Abstract
[Purpose] The aim of this study was to determine the effect of spatial target reaching training (TRT) based on visual biofeedback (VB) on the upper extremity (UE) function of hemiplegic subjects. [Subjects and Methods] Forty subjects between six and eighteen months post-stroke were enrolled in this study. They were randomly allocated to an experimental group (EG, n=20) and a control group (CG, n=20). All subjects received an hour of routine therapy for stroke three times a week for four weeks. Subjects in EG received additional spatial TRT based on VB using a 2-dimensional motion capture analysis system. Both groups were tested at pre and post-intervention. The motor function of each subject’s UE was assessed using the Fugl-Meyer (FM) test of UE and the Wolf Motor Function Test (WMFT). The reaching speed, angle and maximum reach distance were recorded using the motion capture analysis system. The experimental data were analyzed using the paired and independent t-tests. [Results] The mean change scores of the FM Test of UE and WMFT show there was significantly more improvement at post-intervention in EG than in CG. Also, the speed and angle reached showed significantly more increase in the EG compared with the CG. [Conclusions] The findings indicate that UE motor recovery of hemiplegic stroke patients can be enhanced through the use of TRT based on VB.
Key words: Target reaching training, Upper extremity function, Visual biofeedback
INTRODUCTION
Most patients who survive a stroke experience persistent impairment of upper extremity (UE) movement1). UE paresis post-stroke is a leading cause of serious long-term disability2). Cirstea and Levin3) suggested that abnormal post-stroke UE movements contribute to pain, joint contracture and discomfort, which may lead to limb disuse and impede long-term functional recovery. Furthermore, reduced UE function affects stroke victims’ ability to perform activities of daily living4), reduces their independence, and increases the burden of care for caregivers. Following stroke, victims manifest a complex pattern of UE motor impairments resulting in loss of functional abilities, such as reaching4). Therefore, the development and refinement of rehabilitation strategies post-stroke have the potential to improve stroke patients’ function as well as decrease the burden on caregivers and on the health care system.
Various rehabilitation approaches have been used to improve skill reacquisition of the impaired arm5). New therapeutic strategies are under investigation in an effort to improve the functional outcomes for UE, including abduction and flexion training6, 7), robotics8, 9), constraint induced movement therapy10, 11), and electrotherapeutics12,13,14,15). Among these, task-related reaching training (TRT), practice of goal-directed functional movements in a natural environment, has recently become a common rehabilitation approach that addresses these goals. TRT involves a variety of practice to help the individual develop optimal control strategies for solving motor problems16). For UE function, a case study of hemiparetic patients using a variant of TRT found improvement in clinical outcome measures. Furthermore, previous studies involving serial positron emission tomography found that TRT elicits brain plasticity in stroke patients17, 18). Thus, TRT is expected to promote the recovery of reaching in hemiparetic subjects.
Feedback movements are controlled via online sensory feedback that is used to correct ongoing movement19). Biofeedback has been applied to many aspects of stroke rehabilitation, with mixed results. This is largely due to the varying modalities, differences among study designs, and methods of measuring success and progress20). The Ottawa Panel evidence-based clinical practice guidelines for post-stroke rehabilitation recommends biofeedback for the management of several conditions post-stroke21). One benefit of using biofeedback in stroke rehabilitation is that it can give the patient and practitioner access to information about physiological functioning in various domains that might otherwise be too subtle to detect or too subjective to accurately assess and consciously manipulate through visual or auditory feedback. Especially, visual biofeedback (VB) has been adopted in various fields as a motor learning method for acquiring and enhancing motor skills20). A meta-analysis of biofeedback concluded the use of VB for the rehabilitation of UE mobility is appropriate20, 21).
Only a few investigators have used TRT combined with simultaneous VB. There is also some controversy about its functional effects on UE of hemiparetic patients1, 22). Therefore, the purpose of this study was to determine the effect of spatial TRT based on VB on UE function of hemiparetic patients. In this study we examined the effect of clinical evaluation and kinematic analysis, in order to explore the possibility of detecting whether functional improvement is accompanied by a change in motor control during the spatial TRT process based on VB. Assessments were made using the Fugl-Meyer (FM) test of UE, the Wolf Motor Function Test (WMFT), and a 2-D motion capture analysis system to measure the reaching speed (m/s), range of angular shoulder movement (deg), and maximum reach distance (mm).
