Abstract
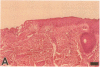
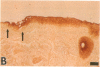
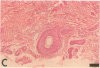
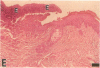
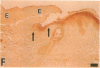
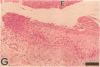
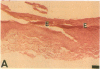
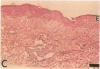
Epidermal growth factor (EGF) along with several related peptide growth factors has been shown both in vivo and in vitro to accelerate events associated with epidermal wound repair. EGF and transforming growth factor alpha act by binding to a common EGF receptor tyrosine kinase thereby initiating a series of events which ultimately regulate cell proliferation. This study examined the immunohistochemical localization of EGF receptor (EGF-R) in burn wound margins, adjacent proliferating epithelium, and closely associated sweat ducts, sebaceous glands, and hair follicles. Tissue specimens removed during surgical debridement were obtained from full and partial thickness burn wounds in 32 patients with total body surface area burns ranging from 2 to 88%. In the early postburn period (days 2-4), prominent staining for EGF-R was found in undifferentiated, marginal keratinocytes, adjacent proliferating, hypertrophic epithelium, and both marginal and nonmarginal hair follicles, sweat ducts, and sebaceous glands. During the late postburn period (days 5-16), EGF-R was depleted along leading epithelial margins; however, immunoreactive EGF-R remained intensely positive in the hypertrophic epithelium and all skin appendages. Increased detection of immunoreactive EGF-R and the presence of [125I]EGF binding in the hypertrophic epithelium correlated positively with proliferating cell nuclear antigen distributions. Thus, the presence of EGF-R in the appropriate keratinocyte populations suggests a functional role for this receptor during wound repair. Dynamic modulation in EGF receptor distribution during the temporal sequence of repair provides further evidence that an EGF/transforming growth factor alpha/EGF-R-mediated pathway is activated during human wound repair.
Full text
PDF

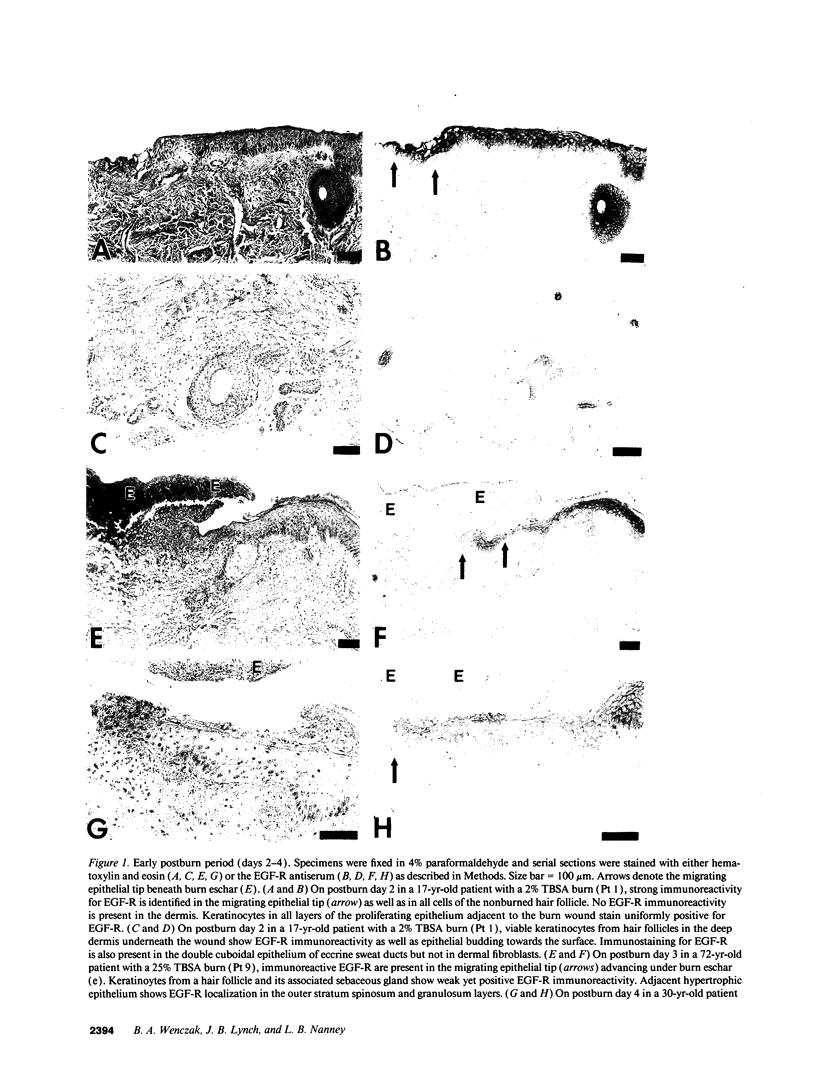

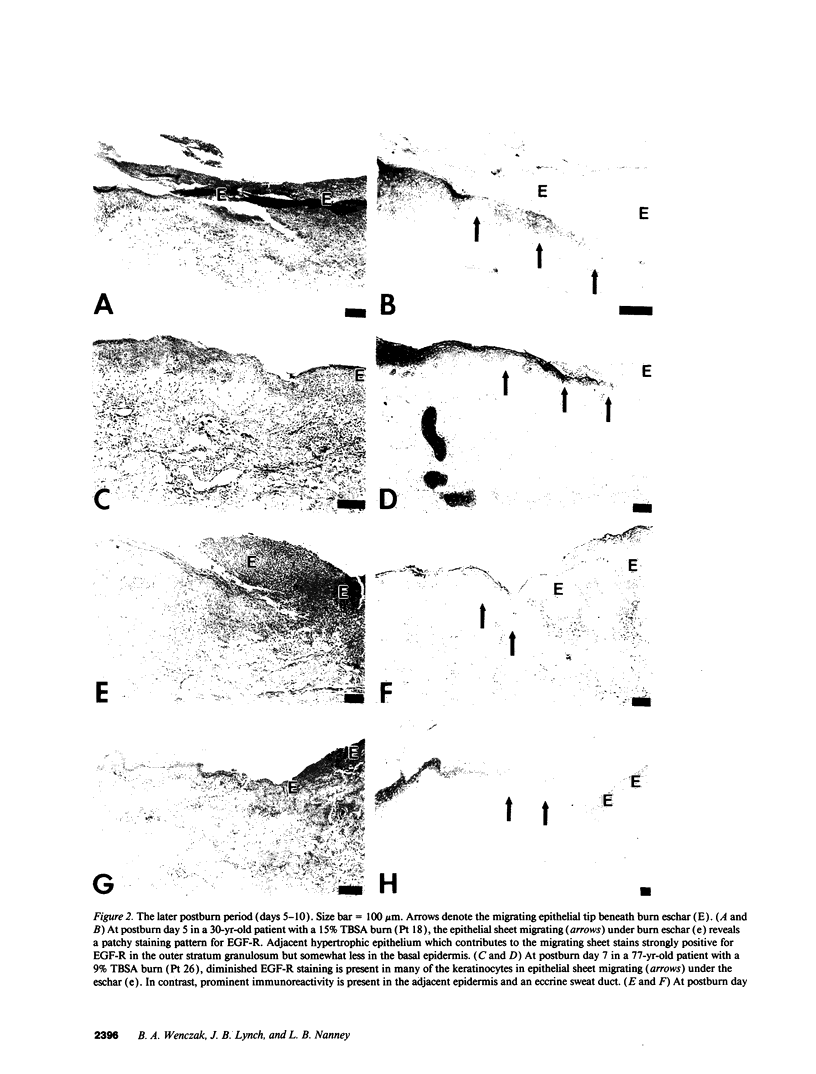
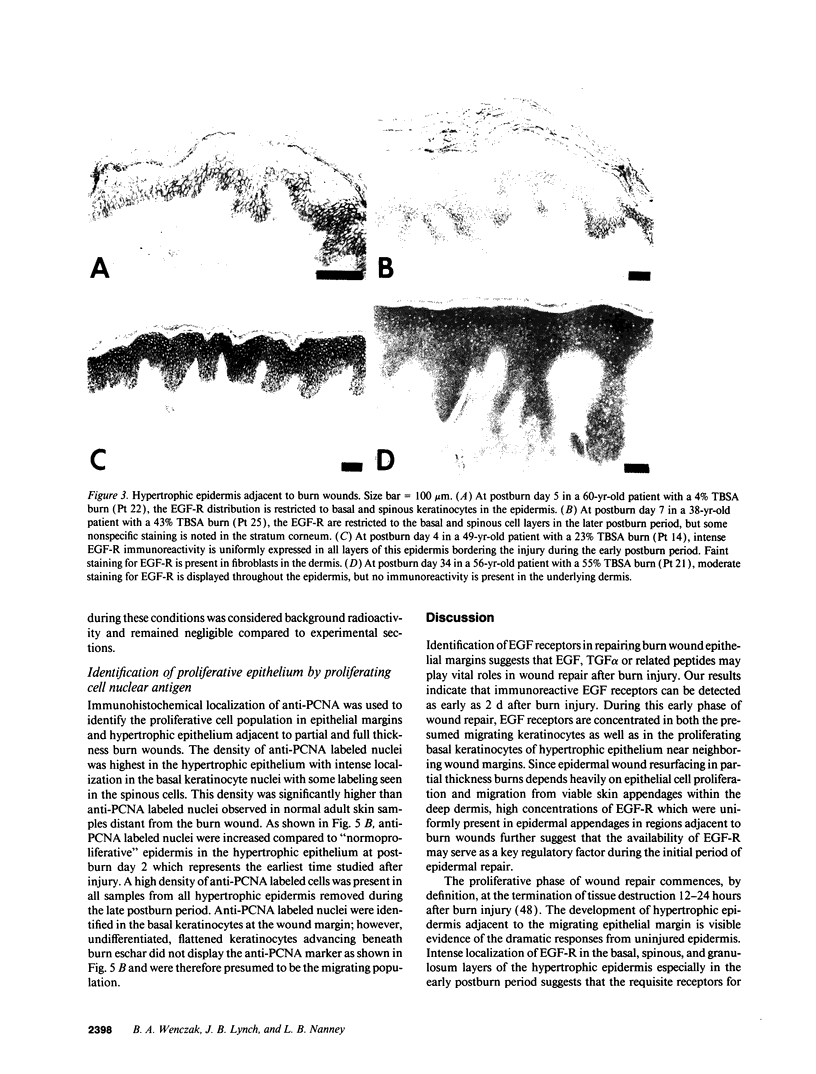



