Highlights
-
•
This is the first report of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a woman with pseudomyxoma peritonei.
-
•
PIPAC achieved clinical and histological disease remission.
-
•
PIPAC with cisplatin and doxorubicin may be effective in pseudomyxoma peritonei.
Keywords: Pseudomyxoma peritonei, Intraperitoneal chemotherapy, Intraabdominal, High pressure, Cisplatin, Doxorubicin
Introduction
Pseudomyxoma peritonei (PMP) is a rare variant of adenocarcinoma with a protracted but ultimately fatal course of disease. PMP has an incidence of 2/1 000 000/year and is characterized by mucinous obstructive ascites and pools of mucin within the abdominal cavity (Smeenk et al., 2008). PMP arises from low- or high-grade mucinous neoplasms of the appendix in > 80% of cases, but ovarian involvement is common in females (Smeenk et al., 2008, Buell-Gutbrod and Gwin, 2013). The distinction between PMP from appendiceal origin and PMP from ovarian origin is often difficult (Buell-Gutbrod and Gwin, 2013), but true ovarian primary carcinoma of pure mucinous morphology presenting as PMP has been described as a distinct clinical entity characterized by low grade and low stage at presentation in the majority of cases (Leen and Singh, 2012). Patients with PMP usually undergo repeated surgical interventions with cytoreductive surgery and removal of myxomatous masses with or without subsequent systemic chemotherapy (Leen and Singh, 2012, Varona et al., 2005). Combination treatment modalities with cytoreductive surgery and intraperitoneal chemotherapy (IPC) have been increasingly used as a potentially valuable therapeutic intervention in women with PMP. In a recent systematic review and meta-analysis of 15 studies, for example, McBride et al. report mean 3-year, 5-year, and 10-year survival rates of 77%, 76%, and 57%, respectively, in women after various combinations of cytoreductive surgery and IPC (McBride et al., 2013). A variant of IPC, hyperthermic intraperitoneal chemoperfusion (HIPEC), with and without peritonectomy has also been successfully applied to women with PMP with sustained treatment responses (Baratti et al., 2008). However, combining cytoreductive surgery with IPC/HIPEC has a high morbidity and a considerable mortality. For example, Saxena et al. reported a 3% mortality rate and grade 3 and 4 morbidity rates of 23% and 22%, respectively, in a series of 145 women with PMP treated with cytoreductive surgery and IPC (Saxena et al., 2010). Also, the potential of IPC to improve survival in women with PMP may be hampered by pharmacological limitations such as poor drug distribution within the abdominal cavity and poor drug penetration into peritoneal nodules (Dedrick and Flessner, 1997). Therefore, it is reasonable to investigate less morbid and potentially more effective IPC concepts overcoming these limitations.
One way to overcome the pharmacokinetic limitations of IPC is to apply IPC as a pressurized aerosol taking advantage of the physical properties of gas and pressure. This approach is based on the observation that application of chemotherapy under pressure significantly enhances tumor drug uptake (Dedrick and Flessner, 1997). Pressurized intraperitoneal aerosol chemotherapy (PIPAC) may therefore be a way to increase the distribution and infiltration depth of intraabdominal chemotherapy. As proof of concept, PIPAC achieved a superior distribution on the peritoneum and a better penetration into peritoneal nodules compared to conventional IPC in an ex vivo model (Reymond et al., 2000, Solass et al., 2012). Based on these experimental data, PIPAC has been tested in humans with recurrent peritoneal carcinomatosis (Solass et al., 2014, Tempfer et al., 2014). In these preliminary applications, PIPAC induced regression of peritoneal nodules with limited hepatic and renal toxicity. In addition, the procedure has been shown to be safe regarding occupational health aspects such as operation theater air contamination with aerosol chemotherapy particles (Solass et al., 2013).
As of yet, there are no data describing PIPAC in patients with PMP. Here we report the case of repeated successful applications of PIPAC with cisplatin and doxorubicin in a patient with recurrent PMP and a long history of repeated surgical interventions.
Case Report
We present the case of a 62 year old woman with PMP and a history of repeated surgical interventions. PMP was first diagnosed in 2004. Initially, the patient underwent laparotomy with hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and removal of mucinous masses. Between 2004 and 2013, laparoscopy and laparotomy were performed 9 times with repeated tumor debulking and removal of mucinous ascites. The patient presented to our clinic in January 2014 with rising CA 125 serum levels (109 U/ml) and suspected disease progression on abdominal sonography. The patient was treated within a compassionate use program, approved by the ethics committee of the Ruhr University Bochum, Bochum, Germany. The treatment was not for research purposes.