SUBJECTS AND METHODS
Forty subjects with post-stroke hemiparesis were recruited from a stroke rehabilitation institute for this study. All procedures used in this study were approved by the local ethics committee of Human Research Sciences registered with the University Clinical Trials Registry. Before the experiment, subjects were provided with sufficient explanation about the study. The subjects signed a consent form before the experiment was conducted. The sample size estimate was based on data collected from previous studies23, 24). A priori power analysis determined that a sample size of 20 post-stroke subjects in each group was required to obtain a statistical power of 0.85 using the general power analysis program 3.1 (University of Kiel, Germany). This was based on an analysis using the independent t-test to compare two groups, with a predetermined coefficient of reliability of 0.9025). Lesion locations were ascertained by computed tomography or by magnetic resonance imaging. The subjects were randomly allocated, 20 subjects each, to an experimental group (EG) and a control group (CG). Inclusion criteria were as follows: ischemic or hemorrhagic post-stroke hemiparesis; discharge from rehabilitation services after first stroke 6 to 18 months earlier; a score of ≥26 in the modified mini-mental status examination Korea version (MMSE-K); Brunnstorm stage III or above for reach of the proximal and distal parts of the arm; no excessive spasticity in the more affected arm, defined as a score of ≤2 on the Modified Ashworth Spasticity scale1); and no excessive pain in the more affected UE, defined as ≤4 on a 10-point Visual Analog Scale1). Subjects were excluded if they had previous neurologic disorders, musculoskeletal abnormalities, confusion, or unilateral neglect. Unilateral neglect was tested by the Star Cancellation Test of visuospatial neglect. Those scoring less than 47 were excluded from the study23).
Motor control and functional performance of UE were evaluated using clinical evaluation and kinematic analysis before and after the four-week intervention period. Two physical and occupational therapists blinded to group allocation conducted the evaluations. Prior to administration of the clinical measures, these blinded evaluators received a 6-hour training session in the administration of the FM test of UE26) and the modified WMFT27). Rater competence was assessed by the primary investigator who has seven years experience of using these measures. The evaluators were trained to conduct the kinematic analysis in accordance with the standardized procedures described below. Subjects were asked not to indicate their treatment assignment to the evaluator.
Post-stroke UE motor impairment was assessed using the UE subsection of the FM assessment test by physical therapists26, 28). The evaluator rated the “normalcy” of 30 voluntary upper extremity movement patterns using a 3-point ordinal scale (0=unable, 1=partial performance, 2=near normal performance) and tested the excitability of 3 tendon-tap reflexes using a 2-point ordinal scale (0=no response, 2=normal or hyperexcitable). Traditionally, the assessment is scored by summing item ratings and reporting the aggregate score out of 66 points, with higher scores representing greater UE motor ability28). The occupational therapists used WMFT to assess the UE motor function. In this test, the participants are timed as they complete 15 activities that involve progressively more difficult arm movements and interactions with objects such as lifting a soda can, stacking checkers, and folding a towel. We report the average times to perform the 15 items29), and performances were rated using a 6-point functional ability scale. The summary score of functional ability is the mean of the item scores. The summary score of the performance time is the median because it is less sensitive to outliers than the mean score.
