Abstract
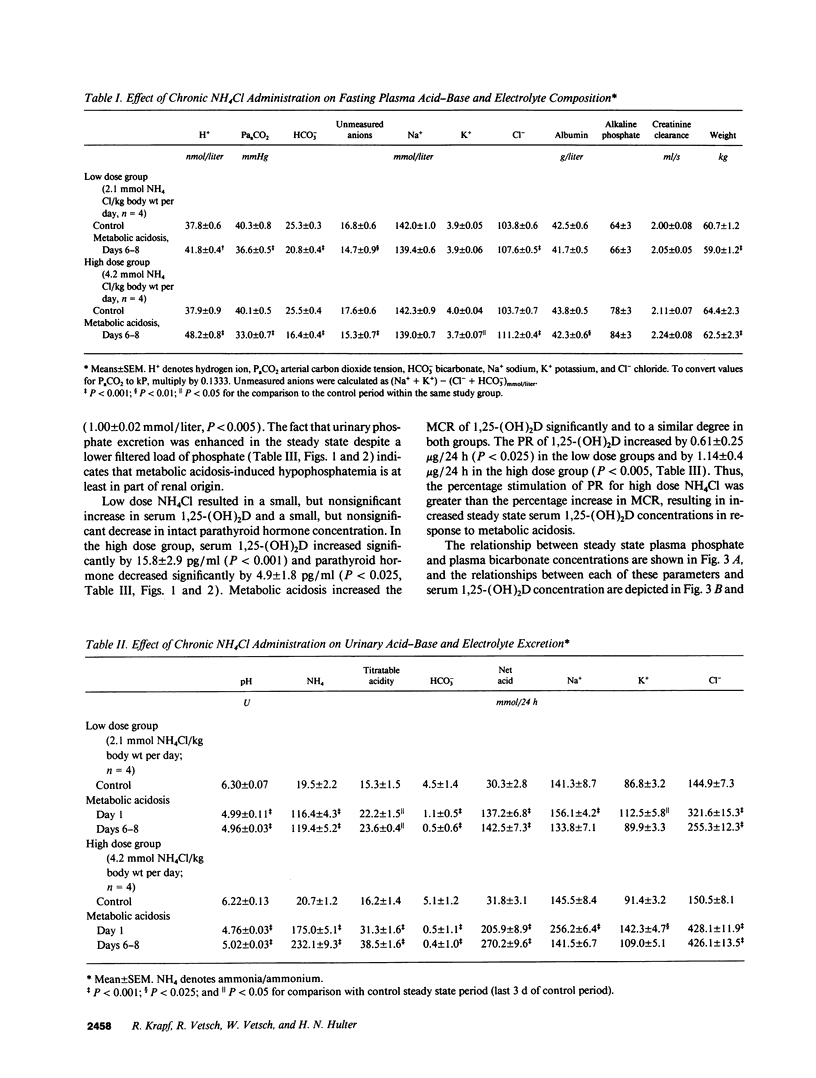
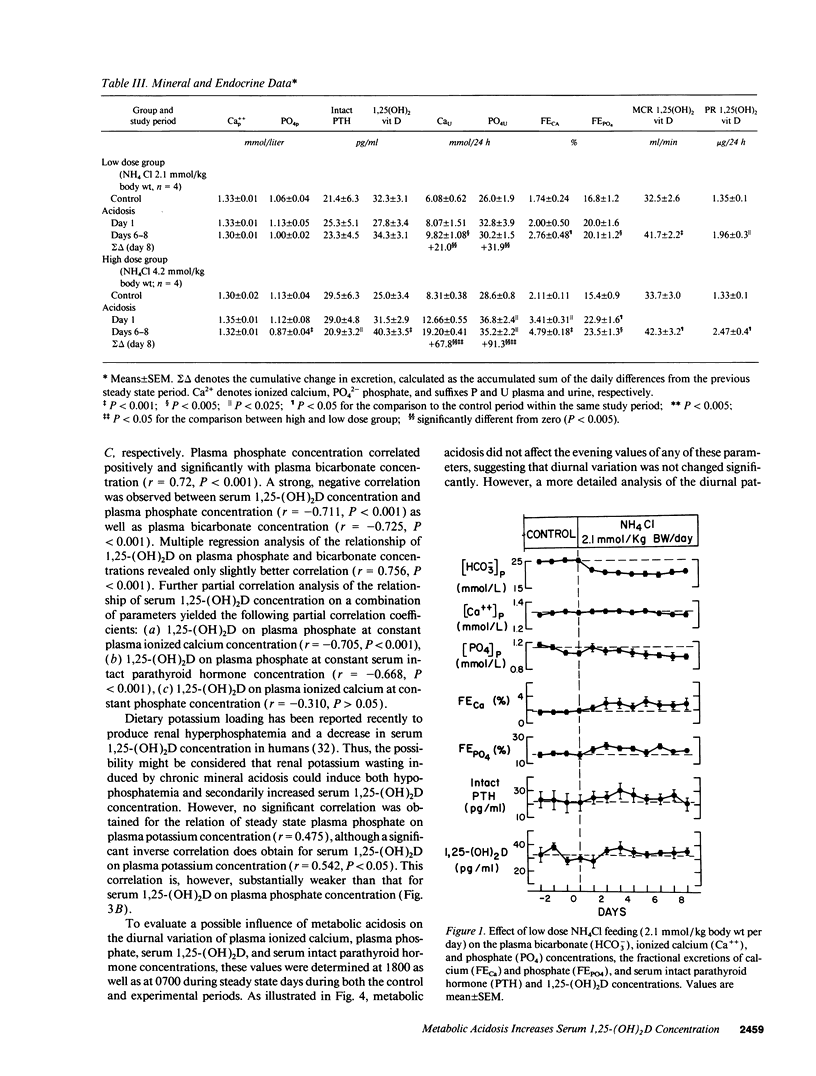
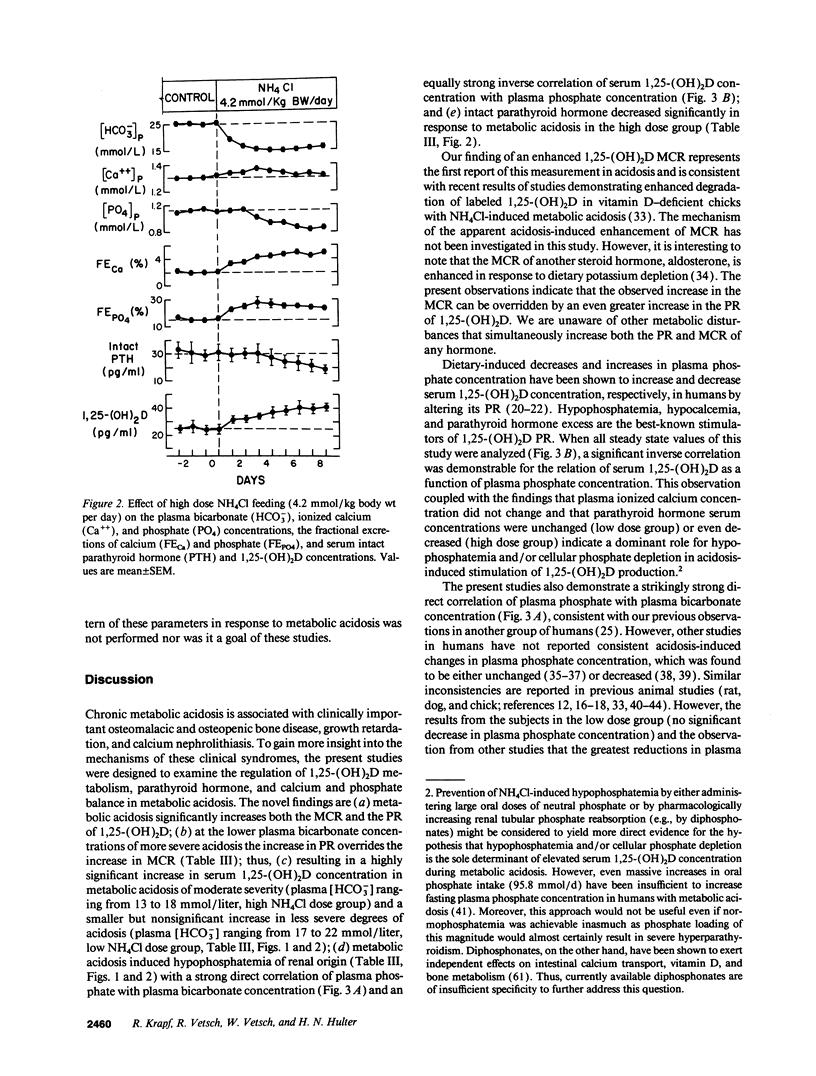
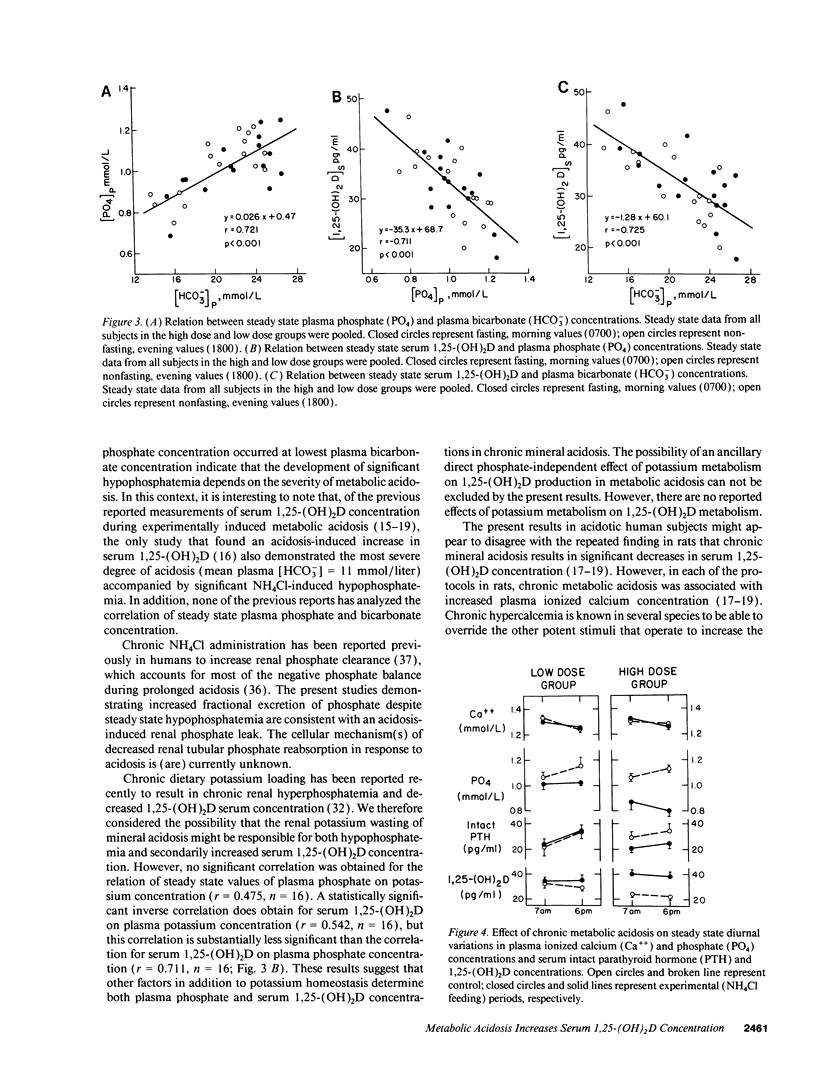
Chronic metabolic acidosis results in metabolic bone disease, calcium nephrolithiasis, and growth retardation. The pathogenesis of each of these sequelae is poorly understood in humans. We therefore investigated the effects of chronic extrarenal metabolic acidosis on the regulation of 1,25-(OH)2D, parathyroid hormone, calcium, and phosphate metabolism in normal humans. Chronic extrarenal metabolic acidosis was induced by administering two different doses of NH4Cl [2.1 (low dose) and 4.2 (high dose) mmol/kg body wt per d, respectively] to four male volunteers each during metabolic balance conditions. Plasma [HCO3-] decreased by 4.5 +/- 0.4 mmol/liter in the low dose and by 9.1 +/- 0.3 mmol/liter (P < 0.001) in the high dose group. Metabolic acidosis induced renal hypophosphatemia, which strongly correlated with the severity of acidosis (Plasma [PO4] on plasma [HCO3-]; r = 0.721, P < 0.001). Both metabolic clearance and production rates of 1,25-(OH)2D increased in both groups. In the high dose group, the percentage increase in production rate was much greater than the percentage increase in metabolic clearance rate, resulting in a significantly increased serum 1,25-(OH)2D concentration. A strong inverse correlation was observed for serum 1,25-(OH)2D concentration on both plasma [PO4] (r = -0.711, P < 0.001) and plasma [HCO3-] (r = -0.725, P < 0.001). Plasma ionized calcium concentration did not change in either group whereas intact serum parathyroid hormone concentration decreased significantly in the high dose group. In conclusion, metabolic acidosis results in graded increases in serum 1,25-(OH)2D concentration by stimulating its production rate in humans. The increased production rate is explained by acidosis-induced hypophosphatemia/cellular phosphate depletion resulting at least in part from decreased renal tubular phosphate reabsorption. The decreased serum intact parathyroid hormone levels in more severe acidosis may be the consequence of hypophosphatemia and/or increased serum 1,25-(OH)2D concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarskog D., Aksnes L. Acute response of plasma 1,25-dihydroxyvitamin D to parathyroid hormone. Lancet. 