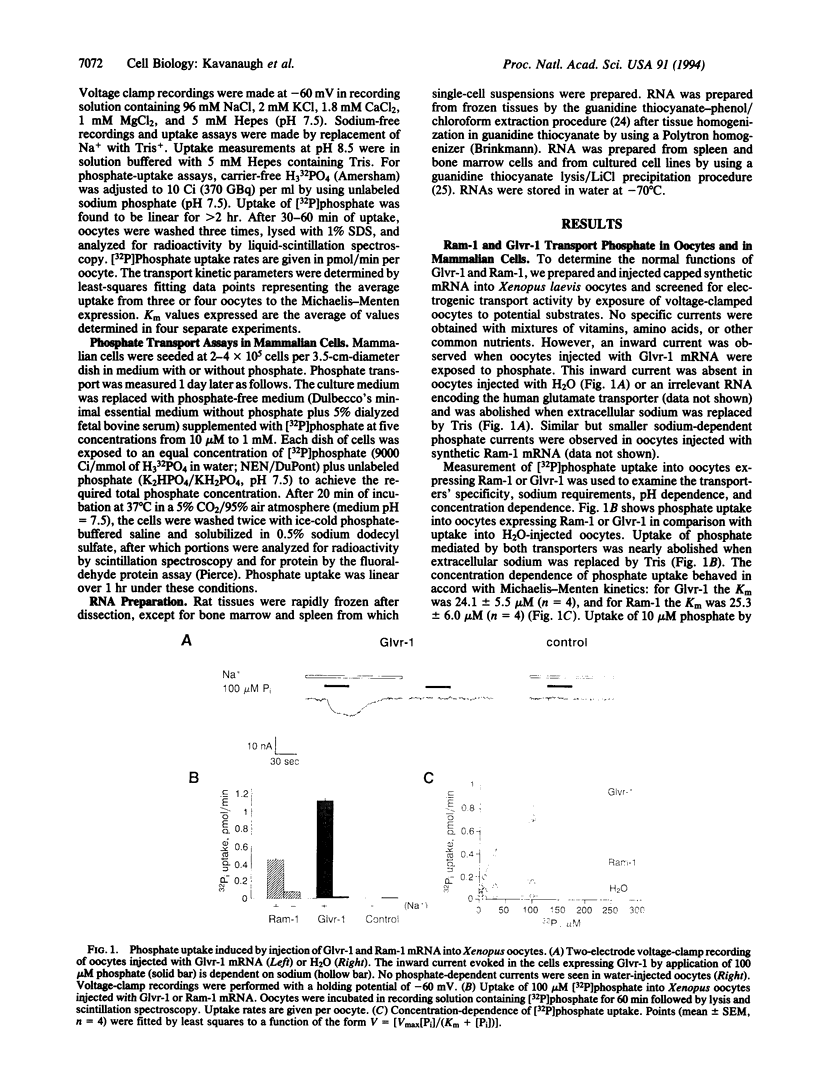
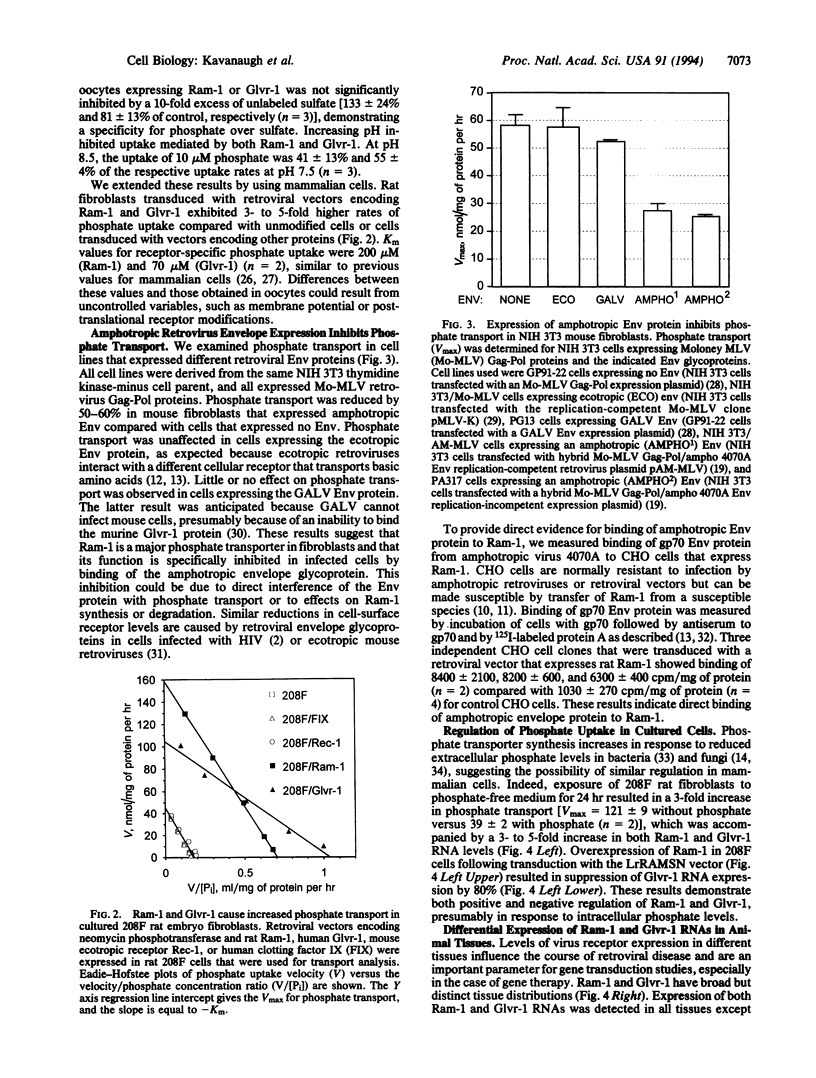
Abstract
Cell surface receptors for gibbon ape leukemia virus (Glvr-1) and murine amphotropic retrovirus (Ram-1) are distinct but related proteins having multiple membrane-spanning regions. Distant homology with a putative phosphate permease of Neurospora crassa suggested that these receptors might serve transport functions. By expression in Xenopus laevis oocytes and in mammalian cells, we have identified Glvr-1 and Ram-1 as sodium-dependent phosphate symporters. Two-electrode voltage-clamp analysis indicates net cation influx, suggesting that phosphate is transported with excess sodium ions. Phosphate uptake was reduced by > 50% in mouse fibroblasts expressing amphotropic envelope glycoprotein, which binds to Ram-1, indicating that Ram-1 is a major phosphate transporter in these cells. RNA analysis shows wide but distinct tissue distributions, with Glvr-1 expression being highest in bone marrow and Ram-1 in heart. Overexpression of Ram-1 severely repressed Glvr-1 synthesis in fibroblasts, suggesting that transporter expression may be controlled by net phosphate accumulation. Accordingly, depletion of extracellular phosphate increased Ram-1 and Glvr-1 expression 3- to 5-fold. These results suggest simple methods to modulate retroviral receptor expression, with possible applications to human gene therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Kavanaugh M. P., Fairman W. A., Wu Y. N., Murdoch G. H., North R. A., Amara S. G. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993 Jul 25;268(21):15329–15332. [PubMed] [Google Scholar]
- Ban J., Portetelle D., Altaner C., Horion B., Milan D., Krchnak V., Burny A., Kettmann R. Isolation and characterization of a 2.3-kilobase-pair cDNA fragment encoding the binding domain of the bovine leukemia virus cell receptor. J Virol. 1993 Feb;67(2):1050–1057. doi: 10.1128/jvi.67.2.1050-1057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P., Young J. A., Varmus H. E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993 Sep 24;74(6):1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991 Jun;11(6):3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Miller A. D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991 Apr 11;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Johann S. V., Gibbons J. J., O'Hara B. GLVR1, a receptor for gibbon ape leukemia virus, is homologous to a phosphate permease of Neurospora crassa and is expressed at high levels in the brain and thymus. J Virol. 