Kinematic analysis was performed using a lateral dynamic reach task23). During the task, subjects sat on a chair with the seat height adjusted to 100% of the lower leg length, measured from the lateral knee joint to the floor while the subject was standing. The trunk was secured to the chairback with a harness in order to minimize compensation movements of trunk flexion and rotation. Adjacent to the chair was a table with its height adjusted to 5 cm below the elbow. The subject rested the affected hand on a pressure sensitive hand switch located at the edge of the table and in line with the subject’s coronal plane. A beverage can, 8 cm in diameter and 12 cm in height, positioned in the patient’s coronal plane, was used as the common target in order to provide the same measurement environment. The reaching distance to the can was standardized to the subject’s arm length (from the medial border of the axilla to the distal wrist crease). If the maximum distance the subject could reach was less than the arm length, the reaching distance to the can was adjusted to the maximum reachable distance. Subjects were instructed to reach for the can at a self-paced speed. A start signal was provided to indicate the start of the task. After one practice trial, three trials were performed and averaged for the analysis. Data were collected using a digital camcorder and a 2-D motion capture analysis system (Dartfish 2-D, KOREA Inc., Seoul, Korea) which was used in conjunction with a personal computer to capture the movement of reference markers during the task and to simultaneously collect one channel of analog signals. The sampling frequency was 240 Hz. The analog signal was the hand switch used to determine the movement onset. The reference marker attached to the can was used to determine the movement end. Thus, movement recording began when the subject’s hand moved off the switch and terminated when the marker attached to the can moved. Five non-invasive, small, infrared light-emitting diode (LED) markers were attached to the ulna styloid process and lateral epicondyle of each subject’s hemiparetic arm, sternum, and both glenohumeral joints; and a scanner unit tracked the movement of the markers23, 24). The following 3 measurements were taken during the reaching task. The speed (m/s) to complete the task; the range of angular shoulder movements (deg), defined as the maximum minus the minimum angle recorded as an individual reached to the farthest point; and the maximum distance reached (mm) to complete the task. The task was repeated 3 times in succession, and the average of the 3 readings of each of the measurements was used in the data analysis23, 30).
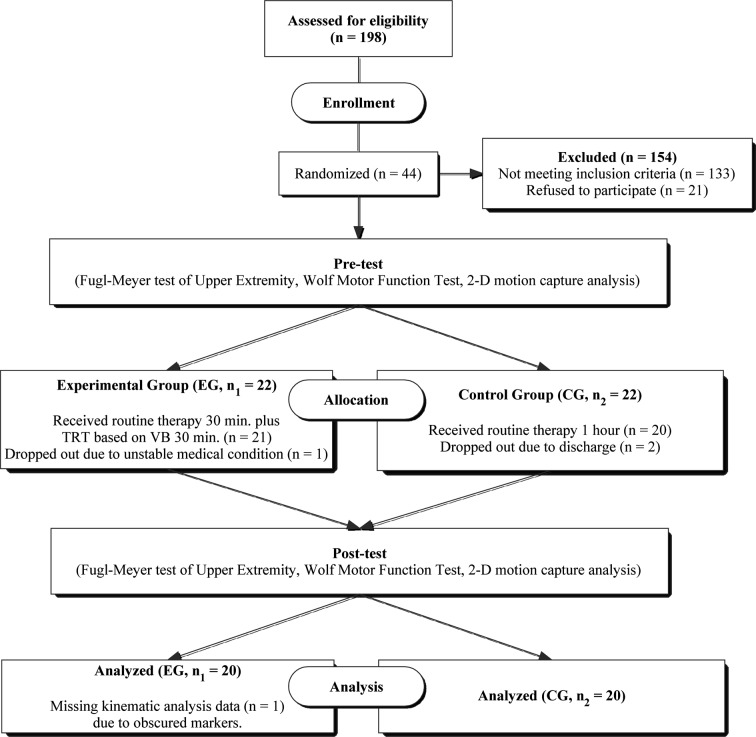
A flow diagram of the test procedure is given in Fig. 1. The subjects were randomly allocated to either EG or CG by selecting a sealed paper marked with either 1 or 2. This was a double-blind study: the subjects and therapists were not aware of their grouping. Over a 4-week period, the subjects in the CG received one hour daily of routine physical and occupational therapy, consisting of compensation techniques for activities of daily living, UE strength, and range of motion and traditional positioning. Subjects in the EG received a half daily routine of physical therapy which included half an hour of TRT based on VB using a 19-inch computer LCD screen connected to the 2-dimensional motion capture analysis system. The training took place in a clinical setting and the subjects performed 12 sessions (30 min each, 150–180 movements per session). Subjects were seated in an armless chair with their trunk restrained to prevent compensatory trunk movement23). Corrective feedback was given when compensatory movements were observed. Other tasks were used to minimize compensatory movements. For example, if excessive shoulder abduction or internal rotation occurred, reaching was intermittently carried out with the paretic arm next to a wall to prevent compensatory movement23). The subjects looked down onto the 19-inch LCD screen, which was placed on top of a wooden stand, and connected to the 2-dimensional motion capture analysis system24, 31). For the reaching task training, familiar objects were used that varied in size, shape, and weight (56–453 g) including: coffee mugs, teacups, plastic balls, books, pitchers with handles32). The subjects were required to look down at the screen during the reaching task training and were only allowed to reach in the frontal plane. Training involved only the paretic limb. The subjects were instructed to move at their preferred speed and to increase that speed as training progressed. In TRT, subjects reached to contact objects that differed in size, shape, and weight.
