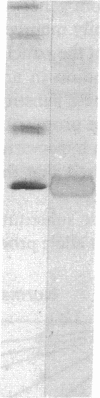
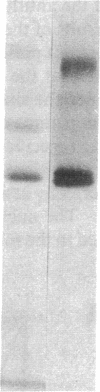
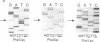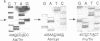Abstract
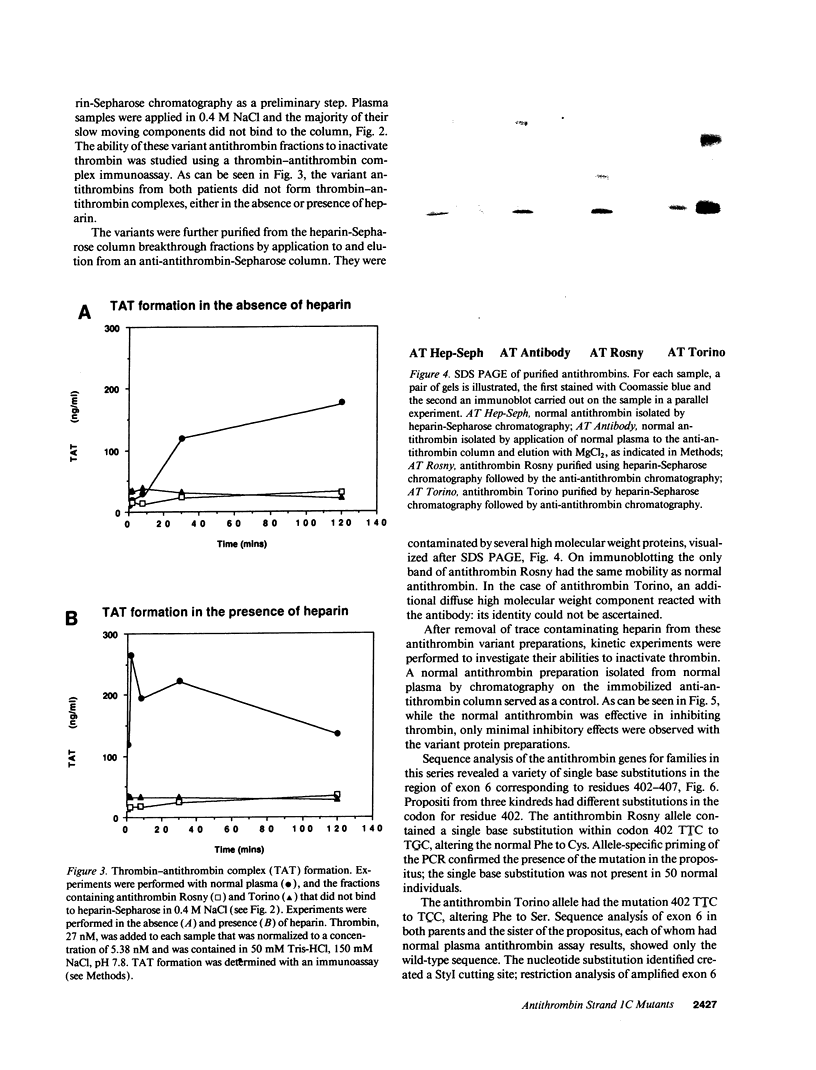
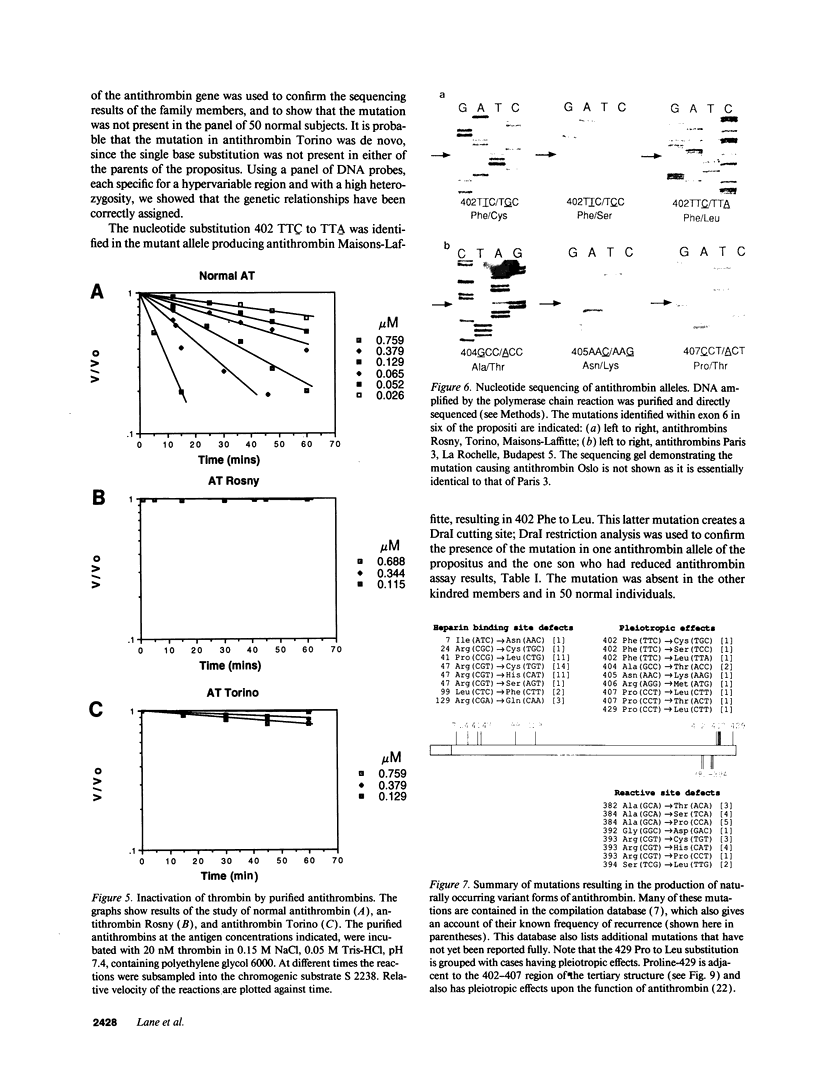
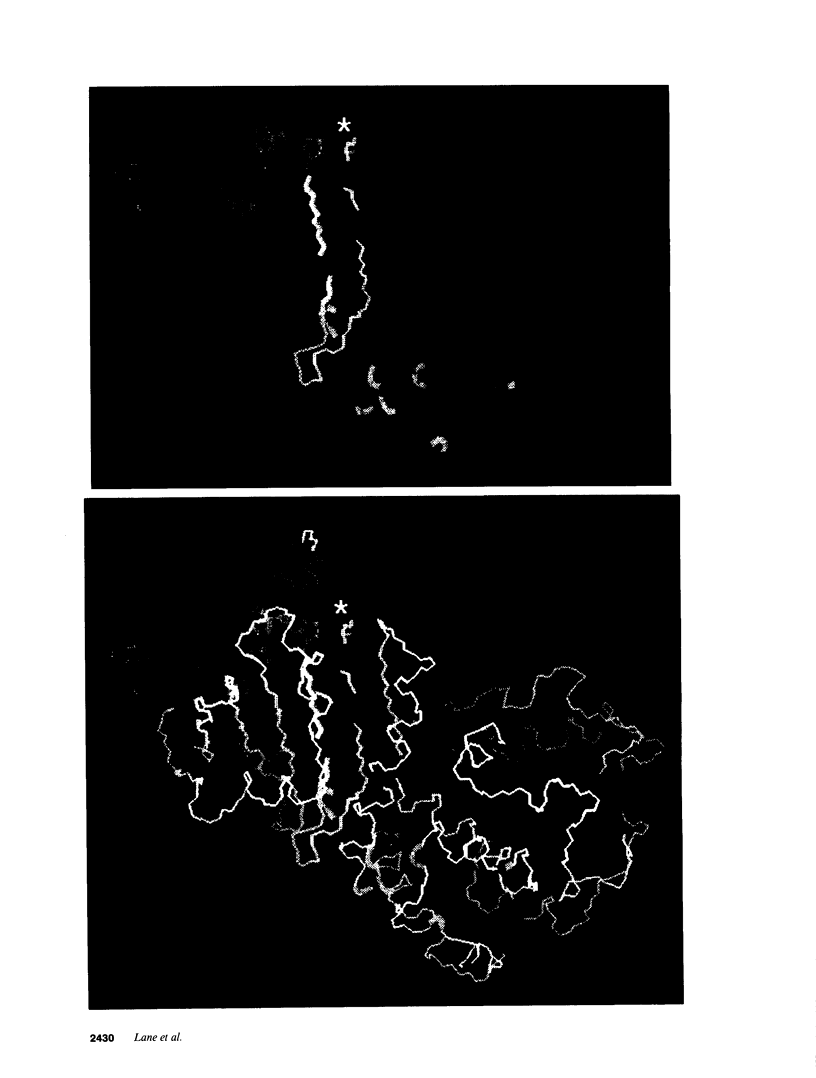
Six different substitution mutations were identified in four different amino acid residues of antithrombin strand 1C and the polypeptide leading into strand 4B (F402S, F402C, F402L, A404T, N405K, and P407T), and are responsible for functional antithrombin deficiency in seven independently ascertained kindreds (Rosny, Torino, Maisons-Laffitte, Paris 3, La Rochelle, Budapest 5, and Oslo) affected by venous thromboembolic disease. In all seven families, variant antithrombins with heparin-binding abnormalities were detected by crossed immunoelectrophoresis, and in six of the kindreds there was a reduced antigen concentration of plasma antithrombin. Two of the variant antithrombins, Rosny and Torino, were purified by heparin-Sepharose and immunoaffinity chromatography, and shown to have greatly reduced heparin cofactor and progressive inhibitor activities in vitro. The defective interactions of these mutants with thrombin may result from proximity of s1C to the reactive site, while reduced circulating levels may be related to s1C proximity to highly conserved internal beta strands, which contain elements proposed to influence serpin turnover and intracellular degradation. In contrast, s1C is spatially distant to the positively charged surface which forms the heparin binding site of antithrombin; altered heparin binding properties of s1C variants may therefore reflect conformational linkage between the reactive site and heparin binding regions of the molecule. This work demonstrates that point mutations in and immediately adjacent to strand 1C have multiple, or pleiotropic, effects on this serpin, leading ultimately to failure of its regulatory function.
Full text
PDF






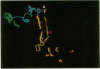
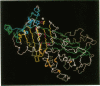
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U. Binding of thrombin to antithrombin III. Scand J Clin Lab Invest. 1969 Aug;24(1):23–27. doi: 10.3109/00365516909080127. [DOI] [PubMed] [Google Scholar]
- Abildgaard U., Fagerhol M. K., Egeberg O. Comparison of progressive antithrombin activity and the concentration of three thrombin inhibitors in human plasma. Scand J Clin Lab Invest. 1970 Dec;26(4):349–354. doi: 10.3109/00365517009046245. [DOI] [PubMed] [Google Scholar]