1980 Feb 16;1(8164):362–363. doi: 10.1016/s0140-6736(80)90904-6. [DOI] [PubMed] [Google Scholar]
- Adams N. D., Gray R. W., Lemann J., Jr The calciuria of increased fixed acid production in humans: evidence against a role for parathyroid hormone and 1,25(OH)2-vitamin D. Calcif Tissue Int. 1979 Nov 6;28(3):233–238. doi: 10.1007/BF02441241. [DOI] [PubMed] [Google Scholar]
- Baran D. T., Lee S. W., Jo O. D., Avioli L. V. Acquired alterations in vitamin D metabolism in the acidotic state. Calcif Tissue Int. 1982 Mar;34(2):165–168. doi: 10.1007/BF02411228. [DOI] [PubMed] [Google Scholar]
- Baylink D., Wergedal J., Stauffer M. Formation, mineralization, and resorption of bone in hypophosphatemic rats. J Clin Invest. 1971 Dec;50(12):2519–2530. doi: 10.1172/JCI106752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck N. Effect of metabolic acidosis on renal response to parathyroid hormone in phosphorus-deprived rats. Am J Physiol. 1981 Jul;241(1):F23–F27. doi: 10.1152/ajprenal.1981.241.1.F23. [DOI] [PubMed] [Google Scholar]
- Bellorin-Font E., Humpierres J., Weisinger J. R., Milanes C. L., Sylva V., Paz-Martinez V. Effect of metabolic acidosis on the PTH receptor-adenylate cyclase system of canine kidney. Am J Physiol. 1985 Oct;249(4 Pt 2):F566–F572. doi: 10.1152/ajprenal.1985.249.4.F566. [DOI] [PubMed] [Google Scholar]
- Bichara M., Mercier O., Borensztein P., Paillard M. Acute metabolic acidosis enhances circulating parathyroid hormone, which contributes to the renal response against acidosis in the rat. J Clin Invest. 1990 Aug;86(2):430–443. doi: 10.1172/JCI114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Metabolic acidosis in the vitamin D-deficient chick. Metabolism. 1977 Oct;26(10):1099–1105. doi: 10.1016/0026-0495(77)90036-1. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Favus M. J., Schneider A. B., Sen P. K., Sherwood L. M., Coe F. L. Effects of metabolic acidosis on PTH and 1,25(OH)2D3 response to low calcium diet. Am J Physiol. 