Fig. 1.
Schema of the study procedure. TRT: target reaching training; VB: visual biofeedback; EG: experimental group; CG: control group
Data were analyzed by using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The values of the experimental and control groups are expressed as means and standard deviations. Since the data samples of this study showed a normal distribution as determined by the Kolmogorov-Smirnov test, parametric methods were used. The independent t-test (age, weight, height, duration, T1–L5 spine and arm length, and star cancellation test) and the χ2 test (gender, side of hemiplegia, and type of stroke) were used to compare the demographic characteristics of the subjects of the two groups. For each group, the difference between the pre-test and post-test results was analyzed using the paired t-test. Comparisons of each variable between the groups were made with the independent t-test. The significance level was chosed as 0.05 for all analyses.
RESULTS
Forty subjects (16 females, 24 males) were recruited for this study, and their characteristics are presented in Table 1. There were no statistically significant differences in the demographic data of the two groups (p > 0.05).
Table 1. Demographic characteristics of the subjects (N = 40).
| Characteristics | EG (n = 20) | CG (n = 20) |
|---|---|---|
| Age (years) | 62.5 ± 9.9 | 58.5 ± 11.8 |
| Weight (kg) | 62.5 ± 5.1 | 66.7 ± 5.3 |
| Height (cm) | 162.1 ± 6.1 | 164.5 ± 6.6 |
| Time since stroke (Months) | 12.2 ± 4.9 | 18.4 ± 13.2 |
| T1–L5 Spine length (cm) | 48.3 ± 4.7 | 46.9 ± 4.8 |
| Arm length (m) | 0.53 ± 0.04 | 0.55 ± 0.03 |
| Star cancellation test (max = 54) | 50.2 ± 1.6 | 51.8 ± 0.6 |
| Gender (Male/Female)a | 13/7 | 11/9 |
| Hemiparetic side (Right/Left)a | 11/9 | 10/10 |
| Types of stroke (Hemorrhage/Infarction)a | 12/8 | 9/11 |
EG: experimental group; CG: control group; Values are mean ± SD; aValues are numbers.
The scores of the FM UE test and WMFT of the EG and CG are listed in Table 2. Significant pre- to post-test differences were found in the FM UE test and WMFT score in the EG and CG (p < 0.05), and there were significant differences in the FM UE test (p = 0.034) and WMFT scores (p = 0.032) of the two groups at post-test.
Table 2. Comparison of clinical measures and kinematic variables between the experimental and control groups.
| EG | CG | |
|---|---|---|
| Clinical measures | ||
| Fugl-Meyer Upper Extremity test (score) | ||
| Pre-test | 30.80 ± 12.50 | 31.67 ± 15.23 |
| Post-test | 44.47 ± 11.22* | 33.93 ± 14.45* |
| Wolf Motor Arm test time (score) | ||
| Pre-test | 39.60 ± 17.53 | 40.93 ± 17.91 |
| Post-test | 53.07 ± 17.25* | 42.33 ± 17.91* |
| Kinematic variables | ||
| Reaching speed (m/s) | ||
| Pre-test | 0.38 ± 0.11 | 0.41 ± 0.10 |
| Post-test | 0.55 ± 0.11* | 0.42 ± 0.10* |
| Reaching angle (˚) | ||
| Pre-test | 67.38 ± 22.96 | 68.07 ± 22.79 |
| Post-test | 86.09 ± 20.58* | 77.92 ± 19.91 |
| Max distance reached (m) | ||
| Pre-test | 0.72 ± 0.16 | 0.74 ± 0.22 |
| Post-test | 0.77 ± 0.14* | 0.77 ± 0.43* |
EG: experimental group; CG: control group; Values are mean ± SD. *Significant difference (p<0.05) between pre- and post-test.
