Abstract
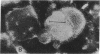
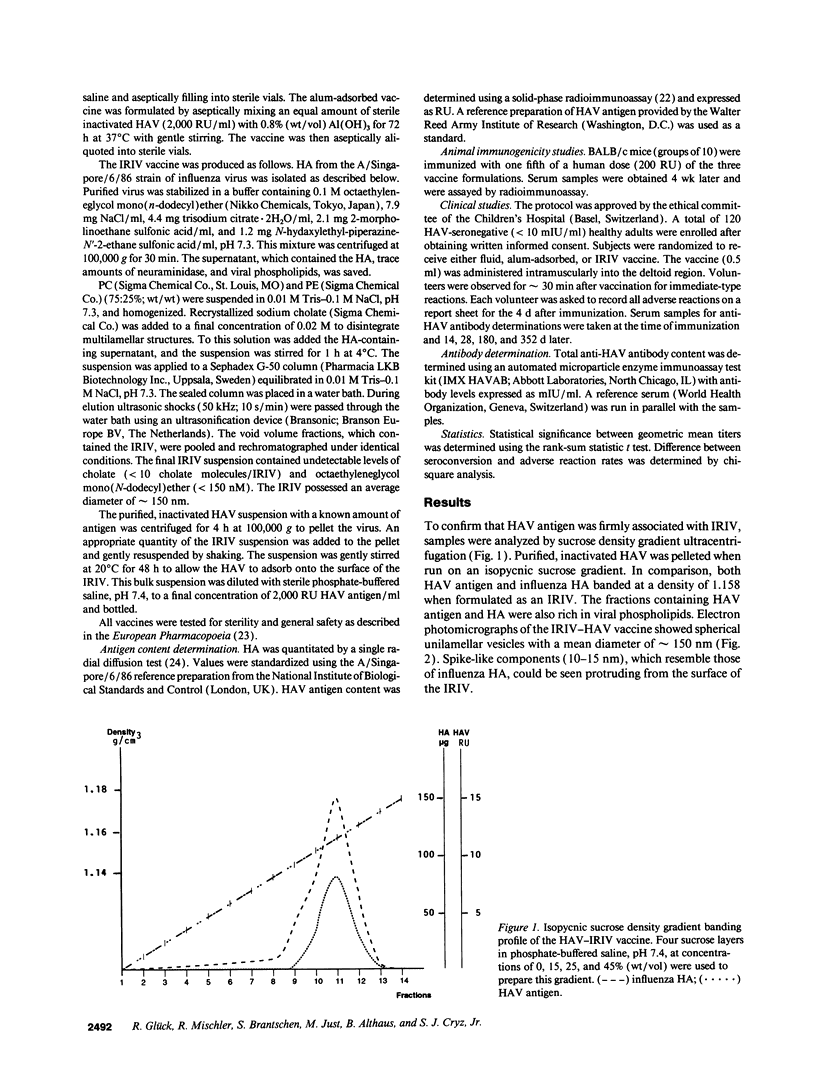
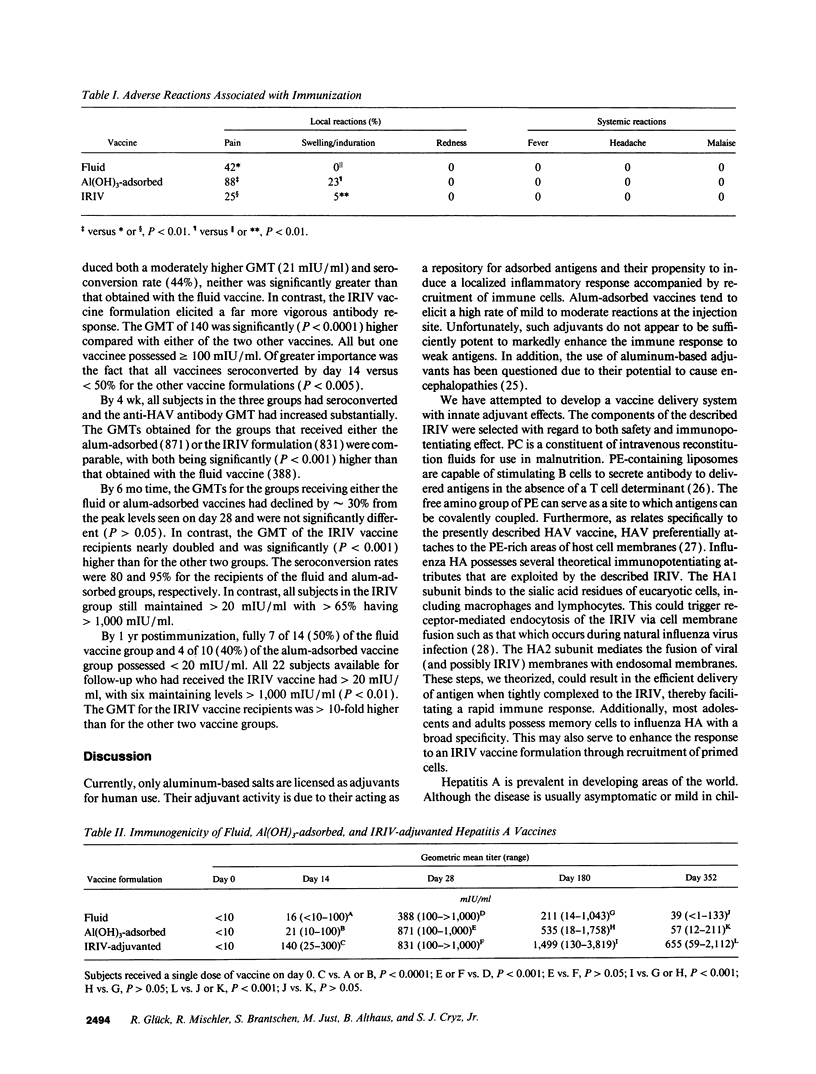
Hepatitis A virus (HAV) was purified from MRC-5 human diploid cell cultures, inactivated with formalin, and evaluated for safety and immunogenicity in humans. Three vaccine formulations were produced: (a) a fluid preparation containing inactivated HAV, (b) inactivated HAV adsorbed to Al(OH)3, and (c) inactivated HAV coupled to novel immunopotentiating reconstituted influenza virosomes (IRIV). IRIV were prepared by combining phosphatidylcholine, phosphatidylethanolamine, phospholipids originating from the influenza virus envelope, influenza virus hemagglutinin, and neuraminidase. The HAV-IRIV appeared as unilamellar vesicles with a diameter of approximately 150 nm when viewed by transmission electron microscopy. Upon intramuscular injection, the alum-adsorbed vaccine was associated with significantly (P < 0.01) more local adverse reactions than either the fluid or IRIV formulations. 14 d after a single dose of vaccine, all the recipients of the IRIV formulation seroconverted (> or = 20 mIU/ml) versus 30 and 44% for those who received the fluid and alum-adsorbed vaccines, respectively (P < 0.001). The geometric mean anti-HAV antibody titer achieved after immunization with the IRIV-HAV vaccine was also significantly higher (P < 0.005) compared with the other two vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Arnon R., Shapira M., Jacob C. O. Synthetic vaccines. J Immunol Methods. 1983 Jul 29;61(3):261–273. doi: 10.1016/0022-1759(83)90220-x. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Progress in vaccines against AIDS. Science. 1989 Dec 8;246(4935):1233–1234. doi: 10.1126/science.2555922. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Heinricy U., Pfisterer M. Immunogenicity of a killed hepatitis A vaccine in seronegative volunteers. Lancet. 