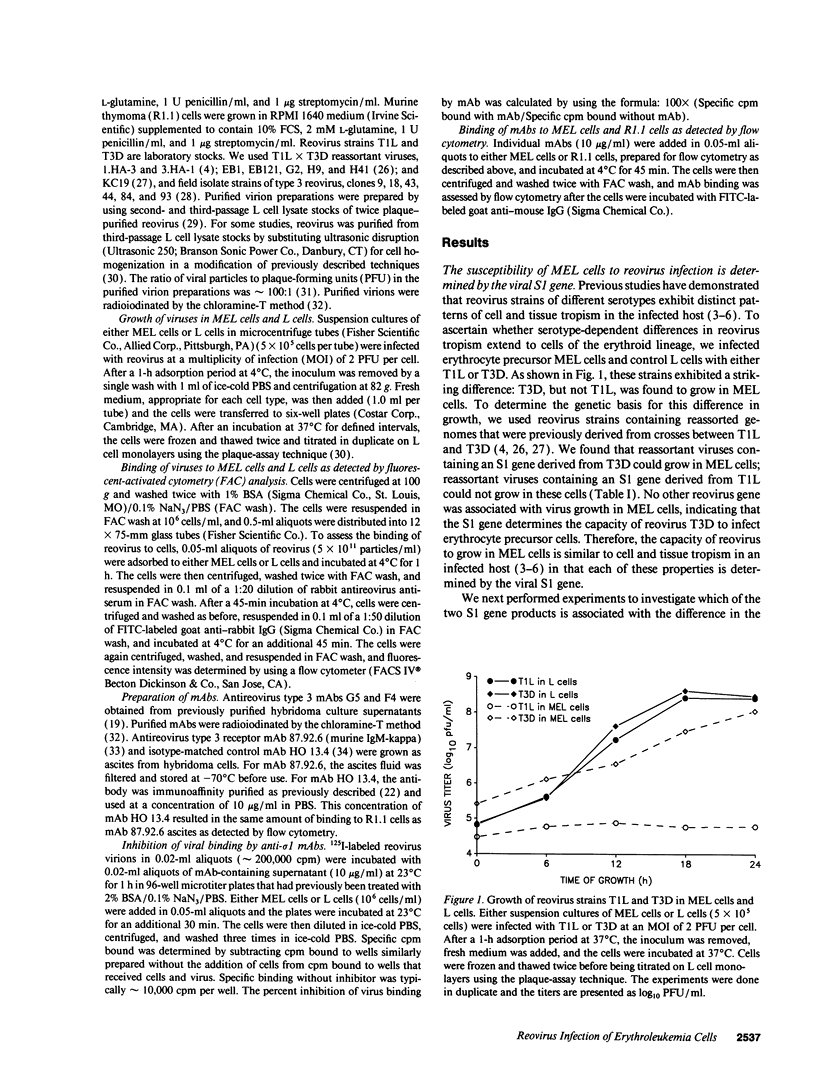
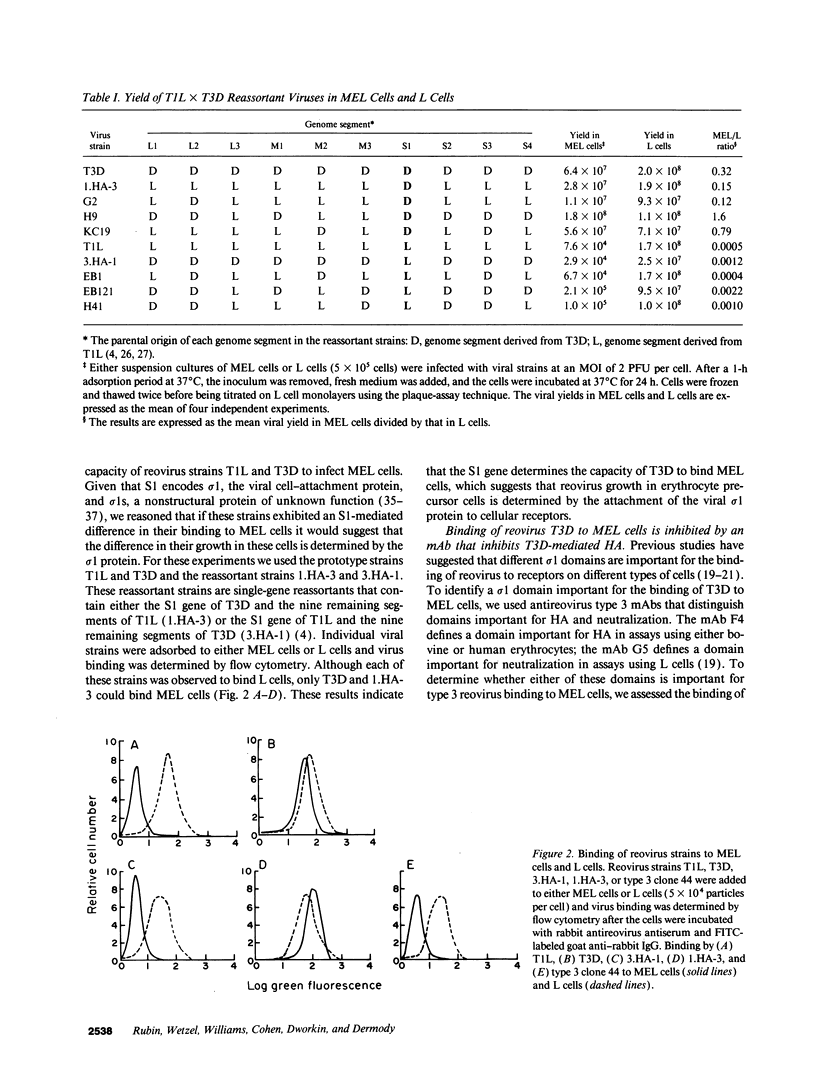
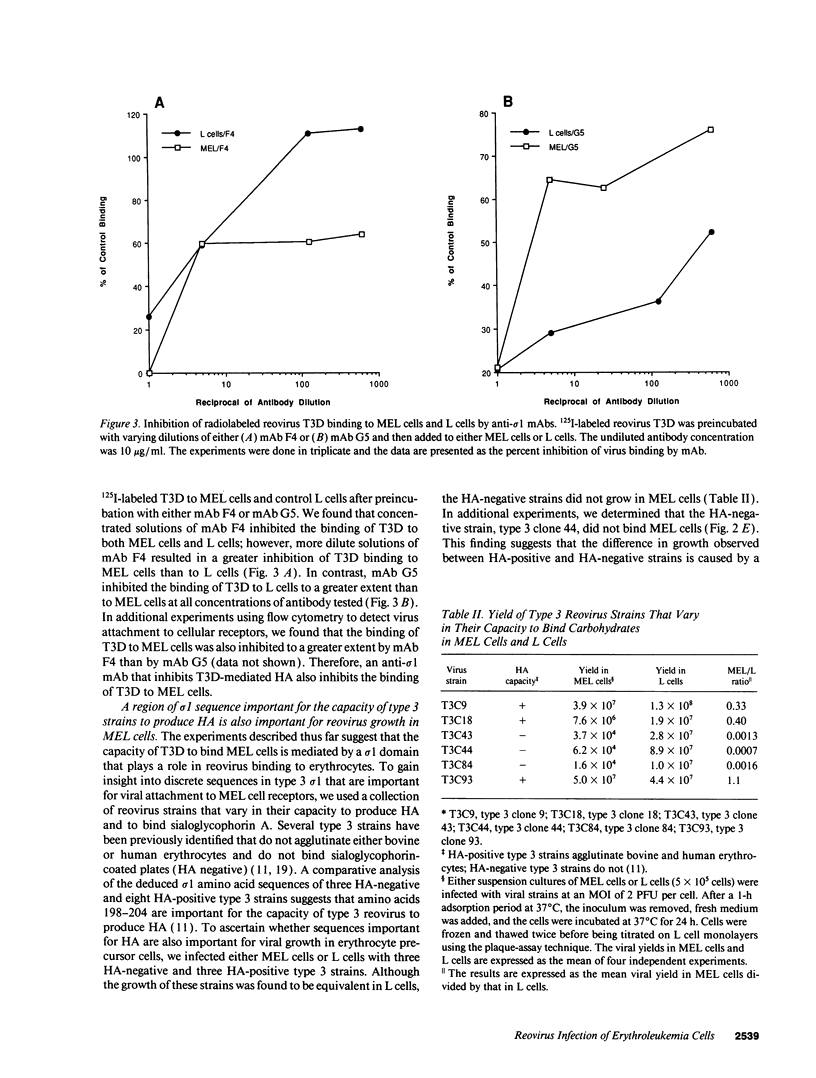
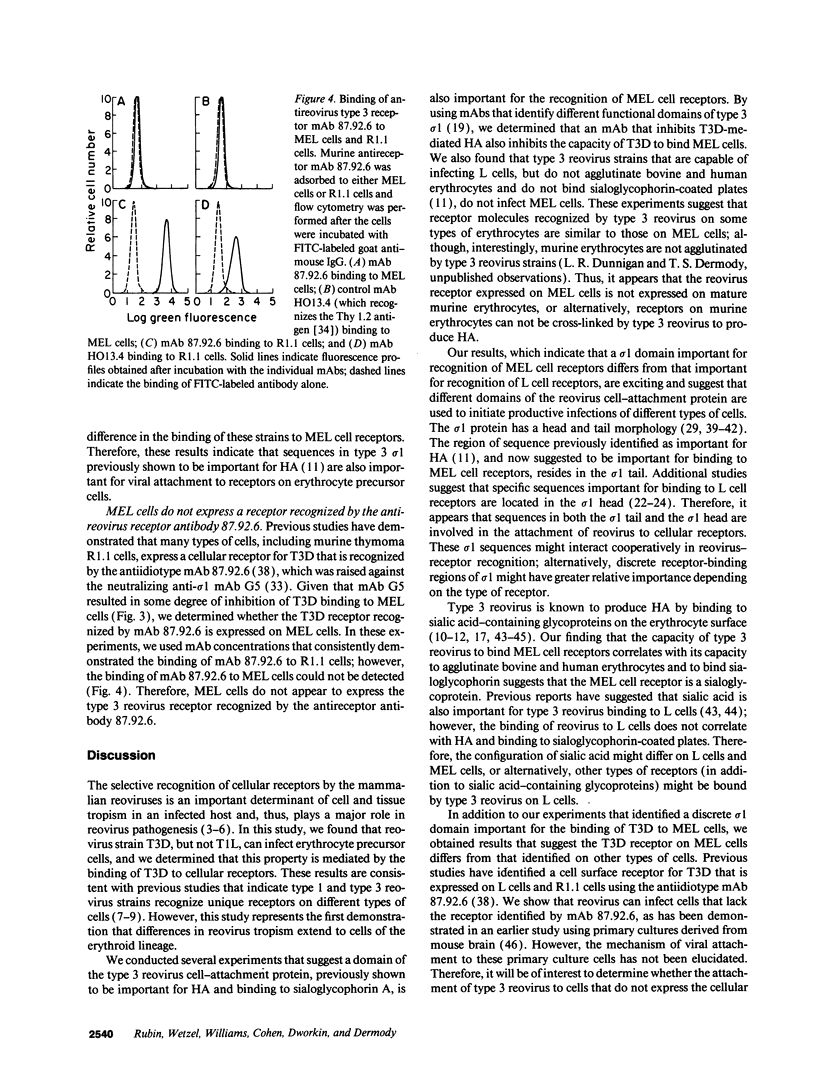
Abstract
The recognition of cellular receptors by the mammalian reoviruses is an important determinant of cell and tissue tropism exhibited by reovirus strains of different serotypes. To extend our knowledge of the role of reovirus-receptor interactions in reovirus tropism, we determined whether type 1 and type 3 reovirus strains can infect cells derived from erythrocyte precursors. We found that reovirus type 3 Dearing (T3D), but not type 1 Lang, can grow in murine erythroleukemia (MEL) cells. This difference in growth was investigated by using reassortant viruses and we found that the capacity of T3D to infect MEL cells is determined by the viral cell-attachment protein, sigma 1. In experiments using murine monoclonal antibodies (mAbs) that bind to different sigma 1 regions, we show that T3D binding to MEL cells is inhibited by a mAb that identifies a domain important for hemagglutination (HA). We also determined that type 3 strains that can infect murine L cells but do not produce HA do not infect MEL cells. These results suggest that type 3 reovirus binds to and infects erythrocyte precursor cells via a sigma 1 domain important for HA. Moreover, this study suggests that different domains of some viral cell-attachment proteins are used to initiate productive infections of different types of cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. D., Paul R. W., Lee P. W. Studies on reovirus receptors of L cells: virus binding characteristics and comparison with reovirus receptors of erythrocytes. Virology. 1984 Oct 15;138(1):37–48. doi: 10.1016/0042-6822(84)90145-4. [DOI] [PubMed] [Google Scholar]