The values of reaching speed, reaching angle, and the maximum reach distance of the EG and CG are also listed in Table 2. In the EG, significant pre- to post-test differences were found in reaching speed (p = 0.000), and reaching angle (p = 0.000), while no significant differences were found in these variables in the CG (p = 0.251 and 0.073, respectively). Significant differences in reaching speed (p = 0.002), and reaching angle (p = 0.037) were found between the two groups at post-test. For the maximum reach distance variable, significant pre- to post-test differences were found in the EG (p = 0.020) and CG (p = 0.044), but there was no significant difference in this variable between the two groups at post-test (p = 0.953).
DISCUSSION
Stroke causes UE motor deficits compromising performance of activities of daily living1, 22). The prognosis for UE recovery following stroke is poor. A systematic review33) concluded that complete motor recovery of the UE occurs in less than 15% of patients with initial paralysis. Of stroke victims, 30 to 66% continue to experience UE motor dysfunctions for > 6 months34), and the subjects of this study, who were more than six months post-stroke, had UE motor dysfunctions. One goal of rehabilitation after stroke is to enhance motor recovery of the paretic UE2, 31). However, the inability to perform tasks involving reaching is a common problem for stroke patients. Reaching is a fundamental component of daily movement that requires the coordination of multiple UE segments30). Therefore, assisting individuals’ adaptation to deficits can improve their function and social participation. On the basis of background information, we carefully applied the spatial TRT based on VB to investigate its effect on the UE function of hemiplegic subjects. Our primary findings indicate that the FM test of UE and WMFT scores showed significantly more improvement in the EG than in the CG. Also, reaching speed and angle showed significantly more improvement in the EG. These results suggest that use of TRT based on VB elicits improvement in the UE function of hemiplegic stroke patients.
We chose to study lateral reaching as a representative movement task because: reaching requires the coordinated movement of multiple UE segments, reaching is a fundamental component of many activities of daily living, and reaching has been extensively studied in adults who are healthy and in people with chronic hemiparesis to better understand UE motor control1, 2). Reaching with the impaired limb improves when familiar objects are used every day and functional goals are emphasized during a single test session35). All the subjects involved in this study used familiar objects to improve reaching with the paretic arm. The functional use of the UE is impaired after loss of movement of approximately 80% of abduction and 40% flexion36, 37).This limits effective movements and the level of activity and participation. It is therefore essential to develop ways of improving paretic arm movement to restore function and minimize disability. The strategy of incorporating muscle activation of abduction and flexion into training therapies may improve paretic arm function. Subjects in this study performed lateral reaching forward in their frontal plane. Previous studies have shown that shoulder abduction and flexion training can lead to improvement in unilateral paretic arm function.12, 27, 38)
Feed-forward movements are performed using a motor plan that is established before movement onset, and an internal model is needed to execute them accurately. On the other hand, feedback movements are used to correct ongoing movement19). There are various kinds of feedback movements: auditory, EMG, kinetic or force feedback39). Among them, visual biofeedback shows the error of movement allowing the subjects to correct abnormal movements. It can also provide support and enhance the movement of subjects with reduced UE function. The positive outcomes of research studies support the use of VB in clinical practices related to the development and recovery of active movement of the UE of stroke patients40). In our study, VB was provided by a computer LCD screen which was placed on top of a wooden stand and connected to a 2-dimensional motion capture analysis system when subjects performed the TRT. We modified the methods of previous studies23, 31). Activities such as reaching require the neural system to preplan a motor plan(s) (i.e. feedforward control) and use sensory feedback (i.e. feedback control) to monitor motor errors and modify ongoing movements19). Stroke patients demonstrate temporal inefficiency in preplanning and executing movements and rely heavily on feedback control in reaching3).