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Birecree E., Whetsell W. O., Jr, Stoscheck C., King L. E., Jr, Nanney L. B. Immunoreactive epidermal growth factor receptors in neuritic plaques from patients with Alzheimer's disease. J Neuropathol Exp Neurol. 1988 Sep;47(5):549–560. doi: 10.1097/00005072-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Woodward S. C., Buckley-Sturrock A., Sato Y., Rifkin D. B., Davidson J. M. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989 Nov;61(5):571–575. [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Twardzik D. R., Marquardt H., Todaro G. J. Vaccinia virus encodes a polypeptide homologous to epidermal growth factor and transforming growth factor. Nature. 1985 Feb 7;313(6002):491–492. doi: 10.1038/313491a0. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor-alpha: structure and biological activities. J Cell Biochem. 1986;32(4):293–304. doi: 10.1002/jcb.240320406. [DOI] [PubMed] [Google Scholar]
- Ellis D. L., Kafka S. P., Chow J. C., Nanney L. B., Inman W. H., McCadden M. E., King L. E., Jr Melanoma, growth factors, acanthosis nigricans, the sign of Leser-Trélat, and multiple acrochordons. A possible role for alpha-transforming growth factor in cutaneous paraneoplastic syndromes. N Engl J Med. 1987 Dec 17;317(25):1582–1587. doi: 10.1056/NEJM198712173172506. [DOI] [PubMed] [Google Scholar]
- Ellis D. L., Nanney L. B., King L. E., Jr Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990 Dec;23(6 Pt 1):1070–1077. doi: 10.1016/0190-9622(90)70335-f. [DOI] [PubMed] [Google Scholar]
- Finzi E., Harkins R., Horn T. TGF-alpha is widely expressed in differentiated as well as hyperproliferative skin epithelium. J Invest Dermatol. 1991 Mar;96(3):328–332. doi: 10.1111/1523-1747.ep12465223. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Chang C. K., Posnett D. N., Fanelli B., Tam J. P. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988 Feb 1;167(2):670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Couchman J. R. Differences in human skin between the epidermal growth factor receptor distribution detected by EGF binding and monoclonal antibody recognition. J Invest Dermatol. 1985 Sep;85(3):239–245. doi: 10.1111/1523-1747.ep12276708. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebda P. A., Klingbeil C. K., Abraham J. A., Fiddes J. C. Basic fibroblast growth factor stimulation of epidermal wound healing in pigs. J Invest Dermatol. 