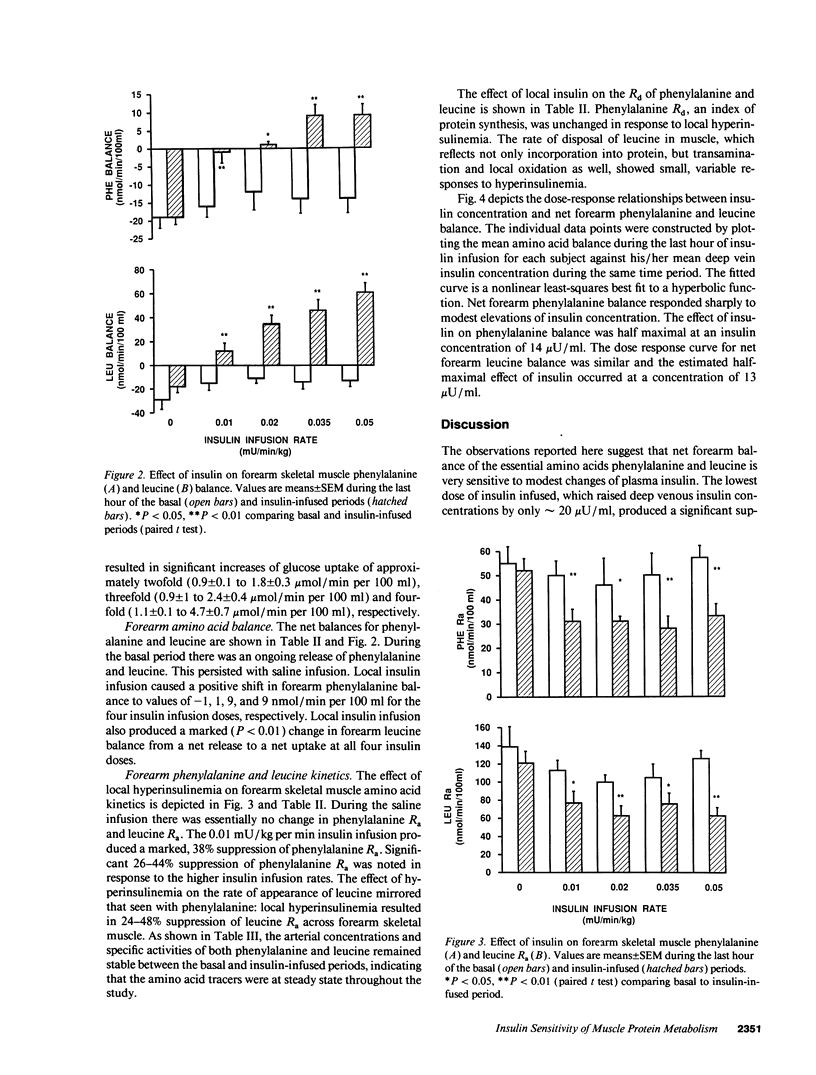
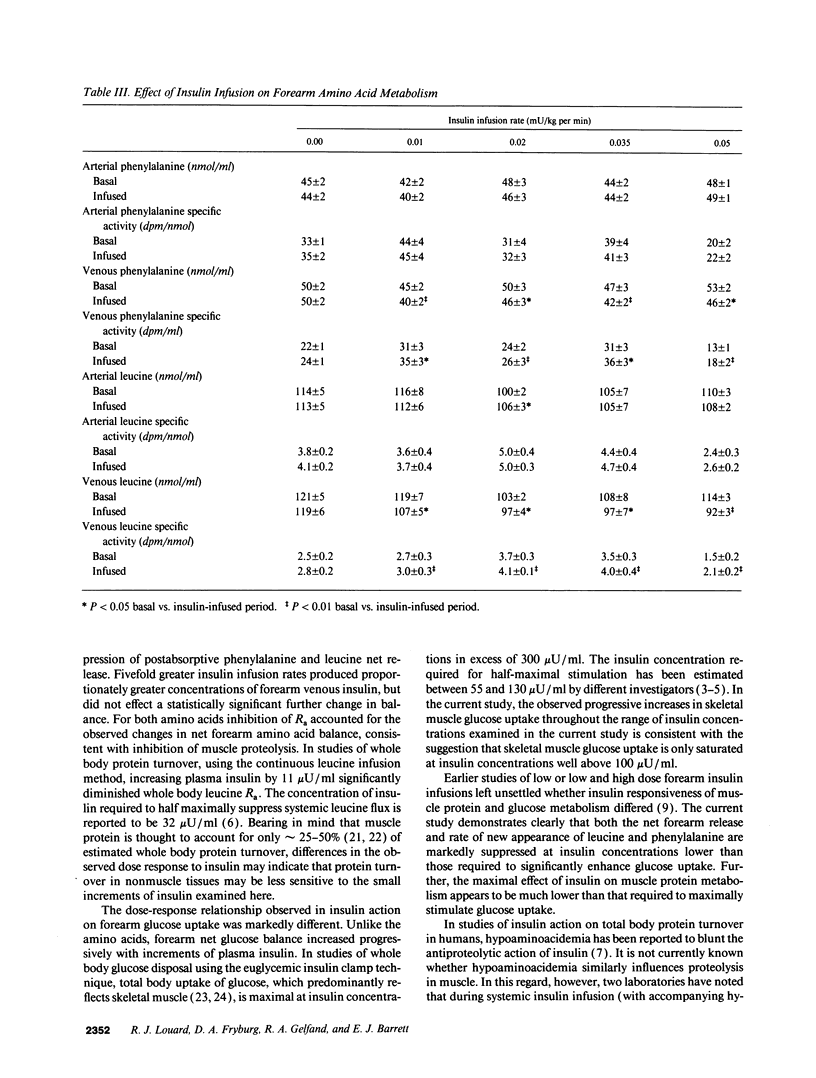
Physiologic increases of insulin promote net amino acid uptake and protein anabolism in forearm skeletal muscle by restraining protein degradation. The sensitivity of this process to insulin is not known. Using the forearm perfusion method, we infused insulin locally in the brachial artery at rates of 0.00 (saline control), 0.01, 0.02, 0.035, or 0.05 mU/min per kg for 150 min to increase local forearm plasma insulin concentration by 0, approximately 20, approximately 35, approximately 60, and approximately 120 microU/ml (n = 35). L-[ring-2,6-3H]phenylalanine and L-[1-14C]leucine were infused systemically, and the net forearm balance, rate of appearance (Ra) and rate of disposal (R(d)) of phenylalanine and leucine, and forearm glucose balance were measured basally and in response to insulin infusion. Compared to saline, increasing rates of insulin infusion progressively increased net forearm glucose uptake from 0.9 mumol/min per 100 ml (saline) to 1.0, 1.8, 2.4, and 4.7 mumol/min per 100 ml forearm, respectively. Net forearm balance for phenylalanine and leucine was significantly less negative than basal (P < 0.01 for each) in response to the lowest dose insulin infusion, 0.01 mU/min per kg, and all higher rates of insulin infusion. Phenylalanine and leucine R(a) declined by approximately 38 and 40% with the lowest dose insulin infusion. Higher doses of insulin produced no greater effect (decline in R(a) varied between 26 and 42% for phenylalanine and 30-50% for leucine). In contrast, R(d) for phenylalanine and leucine did not change with insulin. We conclude that even modest increases of plasma insulin can markedly suppress proteolysis, measured by phenylalanine R(a), in human forearm skeletal muscle. Further increments of insulin within the physiologic range augment glucose uptake but have little additional effect on phenylalanine R(a) or balance. These results suggest that proteolysis in human skeletal muscle is more sensitive than glucose uptake to physiologic increments in insulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfvidsson B., Zachrisson H., Möller-Loswick A. C., Hyltander A., Sandström R., Lundholm K. Effect of systemic hyperinsulinemia on amino acid flux across human legs in postabsorptive state. Am J Physiol. 