1989 May 13;1(8646):1039–1041. doi: 10.1016/s0140-6736(89)92443-4. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Ranke M., Frank H., Gerth H. J. Application of a solid-phase radioimmunoassay and immune electron microscopy for hepatitis A in diagnosis and research. Med Microbiol Immunol. 1978 Nov 17;166(1-4):187–194. doi: 10.1007/BF02121149. [DOI] [PubMed] [Google Scholar]
- Garcon N. M., Six H. R. Universal vaccine carrier. Liposomes that provide T-dependent help to weak antigens. J Immunol. 1991 Jun 1;146(11):3697–3702. [PubMed] [Google Scholar]
- Good M. F., Kumar S., Miller L. H. The real difficulties for malaria sporozoite vaccine development: nonresponsiveness and antigenic variation. Immunol Today. 1988 Nov;9(11):351–355. doi: 10.1016/0167-5699(88)91336-9. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Crabb B. S., Poumbourios P., Tulip W. R., Laver W. G. Three antibody molecules can bind simultaneously to each monomer of the tetramer of influenza virus neuraminidase and the trimer of influenza virus hemagglutinin. Arch Virol. 1991;116(1-4):45–56. doi: 10.1007/BF01319230. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., Ballou W. R., Smith L. F., Wirtz R. A., Zollinger W. D., Hockmeyer W. T. Proteosome-lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science. 1988 May 6;240(4853):800–802. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Milliner D. S., Shinaberger J. H., Shuman P., Coburn J. W. Inadvertent aluminum administration during plasma exchange due to aluminum contamination of albumin-replacement solutions. N Engl J Med. 1985 Jan 17;312(3):165–167. doi: 10.1056/NEJM198501173120307. [DOI] [PubMed] [Google Scholar]
