Abstract
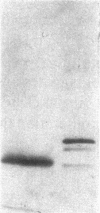
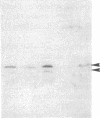
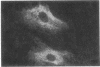
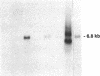
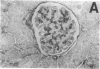
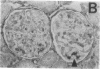
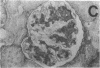
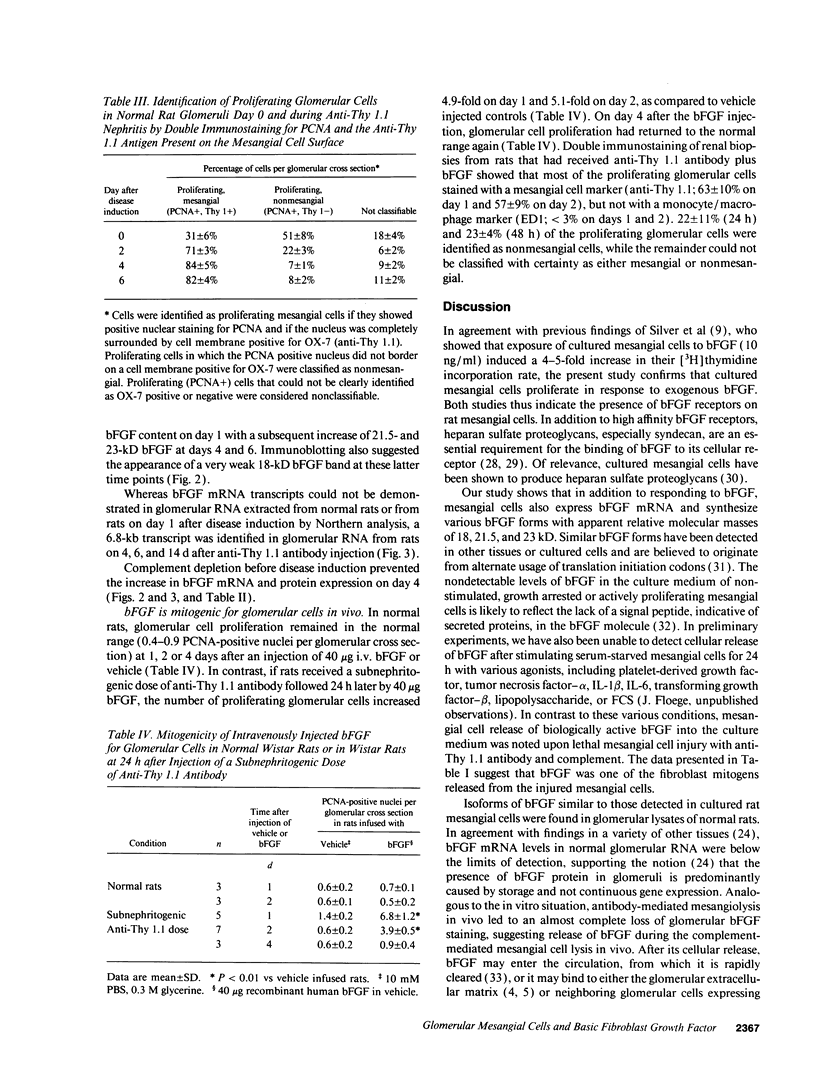
Mesangial injury and cell proliferation are frequent findings in various glomerular diseases in man. Previous studies have demonstrated that basic fibroblast growth factor (bFGF) is a potent mesangial cell mitogen in vitro. To further elucidate the role of bFGF in rat mesangial cell (RMC) proliferation, we examined whether RMC synthesize bFGF in vitro and whether bFGF is involved in mesangial proliferation in vivo. Cultured RMC expressed bFGF protein (23, 21.5, and 18 kD forms) and bFGF mRNA, and released biologically active bFGF into the culture medium after antibody- and complement-mediated injury. Normal rat glomeruli in vivo contained no detectable bFGF mRNA, but bFGF protein (23 and 21.5 kD) could be demonstrated, which immunolocalized to the mesangium. Glomerular bFGF decreased markedly during the acute phase of glomerulonephritis induced by anti-Thy 1.1 antibody, compatible with mesangial bFGF release after complement-mediated mesangiolysis. During the subsequent mesangial proliferative phase, glomerular bFGF protein and mRNA increased above normal. Intrarenal infusion of heparin did not affect the bFGF immunostaining of glomeruli at this stage, indicating a predominantly intracellular localization of the bFGF. The capability of bFGF to mediate proliferation in the anti-Thy 1.1 model was further supported by experiments in which intravenous bFGF given 24 h after a subnephritogenic dose of anti-Thy 1.1 antibody led to a 4.9- to 5.1-fold increase in glomerular cell proliferation (with > 60% of the cells identified as mesangial cells by double immunolabeling). No such increase was observed in normal rats injected with bFGF. These data show that mesangial cells produce and release bFGF after injury and that bFGF is mitogenic for injured mesangial cells in vivo. Release of mesangial cell bFGF thus may be an important mechanism involved in the initiation of mesangial cell proliferation in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Adler S., Baker P. J., Johnson R. J., Ochi R. F., Pritzl P., Couser W. G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986 Mar;77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Gown A. M., Johnson R. J. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992 May;41(5):1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- Baird A., Esch F., Böhlen P., Ling N., Gospodarowicz D. Isolation and partial characterization of an endothelial cell growth factor from the bovine kidney: homology with basic fibroblast growth factor. Regul Pept. 1985 Nov 7;12(3):201–213. doi: 10.1016/0167-0115(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990 Nov;9(3):227–238. doi: 10.1007/BF00046362. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Schnittler H., Nobiling R., Kriz W. Ultrastructural organization of contractile proteins in rat glomerular mesangial cells. Am J Pathol. 1990 Dec;137(6):1343–1351. [PMC free article] [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Floege J., Topley N., Hoppe J., Barrett T. B., Resch K. Mitogenic effect of platelet-derived growth factor in human glomerular mesangial cells: modulation and/or suppression by inflammatory cytokines. Clin Exp Immunol. 1991 Nov;86(2):334–341. doi: 10.1111/j.1365-2249.1991.tb05819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Topley N., Wessel K., Kaever V., Radeke H., Hoppe J., Kishimoto T., Resch K. Monokines and platelet-derived growth factor modulate prostanoid production in growth arrested, human mesangial cells. Kidney Int. 1990 Mar;37(3):859–869. doi: 10.1038/ki.1990.59. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogo A., Hawkins E. P., Berry P. L., Glick A. D., Chiang M. L., MacDonell R. C., Jr, Ichikawa I. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990 Jul;38(1):115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Jacobson H. R., Hoover R. L. Collagen polymorphism in cultured rat kidney mesangial cells. Lab Invest. 1987 Nov;57(5):513–523. [PubMed] [Google Scholar]