- Banerjea A. C., Brechling K. A., Ray C. A., Erikson H., Pickup D. J., Joklik W. K. High-level synthesis of biologically active reovirus protein sigma 1 in a mammalian expression vector system. Virology. 1988 Dec;167(2):601–612. [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K. M., Fields B. N., Harrison S. C. Crystallization of the reovirus type 3 Dearing core. Crystal packing is determined by the lambda 2 protein. J Mol Biol. 1990 Sep 5;215(1):1–5. doi: 10.1016/s0022-2836(05)80089-0. [DOI] [PubMed] [Google Scholar]
- Dermody T. S., Nibert M. L., Bassel-Duby R., Fields B. N. A sigma 1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990 Oct;64(10):5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody T. S., Nibert M. L., Bassel-Duby R., Fields B. N. Sequence diversity in S1 genes and S1 translation products of 11 serotype 3 reovirus strains. J Virol. 1990 Oct;64(10):4842–4850. doi: 10.1128/jvi.64.10.4842-4850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Weiner H. L., Fields B. N., Mitchell G., Noseworthy J., Gaulton G., Greene M. Antiidiotypic antibody to reovirus binds to neurons and protects from viral infection. Ann Neurol. 1986 Jun;19(6):555–558. doi: 10.1002/ana.410190606. [DOI] [PubMed] [Google Scholar]
- Duncan R., Horne D., Cashdollar L. W., Joklik W. K., Lee P. W. Identification of conserved domains in the cell attachment proteins of the three serotypes of reovirus. Virology. 1990 Feb;174(2):399–409. doi: 10.1016/0042-6822(90)90093-7. [DOI] [PubMed] [Google Scholar]
- Duncan R., Horne D., Strong J. E., Leone G., Pon R. T., Yeung M. C., Lee P. W. Conformational and functional analysis of the C-terminal globular head of the reovirus cell attachment protein. Virology. 1991 Jun;182(2):810–819. doi: 10.1016/0042-6822(91)90622-i. [DOI] [PubMed] [Google Scholar]