- Austin R. C., Rachubinski R. A., Ofosu F. A., Blajchman M. A. Antithrombin-III-Hamilton, Ala 382 to Thr: an antithrombin-III variant that acts as a substrate but not an inhibitor of alpha-thrombin and factor Xa. Blood. 1991 May 15;77(10):2185–2189. [PubMed] [Google Scholar]
- Blackburn M. N., Smith R. L., Carson J., Sibley C. C. The heparin-binding site of antithrombin III. Identification of a critical tryptophan in the amino acid sequence. J Biol Chem. 1984 Jan 25;259(2):939–941. [PubMed] [Google Scholar]
- Blajchman M. A., Fernandez-Rachubinski F., Sheffield W. P., Austin R. C., Schulman S. Antithrombin-III-Stockholm: a codon 392 (Gly----Asp) mutation with normal heparin binding and impaired serine protease reactivity. Blood. 1992 Mar 15;79(6):1428–1434. [PubMed] [Google Scholar]
- Bock S. C., Levitan D. J. Characterization of an unusual DNA length polymorphism 5' to the human antithrombin III gene. Nucleic Acids Res. 1983 Dec 20;11(24):8569–8582. doi: 10.1093/nar/11.24.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S. C., Marrinan J. A., Radziejewska E. Antithrombin III Utah: proline-407 to leucine mutation in a highly conserved region near the inhibitor reactive site. Biochemistry. 1988 Aug 9;27(16):6171–6178. doi: 10.1021/bi00416a052. [DOI] [PubMed] [Google Scholar]
- Borg J. Y., Brennan S. O., Carrell R. W., George P., Perry D. J., Shaw J. Antithrombin Rouen-IV 24 Arg----Cys. The amino-terminal contribution to heparin binding. FEBS Lett. 1990 Jun 18;266(1-2):163–166. doi: 10.1016/0014-5793(90)81530-2. [DOI] [PubMed] [Google Scholar]
- Borg J. Y., Owen M. C., Soria C., Soria J., Caen J., Carrell R. W. Proposed heparin binding site in antithrombin based on arginine 47. A new variant Rouen-II, 47 Arg to Ser. J Clin Invest. 1988 Apr;81(4):1292–1296. doi: 10.1172/JCI113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Borg J. Y., George P. M., Soria C., Soria J., Caen J., Carrell R. W. New carbohydrate site in mutant antithrombin (7 Ile----Asn) with decreased heparin affinity. FEBS Lett. 1988 Sep 12;237(1-2):118–122. doi: 10.1016/0014-5793(88)80183-2. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., George P. M., Jordan R. E. Physiological variant of antithrombin-III lacks carbohydrate sidechain at Asn 135. FEBS Lett. 1987 Jul 27;219(2):431–436. doi: 10.1016/0014-5793(87)80266-1. [DOI] [PubMed] [Google Scholar]
- Brodbeck R. M., Brown J. L. Secretion of alpha-1-proteinase inhibitor requires an almost full length molecule. J Biol Chem. 1992 Jan 5;267(1):294–297. [PubMed] [Google Scholar]
