Abstract
Corticotropin-releasing hormone (CRH), the principal regulator of the hypothalamic-pituitary-adrenal axis, is also secreted in peripheral inflammatory sites, where it acts as a local proinflammatory agent. Arthritis-susceptible LEW/N rats have profoundly deficient hypothalamic CRH responses to inflammatory stimuli and other stressors. Arthritis-resistant F344/N rats, on the other hand, have a robust increase in hypothalamic CRH in response to the same stimuli. Contrasting with these hypothalamic CRH responses, we now show that CRH expression is markedly increased in the joints and surrounding tissues of LEW/N rats with streptococcal cell wall- and adjuvant-induced arthritis, whereas it is not increased in similarly treated F344/N rats and is only transiently increased in congenitally athymic nude LEW.rnu/rnu rats. Glucocorticoid treatment suppressed, but did not eliminate, CRH immunoreactivity in the joints of LEW/N rats. CRH mRNA was present in inflamed synovia, as well as in spinal cord, and inflamed synovia also expressed specific CRH-binding sites. We compared CRH expression in inflamed joints with another well-characterized proinflammatory neuropeptide, substance P (SP), and found that SP immunoreactivity paralleled that of CRH. In summary, although LEW/N rats have deficient hypothalamic CRH responses to inflammatory stimuli compared with F344/N rats, they express relatively high levels of CRH at the site of inflammation. Analogous to SP, CRH may be delivered to the inflammatory site by peripheral nerves and/or synthesized at the inflammatory site. These data provide further support for the concept that CRH not only triggers the pituitary-adrenal antiinflammatory cascade, but also functions as an antithetically active local mediator of acute and chronic inflammatory arthritis. These data also illustrate the complex interrelationships of the nervous, endocrine, immune, and inflammatory systems.
Full text
PDF




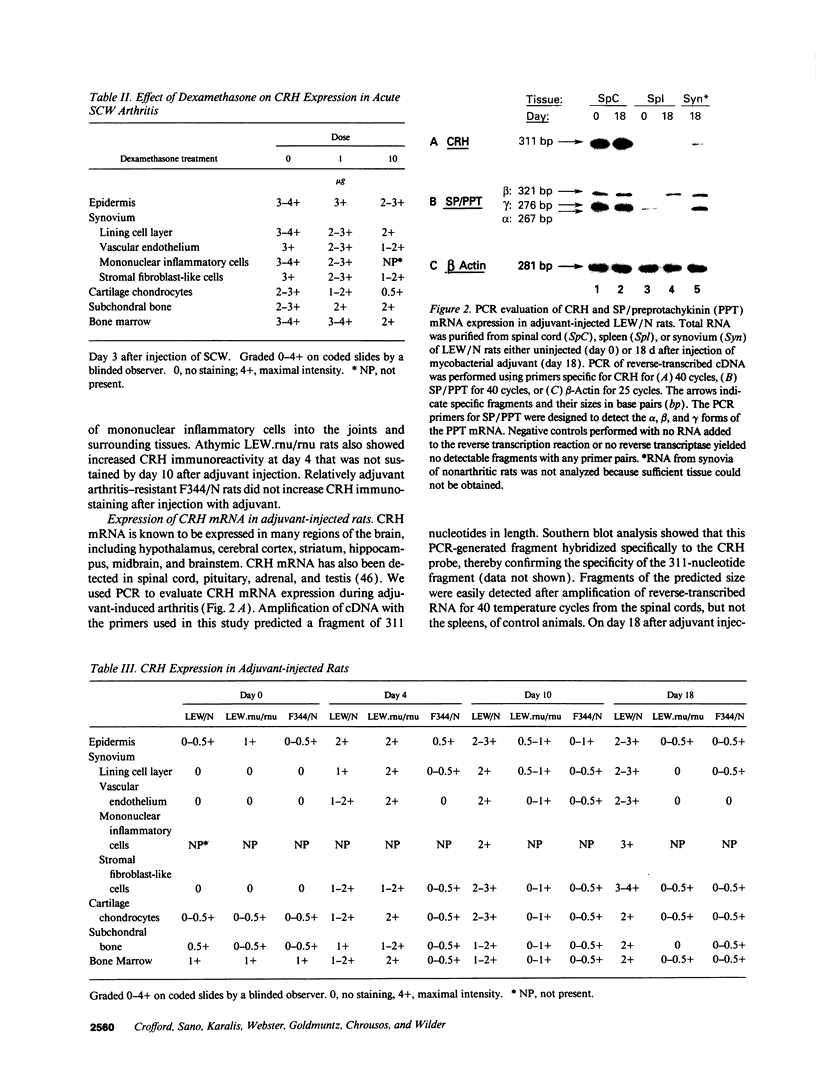
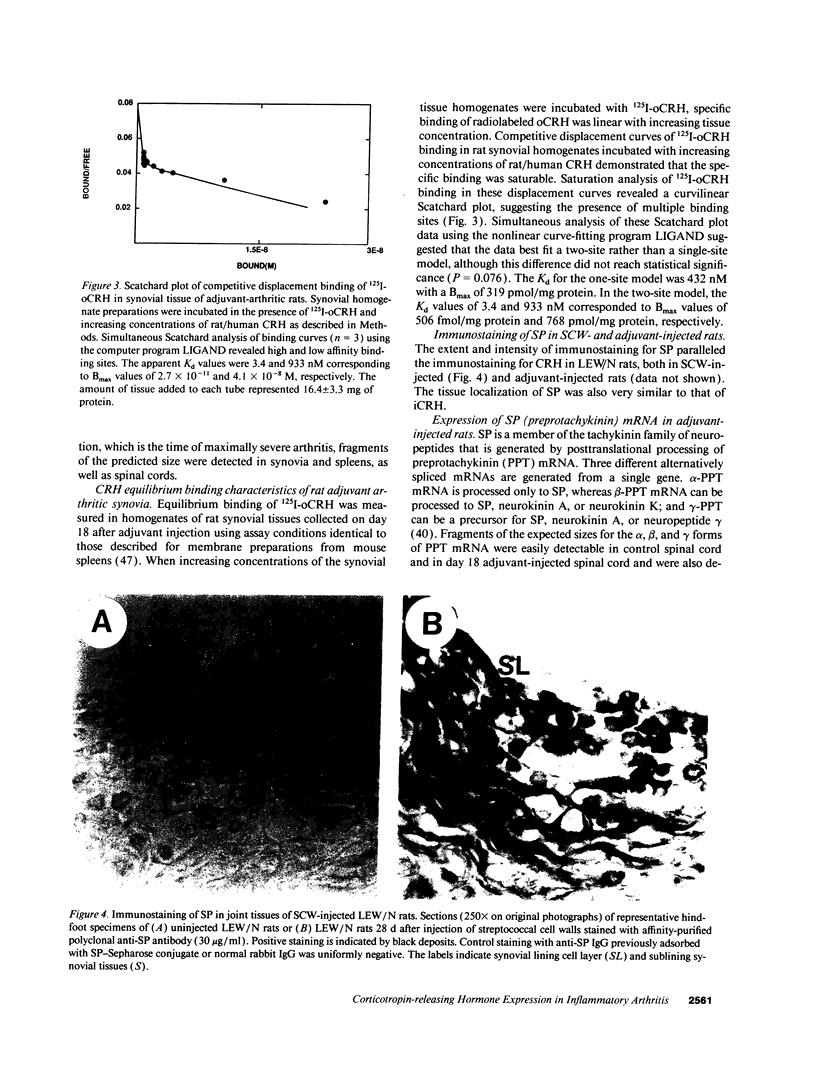



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksentijevich S., Whitfield H. J., Jr, Young W. S., 3rd, Wilder R. L., Chrousos G. P., Gold P. W., Sternberg E. M. Arthritis-susceptible Lewis rats fail to emerge from the stress hyporesponsive period. Brain Res Dev Brain Res. 1992 Jan 17;65(1):115–118. doi: 10.1016/0165-3806(92)90014-n. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Levine J. D. The contribution of the nervous system to inflammation and inflammatory disease. Can J Physiol Pharmacol. 1991 May;69(5):647–651. doi: 10.1139/y91-096. [DOI] [PubMed] [Google Scholar]
- Bateman A., Singh A., Kral T., Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989 Feb;10(1):92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Eisenstein S., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) in genetically resistant rats: PVG rats resist active induction of EAE but are susceptible to and can generate EAE effector T cell lines. J Immunol. 1982 Sep;129(3):918–919. [PubMed] [Google Scholar]