This study used clinical evaluations (FM test of UE and the WMFT) and kinematic analysis to investigate changes in functional performance and control of reaching after TRT based on VB therapy. By combining kinematic analysis and clinical evaluation it is possible to examine whether functional improvement is accompanied by a change in motor control19). Most studies have reported composite scores of standardized tests, such as the FM test or WMFT, rather than determining how the motor control or coordination of arm movements has changed41). Impairment and disability in clinical settings is generally assessed by ordinal scales such as the FM test and WMFT. Although valid clinical measures were used in these studies, kinematics may also provide a more objective, direct, and quantitative method of measuring UE motor changes. Kinematics analysis is a feasible way of measuring motor change, distance, speed, and angles of subjects’ movements. Many studies have used kinematic analysis to investigate the spatiotemporal control of UE reaching movements30, 42, 43). Over the past decade, kinematic studies of subjects with chronic hemiparesis have yielded significant information about how movement control is altered after stroke and provided insight into compensatory movement control strategies44). Compared with clinical rating scales, kinematic studies offer a sensitive, quantitative assessment of the components of abnormal motor performance. Kinematic measures also enable the assessment of spatial and temporal movement characteristics and might thereby provide insights into the spatiotemporal control of movement45). Of the many variables that can be used to quantify reaching performance using kinematic techniques, we chose to quantify the speed, angle, and maximal distance of the reach. We considered these 3 movement characteristics to be important because, presumably, once in a community setting, patients will not use their arm if the movements are not timely or accurate, or if it takes too much effort or too many attempts to perform the movement46). Descriptions of the kinematics of reaching from the acute to the subacute phase after stroke are provided elsewhere22, 41, 47).
The limitations of this study include the following considerations. The present study incorporated critical factors related to motor relearning after stroke, such as type, timing and frequency of feedback, and initial motor and cognitive impairment levels. The relatively small sample size limits generalization of the results. The lack of use of objects of different sizes to investigate the treatment efficacy of reaching might be a limitation in our study. The use of smaller objects might be a more sensitive way of differentiating possible changes after treatment. Further studies are necessary to assess the long-term effects of TRT based on visual biofeedback on the control of reaching using a wider variety of tasks that involve target objects of different sizes. Since training benefits are dependent on the initial level of impairment, further research should systematically evaluate the efficacy of TRT based on VB therapy for stroke patients with different degrees of motor impairment and differences in relevant neurological factors (e.g. neurological severity of stroke). Also, this study did not measure biomechanical parameters, such as relative joint moments and inter-joint coordination of UE. Future studies should employ direct qualitative assessments during fully supported trajectory tracking measurements of the biomechanical parameters and electromyography recordings of the UE muscles. Future trials of TRT based on VB therapy should also incorporate speed and accuracy demands into the training program using individually tailored protocols to investigate their effects on movement efficiency and smoothness.
In conclusion, it is important to establish the efficacy of treatment approaches that are appropriate for post-stroke patients who have UE impairments. This study had provided evidence of greater improvements in UE functional performance of daily activities and motor control during reaching movements after TRT based on VB versus traditional rehabilitation. Based on the findings of the clinical evaluation and kinematic analysis, the use of TRT based on VB appears more effective than traditional rehabilitation at promoting UE functional improvement. Subjects who received TRT based on VB therapy exhibited more efficiency in preplanning the movement of reaching, as indicated by clinical evaluations, and depended more on motor control during reaching, as reflected by the reaching speed and angle variables of kinematic analysis, than those who received traditional rehabilitation. Future research should investigate the benefits of incorporating various task demands into TRT based on VB therapy for task-specific training of movement performance in stroke rehabilitation.
Acknowledgments
The authors are grateful to all the subjects involved in this study as well as authors/publishers/editors of all those articles, journals and books in the literature cited in this article.