1982 Dec;243(6):F570–F575. doi: 10.1152/ajprenal.1982.243.6.F570. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Nalbantian-Brandt C., Favus M. J. Elevated Ca2+ does not inhibit the 1,25(OH)2D3 response to phosphorus restriction. Am J Physiol. 1989 Feb;256(2 Pt 2):F285–F289. doi: 10.1152/ajprenal.1989.256.2.F285. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A. Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol. 1989 May;256(5 Pt 2):F836–F842. doi: 10.1152/ajprenal.1989.256.5.F836. [DOI] [PubMed] [Google Scholar]
- Bushinsky D. A., Riera G. S., Favus M. J., Coe F. L. Evidence that blood ionized calcium can regulate serum 1,25(OH)2D3 independently of parathyroid hormone and phosphorus in the rat. J Clin Invest. 1985 Oct;76(4):1599–1604. doi: 10.1172/JCI112143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushinsky D. A., Riera G. S., Favus M. J., Coe F. L. Response of serum 1,25(OH)2D3 to variation of ionized calcium during chronic acidosis. Am J Physiol. 1985 Sep;249(3 Pt 2):F361–F365. doi: 10.1152/ajprenal.1985.249.3.F361. [DOI] [PubMed] [Google Scholar]
- Caruana R. J., Buckalew V. M., Jr The syndrome of distal (type 1) renal tubular acidosis. Clinical and laboratory findings in 58 cases. Medicine (Baltimore) 1988 Mar;67(2):84–99. doi: 10.1097/00005792-198803000-00002. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Savdie E., Mason R. S., Posen S. The effect of metabolic acidosis on vitamin D metabolites and bone histology in uremic rats. Calcif Tissue Int. 1985 Mar;37(2):158–164. doi: 10.1007/BF02554835. [DOI] [PubMed] [Google Scholar]
- Coe F. L., Firpo J. J., Jr, Hollandsworth D. L., Segil L., Canterbury J. M., Reiss E. Effect of acute and chronic metabolic acidosis on serum immunoreactive parathyroid hormone in man. Kidney Int. 1975 Oct;8(4):263–273. doi: 10.1038/ki.1975.110. [DOI] [PubMed] [Google Scholar]
- Cunningham J., Fraher L. J., Clemens T. L., Revell P. A., Papapoulos S. E. Chronic acidosis with metabolic bone disease. Effect of alkali on bone morphology and vitamin D metabolism. Am J Med. 1982 Aug;73(2):199–204. doi: 10.1016/0002-9343(82)90179-6. [DOI] [PubMed] [Google Scholar]