1990 Dec;95(6):626–631. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- Hebda P. A. Stimulatory effects of transforming growth factor-beta and epidermal growth factor on epidermal cell outgrowth from porcine skin explant cultures. J Invest Dermatol. 1988 Nov;91(5):440–445. doi: 10.1111/1523-1747.ep12476480. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid M., Nanney L. B., Stoscheck C. M., King L. E., Jr Epidermal growth factor binding and receptor distribution in term human placenta. Placenta. 1985 Nov-Dec;6(6):519–526. doi: 10.1016/s0143-4004(85)80005-9. [DOI] [PubMed] [Google Scholar]
- Massagué J. Transforming growth factor-beta modulates the high-affinity receptors for epidermal growth factor and transforming growth factor-alpha. J Cell Biol. 1985 May;100(5):1508–1514. doi: 10.1083/jcb.100.5.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B. Epidermal and dermal effects of epidermal growth factor during wound repair. J Invest Dermatol. 1990 May;94(5):624–629. doi: 10.1111/1523-1747.ep12876204. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Magid M., Stoscheck C. M., King L. E., Jr Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J Invest Dermatol. 1984 Nov;83(5):385–393. doi: 10.1111/1523-1747.ep12264708. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., King L. E. Characterization of binding and receptors for epidermal growth factor in smooth muscle. Cell Tissue Res. 1988 Oct;254(1):125–132. doi: 10.1007/BF00220025. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., King L. E., Jr, Underwood R. A., Holbrook K. A. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990 Jun;94(6):742–748. doi: 10.1111/1523-1747.ep12874601. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., Magid M., King L. E., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986 Mar;86(3):260–265. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Yates R. A., King L. E., Jr Modulation of epidermal growth factor receptors in psoriatic lesions during treatment with topical EGF. J Invest Dermatol. 1992 Mar;98(3):296–301. doi: 10.1111/1523-1747.ep12497963. [DOI] [PubMed] [Google Scholar]
