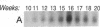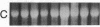Abstract
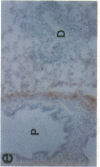
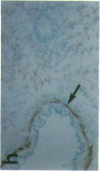
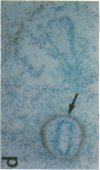
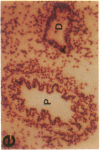
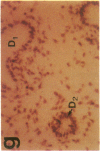
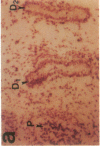
The cell membrane-associated enzyme CD10/neutral endopeptidase 24.11 (CD10/NEP) functions in multiple organ systems to downregulate responses to peptide hormones. Recently, CD10/NEP was found to hydrolyze bombesin-like peptides (BLP), which are mitogens for normal bronchial epithelial cells and small cell lung carcinomas. Growth of BLP-responsive small cell lung carcinomas was potentiated by CD10/NEP inhibition, implicating CD10/NEP in regulation of BLP-mediated tumor growth. BLP are also likely to participate in normal lung development because high BLP levels are found in fetal lung, and bombesin induces proliferation and maturation of human fetal lung in organ cultures and murine fetal lung in utero. To explore potential roles for CD10/NEP in regulating peptide-mediated human fetal lung development, we have characterized temporal and cellular patterns of CD10/NEP expression and effects of CD10/NEP inhibition in organ cultures. Peak CD10/NEP transcript levels are identified at 11-13 wk gestation by Northern blots and localized to epithelial cells and mesenchyme of developing airways by in situ hybridization. CD10/NEP immunostaining is most intense in undifferentiated airway epithelium. In human fetal lung organ cultures, inhibition of CD10/NEP with either phosphoramidon or SCH32615 increases thymidine incorporation by 166-182% (P < 0.025). The specific BLP receptor antagonist, [Leu13-psi(CH2NH)Leu14]bombesin abolishes these effects on fetal lung growth, suggesting that CD10/NEP modulates BLP-mediated proliferation. CD10/NEP expression in the growing front of airway epithelium and the effects of CD10/NEP inhibitors in lung explants implicate the enzyme in the regulation of peptide-mediated fetal lung growth.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo S. M., King T. E., Jr, Waldron J. A., Jr, Sherritt K. M., Kane M. A., Miller Y. E. Increased pulmonary neuroendocrine cells with bombesin-like immunoreactivity in adult patients with eosinophilic granuloma. J Clin Invest. 1990 Sep;86(3):838–844. doi: 10.1172/JCI114782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. A., Gorenstein C. Fluorescent histochemical localization of neutral endopeptidase-24.11 (enkephalinase) in the rat brainstem. J Comp Neurol. 1990 Jun 1;296(1):130–158. doi: 10.1002/cne.902960109. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Shipp M. A., D'Adamio L., Masteller E. L., Reinherz E. L. The common acute lymphoblastic leukemia antigen gene maps to chromosomal region 3 (q21-q27). J Immunol. 1989 Jan 1;142(1):283–287. [PubMed] [Google Scholar]
- Chipkin R. E., Berger J. G., Billard W., Iorio L. C., Chapman R., Barnett A. Pharmacology of SCH 34826, an orally active enkephalinase inhibitor analgesic. J Pharmacol Exp Ther. 1988 Jun;245(3):829–838. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Heinz-Erian P., Jiang N. Y., Sasaki Y., Taylor J., Moreau J. P., Wolfrey W. T., Gardner J. D., Jensen R. T. Probing peptide backbone function in bombesin. A reduced peptide bond analogue with potent and specific receptor antagonist activity. J Biol Chem. 1988 Apr 15;263(11):5056–5060. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- D'Adamio L., Shipp M. A., Masteller E. L., Reinherz E. L. Organization of the gene encoding common acute lymphoblastic leukemia antigen (neutral endopeptidase 24.11): multiple miniexons and separate 5' untranslated regions. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7103–7107. doi: 10.1073/pnas.86.18.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989 Feb;3(2):145–151. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gros C., Souque A., Schwartz J. C., Duchier J., Cournot A., Baumer P., Lecomte J. M. Protection of atrial natriuretic factor against degradation: diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3.4.24.11) by acetorphan. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7580–7584. doi: 10.1073/pnas.86.19.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross I., Freedman R. M., Wilson C. M., Lindsey S. Organotypic culture of fetal rat lung: evaluation and comparison with organ culture. Am Rev Respir Dis. 1981 Mar;123(3):313–319. doi: 10.1164/arrd.1981.123.3.313. [DOI] [PubMed] [Google Scholar]
- Haralambidou S., Melo J. V., Catovsky D. Different reactivity of monoclonal antibodies against common acute lymphoblastic leukaemia antigen (CD10). J Clin Pathol. 1987 May;40(5):490–493. doi: 10.1136/jcp.40.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Ashton J., Schulz W. W., Erdös E. G. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985 Sep;132(3):564–568. doi: 10.1164/arrd.1985.132.3.564. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., O'Hare M. J., Gusterson B. A. Cell-surface peptidases as modulators of growth and differentiation. Lancet. 1989 Sep 30;2(8666):785–787. doi: 10.1016/s0140-6736(89)90841-6. [DOI] [PubMed] [Google Scholar]