1992 Mar;66(3):1635–1640. doi: 10.1128/jvi.66.3.1635-1640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann S. V., van Zeijl M., Cekleniak J., O'Hara B. Definition of a domain of GLVR1 which is necessary for infection by gibbon ape leukemia virus and which is highly polymorphic between species. J Virol. 1993 Nov;67(11):6733–6736. doi: 10.1128/jvi.67.11.6733-6736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh M. P., Arriza J. L., North R. A., Amara S. G. Electrogenic uptake of gamma-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem. 1992 Nov 5;267(31):22007–22009. [PubMed] [Google Scholar]
- Kawakami T. G., Kollias G. V., Jr, Holmberg C. Oncogenicity of gibbon type-C myelogenous leukemia virus. Int J Cancer. 1980 May 15;25(5):641–646. doi: 10.1002/ijc.2910250514. [DOI] [PubMed] [Google Scholar]
- Kawamura I., Koga Y., Oh-Hori N., Onodera K., Kimura G., Nomoto K. Depletion of the surface CD4 molecule by the envelope protein of human immunodeficiency virus expressed in a human CD4+ monocytoid cell line. J Virol. 1989 Sep;63(9):3748–3754. doi: 10.1128/jvi.63.9.3748-3754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kong C. T., Yet S. F., Lever J. E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J Biol Chem. 1993 Jan 25;268(3):1509–1512. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Magagnin S., Werner A., Markovich D., Sorribas V., Stange G., Biber J., Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B. J., Bowman B. J., Grotelueschen J., Metzenberg R. L. Nucleotide sequence of pho-4+, encoding a phosphate-repressible phosphate permease of Neurospora crassa. Gene. 1989 Nov 30;83(2):281–289. doi: 10.1016/0378-1119(89)90114-5. [DOI] [PubMed] [Google Scholar]
- Medina G., Illingworth J. Some factors affecting phosphate transport in a perfused rat heart preparation. Biochem J. 1980 May 15;188(2):297–211. doi: 10.1042/bj1880297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Edwards R. H., Miller A. D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Thompson A. R., Miller A. D. Production of human factor IX in animals by genetically modified skin fibroblasts: potential therapy for hemophilia B. Blood. 1989 Feb;73(2):438–445. [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Quamme G., Biber J., Murer H. Sodium-phosphate cotransport in OK cells: inhibition by PTH and "adaptation" to low phosphate. Am J Physiol. 1989 Dec;257(6 Pt 2):F967–F973. doi: 10.1152/ajprenal.1989.257.6.F967. [DOI] [PubMed] [Google Scholar]
- Robey E., Axel R. CD4: collaborator in immune recognition and HIV infection. Cell. 1990 Mar 9;60(5):697–700. doi: 10.1016/0092-8674(90)90082-p. [DOI] [PubMed] [Google Scholar]