- DePalo D., Theisen A. L., Langman C. B., Bouillon R., Bourdeau J. E. Renal responses to phosphorus deprivation in young rabbits. Miner Electrolyte Metab. 1988;14(6):313–320. [PubMed] [Google Scholar]
- Dominguez J. H., Gray R. W., Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab. 1976 Nov;43(5):1056–1068. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- Eastell R., Riggs B. L., Kumar R. A primed-infusion technique for rapid estimation of the metabolic clearance rate of 1,25(OH)2D3. Am J Physiol. 1987 Sep;253(3 Pt 1):E246–E250. doi: 10.1152/ajpendo.1987.253.3.E246. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Dempsey J. A., Thomson J., Vidruk E., DoPico G. A. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972 Jan;32(1):134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- Frame B., Parfitt A. M. Osteomalacia: current concepts. Ann Intern Med. 1978 Dec;89(6):966–982. doi: 10.7326/0003-4819-89-6-966. [DOI] [PubMed] [Google Scholar]
- Gafter U., Kraut J. A., Lee D. B., Silis V., Walling M. W., Kurokawa K., Haussler M. R., Coburn J. W. Effect of metabolic acidosis in intestinal absorption of calcium and phosphorus. Am J Physiol. 1980 Dec;239(6):G480–G484. doi: 10.1152/ajpgi.1980.239.6.G480. [DOI] [PubMed] [Google Scholar]
- Hollis B. W. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem. 1986 Nov;32(11):2060–2063. [PubMed] [Google Scholar]
- Hulter H. N. Effects and interrelationships of PTH, Ca2+, vitamin D, and Pi in acid-base homeostasis. Am J Physiol. 1985 Jun;248(6 Pt 2):F739–F752. doi: 10.1152/ajprenal.1985.248.6.F739. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Halloran B. P., Toto R. D., Peterson J. C. Long-term control of plasma calcitriol concentration in dogs and humans. Dominant role of plasma calcium concentration in experimental hyperparathyroidism. J Clin Invest. 1985 Aug;76(2):695–702. doi: 10.1172/JCI112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulter H. N., Sebastian A., Sigala J. F., Licht J. H., Glynn R. D., Schambelan M., Biglieri E. G. Pathogenesis of renal hyperchloremic acidosis resulting from dietary potassium restriction in the dog: role of aldosterone. Am J Physiol. 1980 Feb;238(2):F79–F91. doi: 10.1152/ajprenal.1980.238.2.F79. [DOI] [PubMed] [Google Scholar]