- Kondo M., Tamaoki J., Takizawa T. Neutral endopeptidase inhibitor potentiates the tachykinin-induced increase in ciliary beat frequency in rabbit trachea. Am Rev Respir Dis. 1990 Aug;142(2):403–406. doi: 10.1164/ajrccm/142.2.403. [DOI] [PubMed] [Google Scholar]
- LeBien T. W., McCormack R. T. The common acute lymphoblastic leukemia antigen (CD10)--emancipation from a functional enigma. Blood. 1989 Feb 15;73(3):625–635. [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Seon B. K. Marked difference in the in vivo antitumor efficacy between two immunotoxins targeted to different epitopes of common acute lymphoblastic leukemia antigen (CD10). Mechanisms involved in the differential activities of immunotoxins. J Immunol. 1990 Sep 15;145(6):1974–1982. [PubMed] [Google Scholar]
- Martins M. A., Shore S. A., Gerard N. P., Gerard C., Drazen J. M. Peptidase modulation of the pulmonary effects of tachykinins in tracheal superfused guinea pig lungs. J Clin Invest. 1990 Jan;85(1):170–176. doi: 10.1172/JCI114408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H., Haruta Y., Fukukawa T., Barcos M. P., Seon B. K. Unique epitopes of common acute lymphoblastic leukemia antigen detected by new monoclonal antibodies. Cancer Res. 1987 Apr 15;47(8):2160–2166. [PubMed] [Google Scholar]
- Mechtersheimer G., Möller P. Expression of the common acute lymphoblastic leukemia antigen (CD10) in mesenchymal tumors. Am J Pathol. 1989 May;134(5):961–965. [PMC free article] [PubMed] [Google Scholar]
- Nadel J. A., Borson D. B. Modulation of neurogenic inflammation by neutral endopeptidase. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S33–S36. doi: 10.1164/ajrccm/143.3_Pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Nadel J. A. Decreased neutral endopeptidases: possible role in inflammatory diseases of airways. Lung. 1990;168 (Suppl):123–127. doi: 10.1007/BF02718124. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Ronco P., Pollard H., Galceran M., Delauche M., Schwartz J. C., Verroust P. Distribution of enkephalinase (membrane metalloendopeptidase, E.C. 3.4.24.11) in rat organs. Detection using a monoclonal antibody. Lab Invest. 1988 Feb;58(2):210–217. [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles G., Chen C. Y., Reinherz E. L., Shipp M. A. CD10/NEP is expressed on Thy-1low B220+ murine B-cell progenitors and functions to regulate stromal cell-dependent lymphopoiesis. Blood. 1992 Oct 15;80(8):2021–2029. [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Graf P. D., Basbaum C. B., Borson D. B., Nadel J. A. Enkephalinase inhibitor potentiates mammalian tachykinin-induced contraction in ferret trachea. J Pharmacol Exp Ther. 1987 Dec;243(3):1211–1217. [PubMed] [Google Scholar]
- Shipp M. A., Richardson N. E., Sayre P. H., Brown N. R., Masteller E. L., Clayton L. K., Ritz J., Reinherz E. L. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Stefano G. B., D'Adamio L., Switzer S. N., Howard F. D., Sinisterra J., Scharrer B., Reinherz E. L. Downregulation of enkephalin-mediated inflammatory responses by CD10/neutral endopeptidase 24.11. Nature. 1990 Sep 27;347(6291):394–396. doi: 10.1038/347394a0. [DOI] [PubMed] [Google Scholar]
- Shipp M. A., Stefano G. B., Switzer S. N., Griffin J. D., Reinherz E. L. CD10 (CALLA)/neutral endopeptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood. 1991 Oct 1;78(7):1834–1841. [PubMed] [Google Scholar]
- Shipp M. A., Tarr G. E., Chen C. Y., Switzer S. N., Hersh L. B., Stein H., Sunday M. E., Reinherz E. L. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10662–10666. doi: 10.1073/pnas.88.23.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Cellular signaling by peptides of the endothelin gene family. FASEB J. 1990 Sep;4(12):2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- Spindel E. R., Sunday M. E., Hofler H., Wolfe H. J., Habener J. F., Chin W. W. Transient elevation of messenger RNA encoding gastrin-releasing peptide, a putative pulmonary growth factor in human fetal lung. J Clin Invest. 1987 Oct;80(4):1172–1179. doi: 10.1172/JCI113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler-Gerard N. P. Neutral endopeptidase-like enzyme controls the contractile activity of substance P in guinea pig lung. J Clin Invest. 1987 Jun;79(6):1819–1825. doi: 10.1172/JCI113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday M. E., Choi N., Spindel E. R., Chin W. W., Mark E. J. Gastrin-releasing peptide gene expression in small cell and large cell undifferentiated lung carcinomas. Hum Pathol. 1991 Oct;22(10):1030–1039. doi: 10.1016/0046-8177(91)90011-d. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Hua J., Dai H. B., Nusrat A., Torday J. S. Bombesin increases fetal lung growth and maturation in utero and in organ culture. Am J Respir Cell Mol Biol. 1990 Sep;3(3):199–205. doi: 10.1165/ajrcmb/3.3.199. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Kaplan L. M., Motoyama E., Chin W. W., Spindel E. R. Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest. 1988 Jul;59(1):5–24. [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]