- Sheets R. L., Pandey R., Jen W. C., Roy-Burman P. Recombinant feline leukemia virus genes detected in naturally occurring feline lymphosarcomas. J Virol. 1993 Jun;67(6):3118–3125. doi: 10.1128/jvi.67.6.3118-3125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorribas V., Markovich D., Hayes G., Stange G., Forgo J., Biber J., Murer H. Cloning of a Na/Pi cotransporter from opossum kidney cells. J Biol Chem. 1994 Mar 4;269(9):6615–6621. [PubMed] [Google Scholar]
- Takeuchi Y., Simpson G., Vile R. G., Weiss R. A., Collins M. K. Retroviral pseudotypes produced by rescue of a Moloney murine leukemia virus vector by C-type, but not D-type, retroviruses. Virology. 1992 Feb;186(2):792–794. doi: 10.1016/0042-6822(92)90049-u. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lieber M. M., Benveniste R. E., Sherr C. J. Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology. 1975 Oct;67(2):335–343. doi: 10.1016/0042-6822(75)90435-3. [DOI] [PubMed] [Google Scholar]
- Torriani A. From cell membrane to nucleotides: the phosphate regulon in Escherichia coli. Bioessays. 1990 Aug;12(8):371–376. doi: 10.1002/bies.950120804. [DOI] [PubMed] [Google Scholar]
- Tzavaras T., Stewart M., McDougall A., Fulton R., Testa N., Onions D. E., Neil J. C. Molecular cloning and characterization of a defective recombinant feline leukaemia virus associated with myeloid leukaemia. J Gen Virol. 1990 Feb;71(Pt 2):343–354. doi: 10.1099/0022-1317-71-2-343. [DOI] [PubMed] [Google Scholar]
- Van Beusechem V. W., Bakx T. A., Kaptein L. C., Bart-Baumeister J. A., Kukler A., Braakman E., Valerio D. Retrovirus-mediated gene transfer into rhesus monkey hematopoietic stem cells: the effect of viral titers on transduction efficiency. Hum Gene Ther. 1993 Jun;4(3):239–247. doi: 10.1089/hum.1993.4.3-239. [DOI] [PubMed] [Google Scholar]
- Wang H., Dechant E., Kavanaugh M., North R. A., Kabat D. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J Biol Chem. 1992 Nov 25;267(33):23617–23624. [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Wang H., Paul R., Burgeson R. E., Keene D. R., Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991 Dec;65(12):6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle J. P., Pedersen P. L. Phosphate transport processes in eukaryotic cells. J Membr Biol. 1989 Nov;111(3):199–213. doi: 10.1007/BF01871006. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Gibson M., Spear P. G., Scolnick E. M. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J Virol. 1981 Sep;39(3):935–944. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Maldarelli F., Martin M. A., Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992 Dec;66(12):7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M., Johann S. V., Closs E., Cunningham J., Eddy R., Shows T. B., O'Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]