- Brunel F., Duchange N., Fischer A. M., Cohen G. N., Zakin M. M. Antithrombin III Alger: a new case of Arg 47----Cys mutation. Am J Hematol. 1987 Jun;25(2):223–224. doi: 10.1002/ajh.2830250214. [DOI] [PubMed] [Google Scholar]
- Caso R., Lane D. A., Thompson E. A., Olds R. J., Thein S. L., Panico M., Blench I., Morris H. R., Freyssinet J. M., Aiach M. Antithrombin Vicenza, Ala 384 to Pro (GCA to CCA) mutation, transforming the inhibitor into a substrate. Br J Haematol. 1991 Jan;77(1):87–92. doi: 10.1111/j.1365-2141.1991.tb07953.x. [DOI] [PubMed] [Google Scholar]
- Caso R., Lane D. A., Thompson E., Zangouras D., Panico M., Morris H., Olds R. J., Thein S. L., Girolami A. Antithrombin Padua. I: Impaired heparin binding caused by an Arg47 to his (CGT to CAT) substitution. Thromb Res. 1990 Apr 15;58(2):185–190. doi: 10.1016/0049-3848(90)90175-c. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Binding of heparin to human antithrombin III activates selective chemical modification at lysine 236. Lys-107, Lys-125, and Lys-136 are situated within the heparin-binding site of antithrombin III. J Biol Chem. 1989 Feb 25;264(6):3111–3115. [PubMed] [Google Scholar]
- Chang J. Y., Tran T. H. Antithrombin III Basel. Identification of a Pro-Leu substitution in a hereditary abnormal antithrombin with impaired heparin cofactor activity. J Biol Chem. 1986 Jan 25;261(3):1174–1176. [PubMed] [Google Scholar]
- Daly M. E., Perry D. J. DdeI polymorphism in intron 5 of the ATIII gene. Nucleic Acids Res. 1990 Sep 25;18(18):5583–5583. doi: 10.1093/nar/18.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M., Bruce D., Perry D. J., Price J., Harper P. L., O'Meara A., Carrell R. W. Antithrombin Dublin (-3 Val----Glu): an N-terminal variant which has an aberrant signal peptidase cleavage site. FEBS Lett. 1990 Oct 29;273(1-2):87–90. doi: 10.1016/0014-5793(90)81057-u. [DOI] [PubMed] [Google Scholar]
- Devraj-Kizuk R., Chui D. H., Prochownik E. V., Carter C. J., Ofosu F. A., Blajchman M. A. Antithrombin-III-Hamilton: a gene with a point mutation (guanine to adenine) in codon 382 causing impaired serine protease reactivity. Blood. 1988 Nov;72(5):1518–1523. [PubMed] [Google Scholar]
- Duchange N., Chassé J. F., Cohen G. N., Zakin M. M. Antithrombin III tours gene: identification of a point mutation leading to an arginine----cysteine replacement in a silent deficiency. Nucleic Acids Res. 1986 Mar 11;14(5):2408–2408. doi: 10.1093/nar/14.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Ireland H., Di Marzo V., Panico M., Morris H. R., Tripodi A., Mannucci P. M. Antithrombin Milano, single amino acid substitution at the reactive site, Arg393 to Cys. Thromb Haemost. 