- Morein B., Simons K. Subunit vaccines against enveloped viruses: virosomes, micelles and other protein complexes. Vaccine. 1985 Jun;3(2):83–93. doi: 10.1016/0264-410x(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Perrin P., Thibodeau L., Sureau P. Rabies immunosome (subunit vaccine) structure and immunogenicity. Pre- and post-exposure protection studies. Vaccine. 1985 Sep;3(3):325–332. doi: 10.1016/s0264-410x(85)90224-5. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Henry-Aymard M., Pereira H. G. A quantitative, single-radial-diffusion test for immunological studies with influenza virus. J Gen Virol. 1972 Aug;16(2):231–236. doi: 10.1099/0022-1317-16-2-231. [DOI] [PubMed] [Google Scholar]
- Seganti L., Superti F., Orsi N., Gabrieli R., Divizia M., Panà A. Membrane lipid components interacting with hepatitis A virus. Microbiologica. 1989 Jul;12(3):225–230. [PubMed] [Google Scholar]
- Staruch M. J., Wood D. D. The adjuvanticity of interleukin 1 in vivo. J Immunol. 1983 May;130(5):2191–2194. [PubMed] [Google Scholar]
- Taylor A. H., Haberman A. M., Gerhard W., Caton A. J. Structure-function relationships among highly diverse T cells that recognize a determinant from influenza virus hemagglutinin. J Exp Med. 1990 Dec 1;172(6):1643–1651. doi: 10.1084/jem.172.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari E., Dietzschold B., Szokan G., Heber-Katz E. A synthetic peptide induces long-term protection from lethal infection with herpes simplex virus 2. J Exp Med. 1987 Feb 1;165(2):459–470. doi: 10.1084/jem.165.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Martin S. R., Ruigrok R. W., Skehel J. J., Wiley D. C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J Gen Virol. 1988 Aug;69(Pt 8):1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- Wiedermann G., Ambrosch F., Kollaritsch H., Hofmann H., Kunz C., D'Hondt E., Delem A., André F. E., Safary A., Stéphenne J. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine. 1990 Dec;8(6):581–584. doi: 10.1016/0264-410x(90)90013-c. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Dancey G. F., Kinsky S. C. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1234–1236. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]