REFERENCES
- 1.Barcala L, Grecco LA, Colella F, et al. : Visual biofeedback balance training using wii fit after stroke: a randomized controlled trial. J Phys Ther Sci, 2013, 25: 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HS, Kim JU: The effect of self-directed exercise using a task board on pain and function in the upper extremities of stroke patients. J Phys Ther Sci, 2013, 25: 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirstea MC, Levin MF: Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair, 2007, 21: 398–411. [DOI] [PubMed] [Google Scholar]
- 4.Shim S, Kim H, Jung J: Comparison of upper extremity motor recovery of stroke patients with actual physical activity in their daily lives measured with accelerometers. J Phys Ther Sci, 2014, 26: 1009–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirstea CM, Ptito A, Levin MF: Feedback and cognition in arm motor skill reacquisition after stroke. Stroke, 2006, 37: 1237–1242. [DOI] [PubMed] [Google Scholar]
- 6.McCombe Waller S, Harris-Love M, Liu W, et al. : Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res, 2006, 168: 450–454. [DOI] [PubMed] [Google Scholar]
- 7.Whitall J, McCombe Waller S, Silver KH, et al. : Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke, 2000, 31: 2390–2395. [DOI] [PubMed] [Google Scholar]
- 8.Hesse S, Schmidt H, Werner C, et al. : Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol, 2003, 16: 705–710. [DOI] [PubMed] [Google Scholar]
- 9.Masiero S, Carraro E, Celia A, et al. : Robotic therapy: a novel approach in upper-limb neurorehabilitation after stroke. Neurol Sci, 2007, 28: 294. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SL, Thompson PA, Morris DM, et al. : The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair, 2005, 19: 194–205. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Winstein CJ, Miller JP, et al. EXCITE Investigators: Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA, 2006, 296: 2095–2104. [DOI] [PubMed] [Google Scholar]
- 12.Cauraugh JH, Kim S: Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke, 2002, 33: 1589–1594. [DOI] [PubMed] [Google Scholar]
- 13.Cauraugh JH, Kim SB: Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry, 2003, 74: 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose DK, Winstein CJ: Bimanual training after stroke: are two hands better than one? Top Stroke Rehabil, 2004, 11: 20–30. [DOI] [PubMed] [Google Scholar]
- 15.Rose DK, Winstein CJ: The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil, 2005, 19: 452–462. [DOI] [PubMed] [Google Scholar]
- 16.Gentile AM: Skill acquisition: action, movement, and neuromotor processes. In: Carr JH, Shepherd RB, eds. Movement Sciences: Foundation for Physical Therapy in Rehabilitation, 2nd ed. Gaithesburg, 2000, pp 111–187. [Google Scholar]
- 17.Duncan PW, Lai SM, Keighley J: Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology, 2000, 39: 835–841. [DOI] [PubMed] [Google Scholar]
- 18.Spieler JF, Amarenco P: [Socio-economic aspects of stroke management]. Rev Neurol (Paris), 2004, 160: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 19.Desmurget M, Grafton S: Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci, 2000, 4: 423–431. [DOI] [PubMed] [Google Scholar]
- 20.Nelson LA: The role of biofeedback in stroke rehabilitation: past and future directions. Top Stroke Rehabil, 2007, 14: 59–66. [DOI] [PubMed] [Google Scholar]
- 21.Khadilkar A, Phillips K, Jean N, et al. Ottawa Panel: Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil, 2006, 13: 1–269. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Lee Y, Sohng KY: Effects of bilateral passive range of motion exercise on the function of upper extremities and activities of daily living in patients with acute stroke. J Phys Ther Sci, 2014, 26: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell FM, Ashburn AM, Pickering RM, et al. : Head and pelvic movements during a dynamic reaching task in sitting: implications for physical therapists. Arch Phys Med Rehabil, 2001, 82: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 24.Thielman G, Kaminski T, Gentile AM: Rehabilitation of reaching after stroke: comparing 2 training protocols utilizing trunk restraint. Neurorehabil Neural Repair, 2008, 22: 697–705. [DOI] [PubMed] [Google Scholar]