1988 Dec 22;60(3):471–475. [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Ireland H., Panico M., Di Marzo V., Blench I., Morris H. R. Formation of a covalent disulfide-linked antithrombin-albumin complex by an antithrombin variant, antithrombin "Northwick Park". J Biol Chem. 1987 Oct 5;262(28):13381–13384. [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Panico M., Di Marzo V., Morris H. R., Bauer K., Rosenberg R. D. Antithrombin Chicago, amino acid substitution of arginine 393 to histidine. Thromb Res. 1989 Jun 15;54(6):613–619. doi: 10.1016/0049-3848(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Erdjument H., Lane D. A., Panico M., Di Marzo V., Morris H. R. Single amino acid substitutions in the reactive site of antithrombin leading to thrombosis. Congenital substitution of arginine 393 to cysteine in antithrombin Northwick Park and to histidine in antithrombin Glasgow. J Biol Chem. 1988 Apr 25;263(12):5589–5593. [PubMed] [Google Scholar]
- Finazzi G., Caccia R., Barbui T. Different prevalence of thromboembolism in the subtypes of congenital antithrombin III deficiency: review of 404 cases. Thromb Haemost. 1987 Dec 18;58(4):1094–1094. [PubMed] [Google Scholar]
- Gandrille S., Aiach M., Lane D. A., Vidaud D., Molho-Sabatier P., Caso R., de Moerloose P., Fiessinger J. N., Clauser E. Important role of arginine 129 in heparin-binding site of antithrombin III. Identification of a novel mutation arginine 129 to glutamine. J Biol Chem. 1990 Nov 5;265(31):18997–19001. [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Hultin M. B., McKay J., Abildgaard U. Antithrombin Oslo: type Ib classification of the first reported antithrombin-deficient family, with a review of hereditary antithrombin variants. Thromb Haemost. 1988 Jun 16;59(3):468–473. [PubMed] [Google Scholar]
- Ireland H., Lane D. A., Thompson E., Walker I. D., Blench I., Morris H. R., Freyssinet J. M., Grunebaum L., Olds R., Thein S. L. Antithrombin Glasgow II: alanine 382 to threonine mutation in the serpin P12 position, resulting in a substrate reaction with thrombin. Br J Haematol. 1991 Sep;79(1):70–74. doi: 10.1111/j.1365-2141.1991.tb08009.x. [DOI] [PubMed] [Google Scholar]
- JEAN G., RACINE L., MARX R., GAUTIER A. [On the presence of neutral fats in normal and pathological human thrombocytes]. Thromb Diath Haemorrh. 1963 Apr 15;9:1–11. [PubMed] [Google Scholar]
- Joslin G., Fallon R. J., Bullock J., Adams S. P., Perlmutter D. H. The SEC receptor recognizes a pentapeptide neodomain of alpha 1-antitrypsin-protease complexes. J Biol Chem. 1991 Jun 15;266(17):11282–11288. [PubMed] [Google Scholar]