- Hondermarck H., Courty J., Boilly B., Thomas D. Distribution of intravenously administered acidic and basic fibroblast growth factors in the mouse. Experientia. 1990 Sep 15;46(9):973–974. doi: 10.1007/BF01939392. [DOI] [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai-Michaeli R., Eldor A., Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990 Oct;1(11):833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Edelman E. R. Biological and biochemical properties of fibroblast growth factors. Implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989 May-Jun;9(3):269–278. doi: 10.1161/01.atv.9.3.269. [DOI] [PubMed] [Google Scholar]
- Klein D. J., Brown D. M., Kim Y., Oegema T. R., Jr Proteoglycans synthesized by human glomerular mesangial cells in culture. J Biol Chem. 1990 Jun 5;265(16):9533–9543. [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Churg J. Mesangiolysis. Kidney Int. 1983 Jul;24(1):1–9. doi: 10.1038/ki.1983.119. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Metabolism of receptor-bound and matrix-bound basic fibroblast growth factor by bovine capillary endothelial cells. J Cell Biol. 1988 Aug;107(2):753–759. doi: 10.1083/jcb.107.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan L., Warder E., McNeil P. L. Basic fibroblast growth factor is efficiently released from a cytolsolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991 Jul;148(1):1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- O'Donoghue D. J., Darvill A., Ballardie F. W. Mesangial cell autoantigens in immunoglobulin A nephropathy and Henoch-Schönlein purpura. J Clin Invest. 1991 Nov;88(5):1522–1530. doi: 10.1172/JCI115462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R., Sterzel R. B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983 Nov;24(5):626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Pesce C. M., Striker L. J., Peten E., Elliot S. J., Striker G. E. Glomerulosclerosis at both early and late stages is associated with increased cell turnover in mice transgenic for growth hormone. Lab Invest. 1991 Nov;65(5):601–605. [PubMed] [Google Scholar]
- Quarto N., Talarico D., Sommer A., Florkiewicz R., Basilico C., Rifkin D. B. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Reilly T. M., Taylor D. S., Herblin W. F., Thoolen M. J., Chiu A. T., Watson D. W., Timmermans P. B. Monoclonal antibodies directed against basic fibroblast growth factor which inhibit its biological activity in vitro and in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):736–743. doi: 10.1016/0006-291x(89)91521-0. [DOI] [PubMed] [Google Scholar]
- Schulze M., Baker P. J., Perkinson D. T., Johnson R. J., Ochi R. F., Stahl R. A., Couser W. G. Increased urinary excretion of C5b-9 distinguishes passive Heymann nephritis in the rat. Kidney Int. 1989 Jan;35(1):60–68. doi: 10.1038/ki.1989.8. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Shimasaki S., Emoto N., Koba A., Mercado M., Shibata F., Cooksey K., Baird A., Ling N. Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem Biophys Res Commun. 1988 Nov 30;157(1):256–263. doi: 10.1016/s0006-291x(88)80041-x. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Jaffer F. E., Abboud H. E. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker L. J., Peten E. P., Elliot S. J., Doi T., Striker G. E. Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest. 1991 Apr;64(4):446–456. [PubMed] [Google Scholar]
- Thompson R. W., Whalen G. F., Saunders K. B., Hores T., D'Amore P. A. Heparin-mediated release of fibroblast growth factor-like activity into the circulation of rabbits. Growth Factors. 1990;3(3):221–229. doi: 10.3109/08977199009043906. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987 Oct;32(4):514–525. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M. Autocrine regulation of cell growth and transformation by basic fibroblast growth factor. Cancer Metastasis Rev. 1990 Nov;9(3):191–202. doi: 10.1007/BF00046360. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Gordon K., Alpers C. E., Floege J., Pritzl P., Ross R., Couser W. G., Bowen-Pope D. F., Johnson R. J. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991 Sep;40(3):470–476. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]