- Calogero A. E., Gallucci W. T., Gold P. W., Chrousos G. P. Multiple feedback regulatory loops upon rat hypothalamic corticotropin-releasing hormone secretion. Potential clinical implications. J Clin Invest. 1988 Sep;82(3):767–774. doi: 10.1172/JCI113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero A. E., Sternberg E. M., Bagdy G., Smith C., Bernardini R., Aksentijevich S., Wilder R. L., Gold P. W., Chrousos G. P. Neurotransmitter-induced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible Lewis rats: in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone. Neuroendocrinology. 1992 May;55(5):600–608. doi: 10.1159/000126173. [DOI] [PubMed] [Google Scholar]
- Case J. P., Sano H., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in the synovium of rats with experimental erosive arthritis. In situ localization and kinetics of expression of the transformation-associated metalloproteinase in euthymic and athymic Lewis rats. J Clin Invest. 1989 Dec;84(6):1731–1740. doi: 10.1172/JCI114356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Dallman M. F., Helms C., Levine J. D. Epinephrine exacerbates arthritis by an action at presynaptic B2-adrenoceptors. Neuroscience. 1990;34(2):521–523. doi: 10.1016/0306-4522(90)90160-6. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Levine J. D. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989 Jul;62(1):48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Chan A. K., Helms C., Basbaum A. I., Levine J. D. Increasing sympathetic nerve terminal-dependent plasma extravasation correlates with decreased arthritic joint injury in rats. Neuroscience. 1991;40(1):185–189. doi: 10.1016/0306-4522(91)90184-p. [DOI] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- De Souza E. B. Corticotropin-releasing factor receptors in the rat central nervous system: characterization and regional distribution. J Neurosci. 1987 Jan;7(1):88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim D. M., Robinson B. G., Pasieka K. B., Majzoub J. A. Differential regulation of corticotropin-releasing hormone mRNA in rat brain. Am J Physiol. 1990 Apr;258(4 Pt 1):E686–E692. doi: 10.1152/ajpendo.1990.258.4.E686. [DOI] [PubMed] [Google Scholar]
- Griffiths M. M., DeWitt C. W. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984 Jun;132(6):2830–2836. [PubMed] [Google Scholar]
- Hartung H. P., Wolters K., Toyka K. V. Substance P: binding properties and studies on cellular responses in guinea pig macrophages. J Immunol. 1986 May 15;136(10):3856–3863. [PubMed] [Google Scholar]
- Helke C. J., Krause J. E., Mantyh P. W., Couture R., Bannon M. J. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990 Apr 1;4(6):1606–1615. [PubMed] [Google Scholar]
- Herbort C. P., Chan C. C., Nussenblatt R. B. Endotoxin-induced uveitis in the rat: a hypothesis for preferential involvement of the anterior uvea. Curr Eye Res. 1990;9 (Suppl):119–124. doi: 10.3109/02713689008999430. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis K., Sano H., Redwine J., Listwak S., Wilder R. L., Chrousos G. P. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991 Oct 18;254(5030):421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Kavelaars A., Ballieux R. E., Heijnen C. J. The role of IL-1 in the corticotropin-releasing factor and arginine- vasopressin-induced secretion of immunoreactive beta-endorphin by human peripheral blood mononuclear cells. J Immunol. 1989 Apr 1;142(7):2338–2342. [PubMed] [Google Scholar]