- Koide T., Odani S., Takahashi K., Ono T., Sakuragawa N. Antithrombin III Toyama: replacement of arginine-47 by cysteine in hereditary abnormal antithrombin III that lacks heparin-binding ability. Proc Natl Acad Sci U S A. 1984 Jan;81(2):289–293. doi: 10.1073/pnas.81.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. A., Caso R. Antithrombin: structure, genomic organization, function and inherited deficiency. Baillieres Clin Haematol. 1989 Oct;2(4):961–998. doi: 10.1016/s0950-3536(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Lane D. A., Erdjument H., Flynn A., Di Marzo V., Panico M., Morris H. R., Greaves M., Dolan G., Preston F. E. Antithrombin Sheffield: amino acid substitution at the reactive site (Arg393 to His) causing thrombosis. Br J Haematol. 1989 Jan;71(1):91–96. doi: 10.1111/j.1365-2141.1989.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Lane D. A., Erdjument H., Thompson E., Panico M., Di Marzo V., Morris H. R., Leone G., De Stefano V., Thein S. L. A novel amino acid substitution in the reactive site of a congenital variant antithrombin. Antithrombin pescara, ARG393 to pro, caused by a CGT to CCT mutation. J Biol Chem. 1989 Jun 15;264(17):10200–10204. [PubMed] [Google Scholar]
- Lane D. A., Flynn A., Ireland H., Erdjument H., Samson D., Howarth D., Thompson E. Antithrombin III Northwick Park: demonstration of an inactive high MW complex with increased affinity for heparin. Br J Haematol. 1987 Apr;65(4):451–456. doi: 10.1111/j.1365-2141.1987.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Lane D. A., Ireland H., Olds R. J., Thein S. L., Perry D. J., Aiach M. Antithrombin III: a database of mutations. Thromb Haemost. 1991 Dec 2;66(6):657–661. [PubMed] [Google Scholar]
- Lane D. A., Lowe G. D., Flynn A., Thompson E., Ireland H., Erdjument H. Antithrombin III Glasgow: a variant with increased heparin affinity and reduced ability to inactivate thrombin, associated with familial thrombosis. Br J Haematol. 1987 Aug;66(4):523–527. doi: 10.1111/j.1365-2141.1987.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Molho-Sabatier P., Aiach M., Gaillard I., Fiessinger J. N., Fischer A. M., Chadeuf G., Clauser E. Molecular characterization of antithrombin III (ATIII) variants using polymerase chain reaction. Identification of the ATIII Charleville as an Ala 384 Pro mutation. J Clin Invest. 