1991 Jan;260(1 Pt 1):E46–E52. doi: 10.1152/ajpendo.1991.260.1.E46. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988 Dec;255(6 Pt 1):E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Kolterman O. G., Bell J., Mandarino L. J., Olefsky J. M. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985 Nov;76(5):1782–1788. doi: 10.1172/JCI112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. J., Revkin J. H., Young L. H., Zaret B. L., Jacob R., Gelfand R. A. An isotopic method for measurement of muscle protein synthesis and degradation in vivo. Biochem J. 1987 Jul 1;245(1):223–228. doi: 10.1042/bj2450223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Groop L. C., Zych K., Shank M., DeFronzo R. A. Dose-dependent effect of insulin on plasma free fatty acid turnover and oxidation in humans. Am J Physiol. 1990 Nov;259(5 Pt 1):E736–E750. doi: 10.1152/ajpendo.1990.259.5.E736. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Castellino P., Luzi L., Simonson D. C., Haymond M., DeFronzo R. A. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest. 1987 Dec;80(6):1784–1793. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denne S. C., Liechty E. A., Liu Y. M., Brechtel G., Baron A. D. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991 Dec;261(6 Pt 1):E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- Flakoll P. J., Kulaylat M., Frexes-Steed M., Hourani H., Brown L. L., Hill J. O., Abumrad N. N. Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol. 1989 Dec;257(6 Pt 1):E839–E847. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Maycock P. F. Regulation of protein metabolism by a physiological concentration of insulin in mouse soleus and extensor digitorum longus muscles. Effects of starvation and scald injury. Biochem J. 1979 Nov 15;184(2):323–330. doi: 10.1042/bj1840323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryburg D. A., Barrett E. J., Louard R. J., Gelfand R. A. Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol. 1990 Oct;259(4 Pt 1):E477–E482. doi: 10.1152/ajpendo.1990.259.4.E477. [DOI] [PubMed] [Google Scholar]