- Kolasinski S. L., Haines K. A., Siegel E. L., Cronstein B. N., Abramson S. B. Neuropeptides and inflammation. A somatostatin analog as a selective antagonist of neutrophil activation by substance P. Arthritis Rheum. 1992 Apr;35(4):369–375. doi: 10.1002/art.1780350402. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Rees R., Hukkanen M., Grönblad M., Tolvanen E., Gibson S. J., Polak J. M., Brewerton D. A. Nerves in inflammatory synovium: immunohistochemical observations on the adjuvant arthritis rat model. J Rheumatol. 1990 Dec;17(12):1586–1591. [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Capsaicin suppresses substance P-induced joint inflammation in the rat. Neurosci Lett. 1989 Oct 23;105(1-2):155–158. doi: 10.1016/0304-3940(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989 Nov;48(11):928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Moskowitz M. A., Basbaum A. I. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985 Aug;135(2 Suppl):843s–847s. [PubMed] [Google Scholar]
- Lotz M., Carson D. A., Vaughan J. H. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987 Feb 20;235(4791):893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- McGillis J. P., Park A., Rubin-Fletter P., Turck C., Dallman M. F., Payan D. G. Stimulation of rat B-lymphocyte proliferation by corticotropin-releasing factor. J Neurosci Res. 1989 Jul;23(3):346–352. doi: 10.1002/jnr.490230316. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Hynes M. A., Vigh S., Shally A. V., Petrusz P. Immunocytochemical localization of corticotropin releasing factor (CRF) in the rat spinal cord. Brain Res. 1983 Sep 26;275(2):373–377. doi: 10.1016/0006-8993(83)91001-6. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Goetzl E. J. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983 Oct;131(4):1613–1615. [PubMed] [Google Scholar]
- Payan D. G., McGillis J. P., Goetzl E. J. Neuroimmunology. Adv Immunol. 1986;39:299–323. doi: 10.1016/s0065-2776(08)60353-3. [DOI] [PubMed] [Google Scholar]
- Sano H., Forough R., Maier J. A., Case J. P., Jackson A., Engleka K., Maciag T., Wilder R. L. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990 Apr;110(4):1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Hla T., Maier J. A., Crofford L. J., Case J. P., Maciag T., Wilder R. L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992 Jan;89(1):97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A. Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol. 1984 May;82(1):217–222. doi: 10.1111/j.1476-5381.1984.tb16461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmeyer T. H., Avgerinos P. C., Gold P. W., Gallucci W. T., Tomai T. P., Cutler G. B., Jr, Loriaux D. L., Chrousos G. P. Human corticotropin-releasing factor in man: pharmacokinetic properties and dose-response of plasma adrenocorticotropin and cortisol secretion. J Clin Endocrinol Metab. 1984 Dec;59(6):1103–1108. doi: 10.1210/jcem-59-6-1103. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Fudenberg H. H. Binding of [125I]corticotropin releasing factor to blood immunocytes and its reduction in Alzheimer's disease. Immunol Lett. 1988 May;18(1):5–8. doi: 10.1016/0165-2478(88)90061-2. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Leu S. J. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett. 1990 Dec 11;120(2):151–154. doi: 10.1016/0304-3940(90)90025-5. [DOI] [PubMed] [Google Scholar]