- Epstein R. L., Powers M. L., Rogart R. B., Weiner H. L. Binding of 125I-labeled reovirus to cell surface receptors. Virology. 1984 Feb;133(1):46–55. doi: 10.1016/0042-6822(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Ernst H., Shatkin A. J. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc Natl Acad Sci U S A. 1985 Jan;82(1):48–52. doi: 10.1073/pnas.82.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Weiner H. L. Interaction of reovirus with cell surface receptors. II. Generation of suppressor T cells by the hemagglutinin of reovirus type 3. J Immunol. 1980 Dec;125(6):2660–2664. [PubMed] [Google Scholar]
- Fraser R. D., Furlong D. B., Trus B. L., Nibert M. L., Fields B. N., Steven A. C. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J Virol. 1990 Jun;64(6):2990–3000. doi: 10.1128/jvi.64.6.2990-3000.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Pacitti A. F. Differential interaction of reovirus type 3 with sialylated receptor components on animal cells. Virology. 1987 Nov;161(1):245–248. doi: 10.1016/0042-6822(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Pacitti A. F. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J Virol. 1985 Nov;56(2):356–364. doi: 10.1128/jvi.56.2.356-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Weiner H. L. Delayed hypersensitivity in mice infected with reovirus. II. Induction of tolerance and suppressor T cells to viral specific gene products. J Immunol. 1980 Jul;125(1):283–287. [PubMed] [Google Scholar]
- Jacobs B. L., Samuel C. E. Biosynthesis of reovirus-specified polypeptides: the reovirus s1 mRNA encodes two primary translation products. Virology. 1985 May;143(1):63–74. doi: 10.1016/0042-6822(85)90097-2. [DOI] [PubMed] [Google Scholar]