1989 Oct;84(4):1236–1242. doi: 10.1172/JCI114290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy C., Owen M. C., Boswell D. R. Immunoadsorbent purification of antithrombin: active form and inactive variants. Thromb Res. 1989 Sep 1;55(5):657–660. doi: 10.1016/0049-3848(89)90399-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Tanaka S., Tsuji H., Takada O., Uno M., Hashimoto-Gotoh T., Wagatsuma M. Congenital antithrombin III deficiency (AT-III Kyoto): identification of a point mutation altering arginine-406 to methionine behind the reactive site. Thromb Res. 1991 Oct 1;64(1):101–108. doi: 10.1016/0049-3848(91)90209-f. [DOI] [PubMed] [Google Scholar]
- Olds R. J., Lane D. A., Boisclair M., Sas G., Bock S. C., Thein S. L. Antithrombin Budapest 3. An antithrombin variant with reduced heparin affinity resulting from the substitution L99F. FEBS Lett. 1992 Apr 6;300(3):241–246. doi: 10.1016/0014-5793(92)80854-a. [DOI] [PubMed] [Google Scholar]
- Olds R. J., Lane D. A., Caso R., Panico M., Morris H. R., Sas G., Dawes J., Thein S. L. Antithrombin III Budapest: a single amino acid substitution (429Pro to Leu) in a region highly conserved in the serpin family. Blood. 1992 Mar 1;79(5):1206–1212. [PubMed] [Google Scholar]
- Olds R. J., Lane D. A., Finazzi G., Barbui T., Thein S. L. A frameshift mutation leading to type 1 antithrombin deficiency and thrombosis. Blood. 1990 Dec 1;76(11):2182–2186. [PubMed] [Google Scholar]
- Olds R. J., Lane D. A., Ireland H., Leone G., De Stefano V., Wiesel M. L., Cazenave J. P., Thein S. L. Novel point mutations leading to type 1 antithrombin deficiency and thrombosis. Br J Haematol. 1991 Jul;78(3):408–413. doi: 10.1111/j.1365-2141.1991.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Olds R. J., Lane D., Caso R., Tripodi A., Mannucci P. M., Thein S. L. Antithrombin III Milano 2: a single base substitution in the thrombin binding domain detected with PCR and direct genomic sequencing. Nucleic Acids Res. 1989 Dec 25;17(24):10511–10511. doi: 10.1093/nar/17.24.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. C., Borg J. Y., Soria C., Soria J., Caen J., Carrell R. W. Heparin binding defect in a new antithrombin III variant: Rouen, 47 Arg to His. Blood. 1987 May;69(5):1275–1279. [PubMed] [Google Scholar]
- Perry D. J., Daly M., Harper P. L., Tait R. C., Price J., Walker I. D., Carrell R. W. Antithrombin Cambridge II, 384 Ala to Ser. Further evidence of the role of the reactive centre loop in the inhibitory function of the serpins. FEBS Lett. 1991 Jul 22;285(2):248–250. doi: 10.1016/0014-5793(91)80809-h. [DOI] [PubMed] [Google Scholar]
- Peterson C. B., Blackburn M. N. Isolation and characterization of an antithrombin III variant with reduced carbohydrate content and enhanced heparin binding. J Biol Chem. 1985 Jan 10;260(1):610–615. [PubMed] [Google Scholar]