- Hulter H. N., Sebastian A., Toto R. D., Bonner E. L., Jr, Ilnicki L. P. Renal and systemic acid-base effects of the chronic administration of hypercalcemia-producing agents: calcitriol, PTH, and intravenous calcium. Kidney Int. 1982 Mar;21(3):445–458. doi: 10.1038/ki.1982.45. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Kraut J. A., Kurokawa K. Metabolic acidosis suppresses 25-hydroxyvitamin in D3-1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J Clin Invest. 1982 Jul;70(1):135–140. doi: 10.1172/JCI110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf R., Beeler I., Hertner D., Hulter H. N. Chronic respiratory alkalosis. The effect of sustained hyperventilation on renal regulation of acid-base equilibrium. N Engl J Med. 1991 May 16;324(20):1394–1401. doi: 10.1056/NEJM199105163242003. [DOI] [PubMed] [Google Scholar]
- Kraut J. A., Gordon E. M., Ransom J. C., Horst R., Slatopolsky E., Coburn J. W., Kurokawa K. Effect of chronic metabolic acidosis on vitamin D metabolism in humans. Kidney Int. 1983 Nov;24(5):644–648. doi: 10.1038/ki.1983.206. [DOI] [PubMed] [Google Scholar]
- Kraut J. A., Mishler D. R., Singer F. R., Goodman W. G. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986 Nov;30(5):694–700. doi: 10.1038/ki.1986.242. [DOI] [PubMed] [Google Scholar]
- Langman C. B. Calcitriol metabolism during chronic metabolic acidosis. Semin Nephrol. 1989 Mar;9(1):65–71. [PubMed] [Google Scholar]
- Lau K., Rodriguez Nichols F., Tannen R. L. Renal excretion of divalent ions in response to chronic acidosis: evidence that systemic pH is not the controlling variable. J Lab Clin Med. 1987 Jan;109(1):27–33. [PubMed] [Google Scholar]
- Lee S. W., Russell J., Avioli L. V. 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: conversion impaired by systemic metabolic acidosis. Science. 1977 Mar 11;195(4282):994–996. doi: 10.1126/science.841324. [DOI] [PubMed] [Google Scholar]
