Abstract
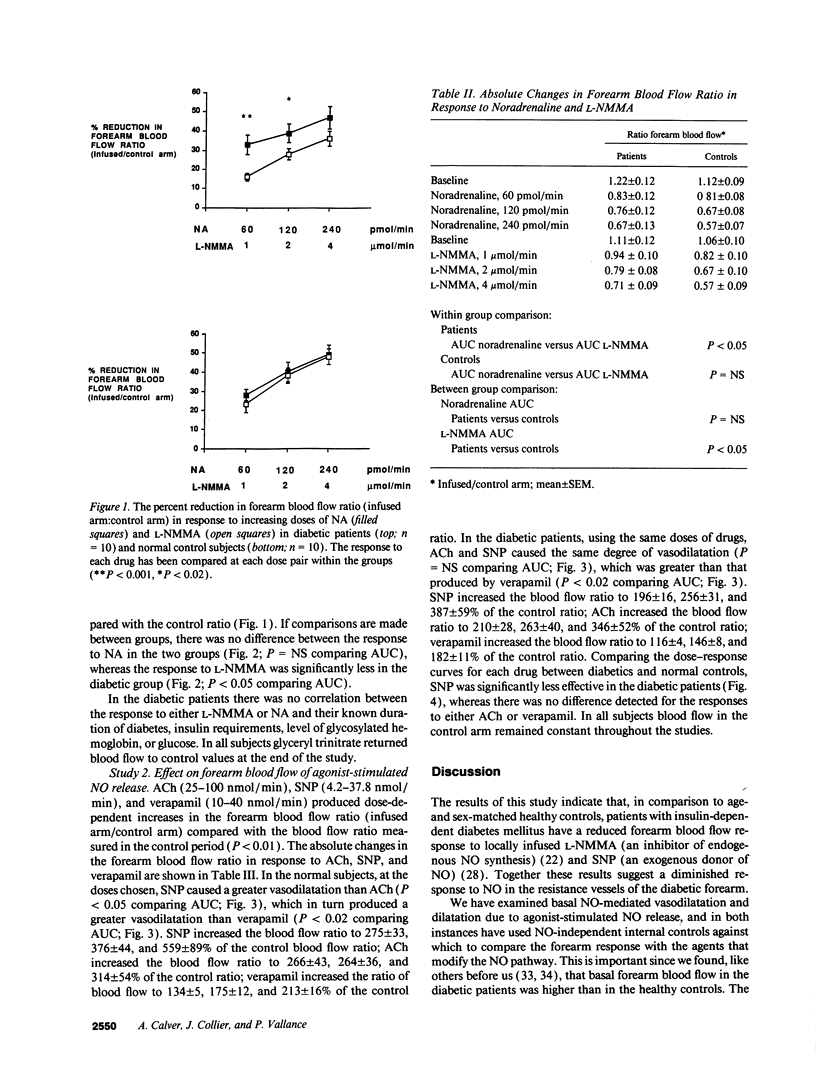
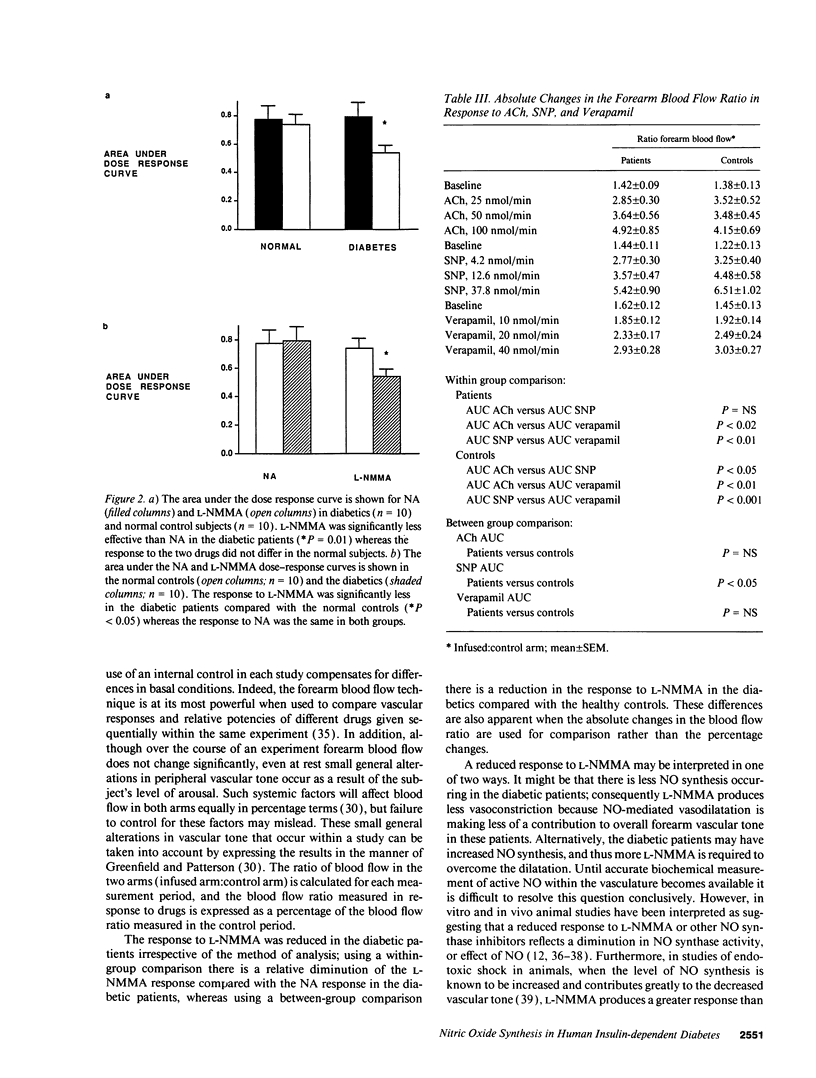
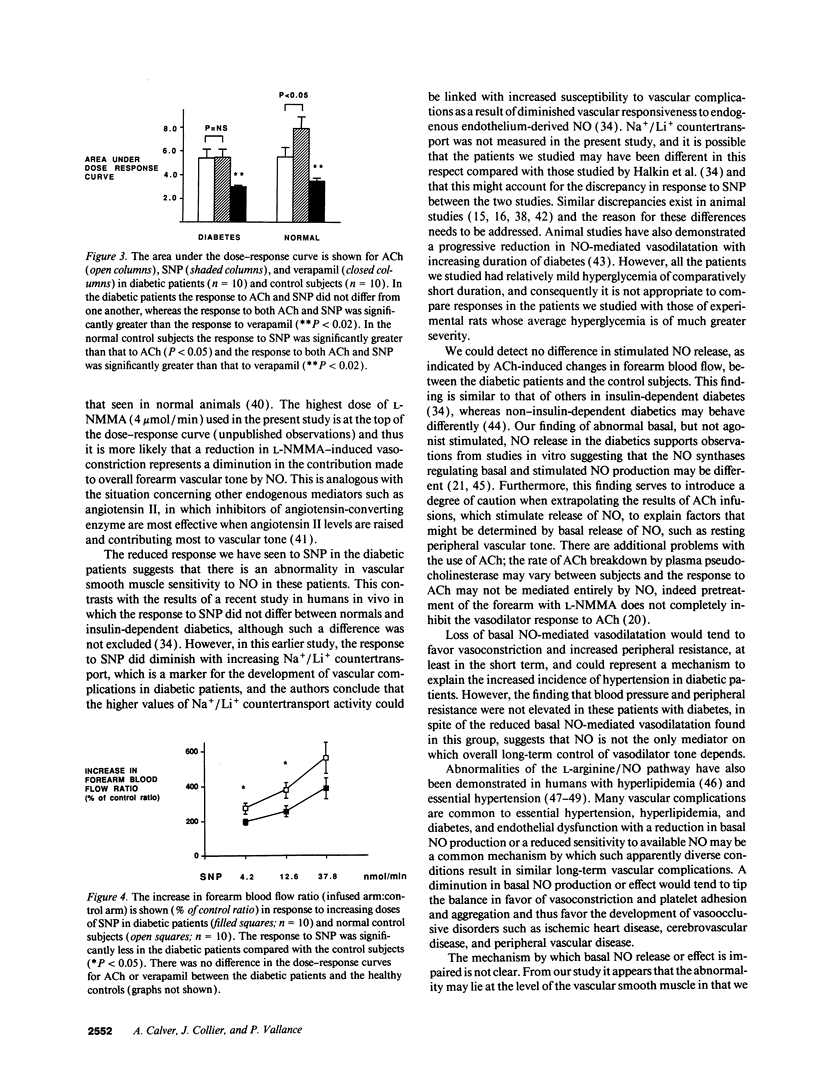
Patients with insulin-dependent diabetes mellitus have an increased mortality and morbidity due to vascular complications. Nitric oxide from the vascular endothelium contributes to the control of normal vascular tone, and endothelial dysfunction has been implicated in the pathogenesis of diabetic vascular disease. In this study we have examined basal and stimulated nitric oxide-mediated vasodilatation in insulin-dependent diabetics and age- and sex-matched healthy controls. Drugs were infused locally into the brachial artery and forearm blood flow measured using venous occlusion plethysmography. Noradrenaline and NG-monomethyl-L-arginine produced similar reductions in resting forearm blood flow in healthy controls. However, in the diabetics, NG-monomethyl-L-arginine was significantly less effective than noradrenaline. Comparing between groups, the response to NG-monomethyl-L-arginine was also significantly less in the diabetics compared with the healthy controls. The response to sodium nitroprusside was significantly less in the diabetics compared with the healthy controls, whereas the responses to both acetylcholine and verapamil were the same in the two groups. The results provide evidence for an abnormality of basal nitric oxide-mediated dilatation in the forearm arterial bed of patients with insulin-dependent diabetes mellitus, and suggest that the vascular smooth muscle is less sensitive to nitric oxide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver A., Collier J., Moncada S., Vallance P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens. 1992 Sep;10(9):1025–1031. [PubMed] [Google Scholar]
- Case D. B., Wallace J. M., Keim H. J., Weber M. A., Drayer J. I., White R. P., Sealey J. E., Laragh J. H. Estimating renin participation in hypertension: superiority of converting enzyme inhibitor over saralasin. Am J Med. 1976 Nov;61(5):790–796. doi: 10.1016/0002-9343(76)90160-1. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Giugliano D., Quatraro A., Dello Russo P., Lefèbvre P. J. Metabolic control may influence the increased superoxide generation in diabetic serum. Diabet Med. 1991 Jul;8(6):540–542. doi: 10.1111/j.1464-5491.