- Odland G., Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968 Oct;39(1):135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olashaw N. E., O'Keefe E. J., Pledger W. J. Platelet-derived growth factor modulates epidermal growth factor receptors by a mechanism distinct from that of phorbol esters. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3834–3838. doi: 10.1073/pnas.83.11.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Richards C. S., Hendler F., Burns D., Gusterson B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986 May;149(1):9–14. doi: 10.1002/path.1711490104. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Ditesheim J. A., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol. 1991 Jul;97(1):34–42. [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Characteristics of antibodies to the epidermal growth factor receptor-kinase. Arch Biochem Biophys. 1983 Dec;227(2):457–468. doi: 10.1016/0003-9861(83)90476-9. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., King L. E., Jr Functional and structural characteristics of EGF and its receptor and their relationship to transforming proteins. J Cell Biochem. 1986;31(2):135–152. doi: 10.1002/jcb.240310206. [DOI] [PubMed] [Google Scholar]
- Taylor H. C., Lightner V. A., Beyer W. F., Jr, McCaslin D., Briscoe G., Erickson H. P. Biochemical and structural studies of tenascin/hexabrachion proteins. J Cell Biochem. 1989 Oct;41(2):71–90. doi: 10.1002/jcb.240410204. [DOI] [PubMed] [Google Scholar]
- Thaete L. G., Ahnen D. J., Malkinson A. M. Proliferating cell nuclear antigen (PCNA/cyclin) immunocytochemistry as a labeling index in mouse lung tissues. Cell Tissue Res. 1989 Apr;256(1):167–173. doi: 10.1007/BF00224731. [DOI] [PubMed] [Google Scholar]
- Van Winkle W., Jr The epithelium in wound healing. Surg Gynecol Obstet. 1968 Nov;127(5):1089–1115. [PubMed] [Google Scholar]
- Werner M. H., Nanney L. B., Stoscheck C. M., King L. E. Localization of immunoreactive epidermal growth factor receptors in human nervous system. J Histochem Cytochem. 1988 Jan;36(1):81–86. doi: 10.1177/36.1.3275713. [DOI] [PubMed] [Google Scholar]
- deCamara D. L., Raine T. J., London M. D., Robson M. C., Heggers J. P. Progression of thermal injury: a morphologic study. Plast Reconstr Surg. 1982 Mar;69(3):491–499. doi: 10.1097/00006534-198203000-00016. [DOI] [PubMed] [Google Scholar]