- Lemann J., Jr, Litzow J. R., Lennon E. J. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966 Oct;45(10):1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemann J., Litzow J. R., Lennon E. J. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967 Aug;46(8):1318–1328. doi: 10.1172/JCI105624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautalen C., Montoreano R., Labarrere C. Early skeletal effect of alkali therapy upon the osteomalacia of renal tubular acidosis. J Clin Endocrinol Metab. 1976 May;42(5):875–881. doi: 10.1210/jcem-42-5-875. [DOI] [PubMed] [Google Scholar]
- McSherry E., Morris R. C., Jr Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest. 1978 Feb;61(2):509–527. doi: 10.1172/JCI108962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum S. R., Zahradnik R. J., Lavigne J. R., Brennan G. L., Nozawa-Ung K., Kim L. Y., Keutmann H. T., Wang C. A., Potts J. T., Jr, Segre G. V. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987 Aug;33(8):1364–1367. [PubMed] [Google Scholar]
- PINES K. L., MUDGE G. H. Renal tubular acidosis with osteomalacia; report of 3 cases. Am J Med. 1951 Sep;11(3):302–311. doi: 10.1016/0002-9343(51)90167-2. [DOI] [PubMed] [Google Scholar]
- Perry W., Allen L. N., Stamp T. C., Walker P. G. Vitamin D resistance in osteomalacia after ureterosigmoidostomy. N Engl J Med. 1977 Nov 17;297(20):1110–1112. doi: 10.1056/NEJM197711172972008. [DOI] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Morris R. C., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987 Oct;80(4):1147–1154. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Morris R. C., Jr Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989 May;83(5):1494–1499. doi: 10.1172/JCI114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Murphy M. M., Morris R. C., Jr Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986 Jan;77(1):7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Holick M. F., DeLuca H. F. 1,25-dihydroxycholecalciferol: a potent stimulator of bone resorption in tissue culture. Science. 1972 Feb 18;175(4023):768–769. doi: 10.1126/science.175.4023.768. [DOI] [PubMed] [Google Scholar]