- Fryburg D. A., Louard R. J., Gerow K. E., Gelfand R. A., Barrett E. J. Growth hormone stimulates skeletal muscle protein synthesis and antagonizes insulin's antiproteolytic action in humans. Diabetes. 1992 Apr;41(4):424–429. doi: 10.2337/diab.41.4.424. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Glickman M. G., Castellino P., Louard R. J., DeFronzo R. A. Measurement of L-[1-14C]leucine kinetics in splanchnic and leg tissues in humans. Effect of amino acid infusion. Diabetes. 1988 Oct;37(10):1365–1372. doi: 10.2337/diab.37.10.1365. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louard R. J., Barrett E. J., Gelfand R. A. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci (Lond) 1990 Nov;79(5):457–466. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Halliday D., Griggs R. C. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988 Feb;254(2 Pt 1):E208–E213. doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Beaufrere B., Koziet J., Desjeux J. F., Bier D. M., Young V. R., Lestradet H. Whole body de novo amino acid synthesis in type I (insulin-dependent) diabetes studied with stable isotope-labeled leucine, alanine, and glycine. Diabetes. 1985 Jan;34(1):67–73. doi: 10.2337/diab.34.1.67. [DOI] [PubMed] [Google Scholar]
- Stirewalt W. S., Low R. B., Slaiby J. M. Insulin sensitivity and responsiveness of epitrochlearis and soleus muscles from fed and starved rats. Recognition of differential changes in insulin sensitivities of protein synthesis and glucose incorporation into glycogen. Biochem J. 1985 Apr 15;227(2):355–362. doi: 10.1042/bj2270355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P., Inchiostro S., Biolo G., Vincenti E., Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest. 1991 Jul;88(1):27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpleby A. M., Boroujerdi M. A., Brown P. M., Carson E. R., Sönksen P. H. The effect of metabolic control on leucine metabolism in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1986 Mar;29(3):131–141. doi: 10.1007/BF02427082. [DOI] [PubMed] [Google Scholar]
- Williams I. H., Sugden P. H., Morgan H. E. Use of aromatic amino acids as monitors of protein turnover. Am J Physiol. 1981 Jun;240(6):E677–E681. doi: 10.1152/ajpendo.1981.240.6.E677. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman R. J., Ludemann R., Easton T. G., Etlinger J. D. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol. 1988 Jun;254(6 Pt 1):E726–E732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]