- Jansson L. T., Kling S., Dallman P. R. Anemia in children with acute infections seen in a primary care pediatric outpatient clinic. Pediatr Infect Dis. 1986 Jul-Aug;5(4):424–427. doi: 10.1097/00006454-198607000-00009. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Nagata L., Masri S. A., Pon R. T., Lee P. W. Analysis of functional domains on reovirus cell attachment protein sigma 1 using cloned S1 gene deletion mutants. Virology. 1987 Sep;160(1):162–168. doi: 10.1016/0042-6822(87)90056-0. [DOI] [PubMed] [Google Scholar]
- Nibert M. L., Dermody T. S., Fields B. N. Structure of the reovirus cell-attachment protein: a model for the domain organization of sigma 1. J Virol. 1990 Jun;64(6):2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Onodera T., Toniolo A., Ray U. R., Jenson A. B., Knazek R. A., Notkins A. L. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J Exp Med. 1981 Jun 1;153(6):1457–1473. doi: 10.1084/jem.153.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. W., Choi A. H., Lee P. W. The alpha-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology. 1989 Sep;172(1):382–385. doi: 10.1016/0042-6822(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Paul R. W., Lee P. W. Glycophorin is the reovirus receptor on human erythrocytes. Virology. 1987 Jul;159(1):94–101. doi: 10.1016/0042-6822(87)90351-5. [DOI] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Eaton M. A., Anderson A. O. Reovirus infection in adult mice: the virus hemagglutinin determines the site of intestinal disease. Microb Pathog. 1986 Feb;1(1):79–87. doi: 10.1016/0882-4010(86)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. H., Kornstein M. J., Anderson A. O. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985 Feb;53(2):391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Pelletier J., Bassel-Duby R., Jayasuriya A., Fields B. N., Sonenberg N. Identification of a new polypeptide coded by reovirus gene S1. J Virol. 1985 Jun;54(3):720–725. doi: 10.1128/jvi.54.3.720-725.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Duncan R., Lee P. W. Site-directed mutagenesis of the C-terminal portion of reovirus protein sigma 1: evidence for a conformation-dependent receptor binding domain. Virology. 1992 Jan;186(1):219–227. doi: 10.1016/0042-6822(92)90076-2. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Girard K., Williams W. V., McPhillips T., Rubin D. H. Reovirus type 1 and type 3 differ in their binding to isolated intestinal epithelial cells. Microb Pathog. 1988 Jul;5(1):29–40. doi: 10.1016/0882-4010(88)90078-2. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ault K. A., Fields B. N. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J Immunol. 1980 May;124(5):2143–2148. [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Guy H. R., Rubin D. H., Robey F., Myers J. N., Kieber-Emmons T., Weiner D. B., Greene M. I. Sequences of the cell-attachment sites of reovirus type 3 and its anti-idiotypic/antireceptor antibody: modeling of their three-dimensional structures. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6488–6492. doi: 10.1073/pnas.85.17.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. C., Lim D., Duncan R., Shahrabadi M. S., Cashdollar L. W., Lee P. W. The cell attachment proteins of type 1 and type 3 reovirus are differentially susceptible to trypsin and chymotrypsin. Virology. 1989 May;170(1):62–70. doi: 10.1016/0042-6822(89)90352-8. [DOI] [PubMed] [Google Scholar]