- Reddy G. S., Jones G., Kooh S. W., Fraser D. Inhibition of 25-hydroxyvitamin D3-1-hydroxylase by chronic metabolic acidosis. Am J Physiol. 1982 Oct;243(4):E265–E271. doi: 10.1152/ajpendo.1982.243.4.E265. [DOI] [PubMed] [Google Scholar]
- Richards P., Chamberlain M. J., Wrong O. M. Treatment of osteomalacia of renal tubular acidosis by sodium bicarbonate alone. Lancet. 1972 Nov 11;2(7785):994–997. doi: 10.1016/s0140-6736(72)92405-1. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A., Hernandez R. E., Portale A. A., Colman J., Tatsuno J., Morris R. C., Jr Dietary potassium influences kidney maintenance of serum phosphorus concentration. Kidney Int. 1990 May;37(5):1341–1349. doi: 10.1038/ki.1990.120. [DOI] [PubMed] [Google Scholar]
- Silver J., Naveh-Many T., Mayer H., Schmelzer H. J., Popovtzer M. M. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986 Nov;78(5):1296–1301. doi: 10.1172/JCI112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT J. F., LITTLE B., TAIT S. A., FLOOD C. The metabolic clearance rate of aldosterone in pregnant and nonpregnant subjects estimated by both single-injection and constant-infusion methods. J Clin Invest. 1962 Dec;41:2093–2100. doi: 10.1172/JCI104667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. Bone mineral mobilization activity of 1,25-dihydroxycholecalciferol, a metabolite of vitamin D. Arch Biochem Biophys. 1971 Oct;146(2):574–578. doi: 10.1016/0003-9861(71)90163-9. [DOI] [PubMed] [Google Scholar]
- Weber H. P., Gray R. W., Dominguez J. H., Lemann J., Jr The lack of effect of chronic metabolic acidosis on 25-OH-vitamin D metabolism and serum parathyroid hormone in humans. J Clin Endocrinol Metab. 1976 Nov;43(5):1047–1055. doi: 10.1210/jcem-43-5-1047. [DOI] [PubMed] [Google Scholar]
- Weisinger J. R., Favus M. J., Langman C. B., Bushinsky D. A. Regulation of 1,25-dihydroxyvitamin D3 by calcium in the parathyroidectomized, parathyroid hormone-replete rat. J Bone Miner Res. 1989 Dec;4(6):929–935. doi: 10.1002/jbmr.5650040618. [DOI] [PubMed] [Google Scholar]