- Singh V. K. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol. 1989 Aug;23(3):257–262. doi: 10.1016/0165-5728(89)90058-1. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Hamill G. S., Jacobowitz D. M. Capsaicin depletes corticotropin-releasing factor-like immunoreactive neurons in the rat spinal cord and medulla oblongata. Neuroendocrinology. 1984 Jun;38(6):514–517. doi: 10.1159/000123942. [DOI] [PubMed] [Google Scholar]
- Stephanou A., Jessop D. S., Knight R. A., Lightman S. L. Corticotrophin-releasing factor-like immunoreactivity and mRNA in human leukocytes. Brain Behav Immun. 1990 Mar;4(1):67–73. doi: 10.1016/0889-1591(90)90007-d. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Glowa J. R., Smith M. A., Calogero A. E., Listwak S. J., Aksentijevich S., Chrousos G. P., Wilder R. L., Gold P. W. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992 Jan 20;570(1-2):54–60. doi: 10.1016/0006-8993(92)90563-o. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Young W. S., 3rd, Bernardini R., Calogero A. E., Chrousos G. P., Gold P. W., Wilder R. L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Tomori N., Tozawa F., Demura H., Shizume K., Mouri T., Miura Y., Sasano N. Immunoreactive corticotropin and corticotropin-releasing factor in human hypothalamus, adrenal, lung cancer, and pheochromocytoma. J Clin Endocrinol Metab. 1984 May;58(5):919–924. doi: 10.1210/jcem-58-5-919. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Harwood J. P., Millan M. A., Chrousos G. P., Goldstein D. S., Zimlichman R., Catt K. J., Aguilera G. Functional corticotropin releasing factor receptors in the primate peripheral sympathetic nervous system. Nature. 1986 Jan 9;319(6049):147–150. doi: 10.1038/319147a0. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Offner H., Reshef T., Fritz R., Chou C. H., Cohen I. R. Specificity of T lymphocyte lines for peptides of myelin basic protein. J Immunol. 1985 Jul;135(1):229–233. [PubMed] [Google Scholar]
- Webster E. L., De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification, autoradiographic localization, and regulation by divalent cations and guanine nucleotides. Endocrinology. 1988 Feb;122(2):609–617. doi: 10.1210/endo-122-2-609. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Tracey D. E., Jutila M. A., Wolfe S. A., Jr, De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990 Jul;127(1):440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Hansen C. Thymus-dependent and -independent regulation of Ia antigen expression in situ by cells in the synovium of rats with streptococcal cell wall-induced arthritis. Differences in site and intensity of expression in euthymic, athymic, and cyclosporin A-treated LEW and F344 rats. J Clin Invest. 1987 Apr;79(4):1160–1171. doi: 10.1172/JCI112933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
