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Chester A. H., O'Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990 Oct 13;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. A reversible vascular abnormality associated with diabetic ketosis. Clin Sci. 1970 Nov;39(5):539–548. doi: 10.1042/cs0390539. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R. Diabetes and hypertensive vascular disease. Mechanisms and treatment. Am J Cardiol. 1973 Sep 20;32(4):592–606. doi: 10.1016/s0002-9149(73)80051-7. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Cooke J. P., Mendelsohn M. E., Gallagher S. J., Coleman S. M., Loscalzo J., Dzau V. J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990 Jul;86(1):228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi Y., Thiel M. A., Bühler F. R., Lüscher T. F. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990 Aug;16(2):170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GREENFIELD A. D., PATTERSON G. C. Reactions of the blood vessels of the human forearm to increases in transmural pressure. J Physiol. 1954 Sep 28;125(3):508–524. doi: 10.1113/jphysiol.1954.sp005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Halkin A., Benjamin N., Doktor H. S., Todd S. D., Viberti G., Ritter J. M. Vascular responsiveness and cation exchange in insulin-dependent diabetes. Clin Sci (Lond) 1991 Aug;81(2):223–232. doi: 10.1042/cs0810223. [DOI] [PubMed] [Google Scholar]
- Jensen T., Bjerre-Knudsen J., Feldt-Rasmussen B., Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet. 1989 Mar 4;1(8636):461–463. doi: 10.1016/s0140-6736(89)91365-2. [DOI] [PubMed] [Google Scholar]
- Jensen T., Borch-Johnsen K., Kofoed-Enevoldsen A., Deckert T. Coronary heart disease in young type 1 (insulin-dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987 Mar;30(3):144–148. doi: 10.1007/BF00274218. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff R. J., Gardiner S. M., Compton A. M., Bennett T. Selective impairment of hindquarters vasodilator responses to bradykinin in conscious Wistar rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Jun;103(2):1357–1362. doi: 10.1111/j.1476-5381.1991.tb09793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff R. J., Gardiner S. M., Compton A. M., Bennett T. The effects of endothelin-1 and NG-nitro-L-arginine methyl ester on regional haemodynamics in conscious rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Jun;103(2):1321–1326. doi: 10.1111/j.1476-5381.1991.tb09787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Linder L., Kiowski W., Bühler F. R., Lüscher T. F. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990 Jun;81(6):1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Cagliero E. Pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes. 1991 Jun;40(6):653–659. doi: 10.2337/diab.40.6.653. [DOI] [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G. R., Crook P., Moore P. K., Page C. P. The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br J Pharmacol. 1991 Mar;102(3):759–763. doi: 10.1111/j.1476-5381.1991.tb12246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan W. G. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989 Mar;256(3 Pt 2):H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Panza J. A., Quyyumi A. A., Brush J. E., Jr, Epstein S. E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990 Jul 5;323(1):22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Porta M., La Selva M., Molinatti P., Molinatti G. M. Endothelial cell function in diabetic microangiopathy. Diabetologia. 1987 Aug;30(8):601–609. doi: 10.1007/BF00277315. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br J Pharmacol. 1990 Oct;101(2):325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. F., Dobbs R. J., Bayley S. Response of forearm resistance vessels to verapamil and sodium nitroprusside in normotensive and hypertensive men: evidence for a functional abnormality of vascular smooth muscle in primary hypertension. Clin Sci (Lond) 1982 Jul;63(1):33–42. doi: 10.1042/cs0630033. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- WHITNEY R. J. The measurement of volume changes in human limbs. J Physiol. 1953 Jul;121(1):1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]