- 25.Faul F, Erdfelder E, Lang AG, et al. : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods, 2007, 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 26.Coderre AM, Zeid AA, Dukelow SP, et al. : Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair, 2010, 24: 528–541. [DOI] [PubMed] [Google Scholar]
- 27.Whitall J, Savin DN, Jr, Harris-Love M, et al. : Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper-extremity hemiparesis. Arch Phys Med Rehabil, 2006, 87: 656–660. [DOI] [PubMed] [Google Scholar]
- 28.Fugl-Meyer AR, Jääskö L, Leyman I, et al. : The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med, 1975, 7: 13–31. [PubMed] [Google Scholar]
- 29.Wolf SL, Catlin PA, Ellis M, et al. : Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke, 2001, 32: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 30.Lin KC, Wu CY, Wei TH, et al. : Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study. Clin Rehabil, 2007, 21: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Kudo A, Sugihara S, et al. : A study of upper extremity training for patients with stroke using a virtual environment system. J Phys Ther Sci, 2013, 25: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheidt RA, Conditt MA, Secco EL, et al. : Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol, 2005, 93: 3200–3213. [DOI] [PubMed] [Google Scholar]
- 33.Hendricks HT, van Limbeek J, Geurts AC, et al. : Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil, 2002, 83: 1629–1637. [DOI] [PubMed] [Google Scholar]
- 34.Richards L, Pohl P: Therapeutic interventions to improve upper extremity recovery and function. Clin Geriatr Med, 1999, 15: 819–832. [PubMed] [Google Scholar]
- 35.Wu SW, Trommershäuser J, Maloney LT, et al. : Limits to human movement planning in tasks with asymmetric gain landscapes. J Vis, 2006, 6: 53–63. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski TR, Bock C, Gentile AM: The coordination between trunk and arm motion during pointing movements. Exp Brain Res, 1995, 106: 457–466. [DOI] [PubMed] [Google Scholar]
- 37.Nozaki D, Kurtzer I, Scott SH: Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci, 2006, 9: 1364–1366. [DOI] [PubMed] [Google Scholar]
- 38.Mudie MH, Matyas TA: Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil, 2000, 22: 23–37. [DOI] [PubMed] [Google Scholar]
- 39.McCombe PA, Read SJ: Immune and inflammatory responses to stroke: good or bad? Int J Stroke, 2008, 3: 254–265. [DOI] [PubMed] [Google Scholar]
- 40.Shumway-Cook A, Woollacott MH: Motor Control: Translating Research into Clinical Practice, 3nd ed. Philadelphia: Lippincott Williams & Wilkins, 2007, pp 518–545. [Google Scholar]
- 41.McCombe Waller S, Liu W, Whitall J: Temporal and spatial control following bilateral versus unilateral training. Hum Mov Sci, 2008, 27: 749–758. [DOI] [PubMed] [Google Scholar]
- 42.Hakim RM, Kelly SJ, Grant-Beuttler M, et al. : Case report: a modified constraint-induced therapy (mCIT) program for the upper extremity of a person with chronic stroke. Physiother Theory Pract, 2005, 21: 243–256. [DOI] [PubMed] [Google Scholar]
- 43.Huang YY, Wu CY, Hong WH, et al. : A kinematic study of modified constraint-induced movement therapy in patients with stroke. Formos J Med, 2006, 10: 319–327. [Google Scholar]
- 44.Cirstea MC, Levin MF: Compensatory strategies for reaching in stroke. Brain, 2000, 123: 940–953. [DOI] [PubMed] [Google Scholar]
- 45.Platz T, Bock S, Prass K: Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia, 2001, 39: 687–698. [DOI] [PubMed] [Google Scholar]
- 46.Reinkensmeyer DJ, Pang CT, Nessler JA, et al. : Web-based telerehabilitation for the upper extremity after stroke. IEEE Trans Neural Syst Rehabil Eng, 2002, 10: 102–108. [DOI] [PubMed] [Google Scholar]
- 47.Ward RP, Don CW, Furlong KT, et al. : Predictors of long-term mortality in patients with ischemic stroke referred for transesophageal echocardiography. Stroke, 2006, 37: 204–208. [DOI] [PubMed] [Google Scholar]