- Prochownik E. V., Antonarakis S., Bauer K. A., Rosenberg R. D., Fearon E. R., Orkin S. H. Molecular heterogeneity of inherited antithrombin III deficiency. N Engl J Med. 1983 Jun 30;308(26):1549–1552. doi: 10.1056/NEJM198306303082601. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Groeneveld E., Kluft C., Nieuwenhuis H. K. Alpha 2-antiplasmin Enschede is not an inhibitor, but a substrate, of plasmin. Biochem J. 1988 Oct 15;255(2):609–615. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Sas G. Hereditary antithrombin III deficiency: biochemical aspects. Haematologia (Budap) 1984;17(1):81–86. [PubMed] [Google Scholar]
- Sas G., Pepper D. S., Cash J. D. Plasma and serum antithrombin - III: differentiation by crossed immunoelectrophoresis. Thromb Res. 1975 Jan;6(1):87–91. doi: 10.1016/0049-3848(75)90153-x. [DOI] [PubMed] [Google Scholar]
- Sas G., Petö I., Bánhegyi D., Blaskó G., Domján G. Heterogeneity of the "classical" antithrombin III deficiency. Thromb Haemost. 1980 Jun 18;43(2):133–136. [PubMed] [Google Scholar]
- Skriver K., Wikoff W. R., Patston P. A., Tausk F., Schapira M., Kaplan A. P., Bock S. C. Substrate properties of C1 inhibitor Ma (alanine 434----glutamic acid). Genetic and structural evidence suggesting that the P12-region contains critical determinants of serine protease inhibitor/substrate status. J Biol Chem. 1991 May 15;266(14):9216–9221. [PubMed] [Google Scholar]
- Stein P. E., Leslie A. G., Finch J. T., Carrell R. W. Crystal structure of uncleaved ovalbumin at 1.95 A resolution. J Mol Biol. 1991 Oct 5;221(3):941–959. doi: 10.1016/0022-2836(91)80185-w. [DOI] [PubMed] [Google Scholar]
- Stephens A. W., Thalley B. S., Hirs C. H. Antithrombin-III Denver, a reactive site variant. J Biol Chem. 1987 Jan 25;262(3):1044–1048. [PubMed] [Google Scholar]
- Sun X. J., Chang J. Y. Evidence that arginine-129 and arginine-145 are located within the heparin binding site of human antithrombin III. Biochemistry. 1990 Sep 25;29(38):8957–8962. doi: 10.1021/bi00490a011. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Hinton J. A simple and rapid method of direct sequencing using Dynabeads. Br J Haematol. 1991 Sep;79(1):113–115. doi: 10.1111/j.1365-2141.1991.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Wong Z., Wilson V., Patel I., Povey S., Jeffreys A. J. Characterization of a panel of highly variable minisatellites cloned from human DNA. Ann Hum Genet. 1987 Oct;51(Pt 4):269–288. doi: 10.1111/j.1469-1809.1987.tb01062.x. [DOI] [PubMed] [Google Scholar]