The patient underwent three courses q 28–42 days of PIPAC with cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 at 12 mm Hg and 37 °C for 30 min. The PIPAC procedure was performed as described before (Solass et al., 2014). Briefly, after insufflation of a 12 mm Hg CO2 pneumoperitoneum, two balloon safety trocars (5 and 12 mm, Applied Medical, Düsseldorf', Germany) were inserted into the abdominal wall in an operating room equipped with laminar airflow. Video documentation was started and biopsies were taken for histologic confirmation of PMP during the first procedure and the following procedures in order to ascertain tumor regression. Ascites volume was documented and ascites was removed. Then, a nebulizer (Reger Medizintechnik, Rottweil, Germany) was connected to an intravenous high-pressure injector (Injektron 82M, MedTron, Saarbruecken, Germany) and inserted into the abdomen. The tightness of the abdomen was documented via a zero-flow of CO2. A pressurized aerosol containing cisplatin at a dose of 7.5 mg/m2 body surface in a 150 ml NaCl 0.9% solution followed by doxorubicin at a dose of 1.5 mg/m2 body surface in a 50 ml NaCl 0.9% solution was applied via nebulizer and injector. The dosage used in this cohort study was based on previous clinical experience in patients with peritoneal carcinomatosis treated with PIPAC in this dosage and formulation (Solass et al., 2014, Tempfer et al., 2014). Injection parameters were set at a flow rate of 30 ml/min and a maximum upstream pressure of 200 psi in the high-pressure injector. The injection was remote-controlled to minimize personnel exposure. The therapeutic capnoperitoneum was maintained for 30 min at a temperature of 37 °C. Then, the chemotherapy aerosol was exsufflated via a closed line over two sequential microparticle filters into the airwaste system of the hospital. Finally, trocars were retracted and laparoscopy was ended. No drainage of the abdomen was applied. The PIPAC procedure was repeated after 4–6 weeks. The peritoneal carcinomatosis index (PCI) was determined according to Sugarbaker, based on lesion size and distribution (Mazzei et al., 2013). Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (Common Terminology Criteria for Adverse Events (CTCAE)).
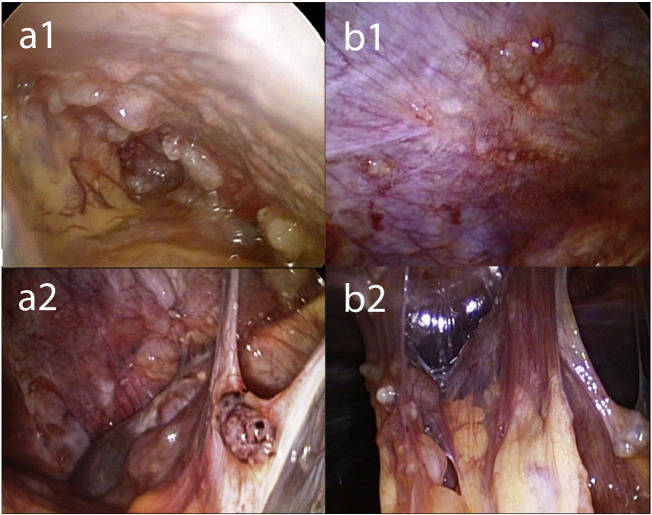
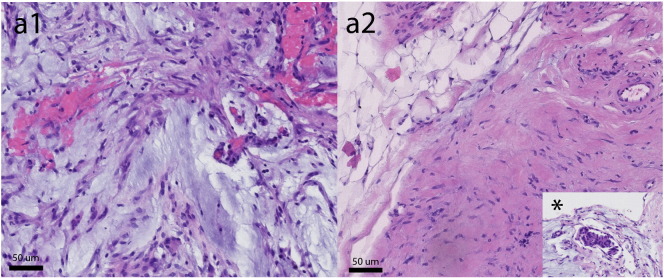
Fig. 1 shows snapshots of the video-laparoscopy during the first and third PIPACs demonstrating PMP tumor nodules. After therapy, mucus had largely disappeared from the abdomen, and ascites had disappeared. The figures are snapshots taken during different PIPAC procedures. Thus, the selection was subjective. We aimed to take the snapshot pairs a1 and b1 as well as a2 and b2 from the same quadrants (upper left and upper right, respectively), exemplifying minimization and scarring of tumor nodules. Specifically, nodular sclerosis of peritoneal nodules was observed, as well as reticular scarring of the visceral and the parietal peritoneum. Objective tumor response was noted in this patient after the third PIPAC, defined as tumor regression on histology and PCI improvement on repeated video-laparoscopy. Specifically, major pathological tumor response with rare residual cancer cells scattered throughout the fibrosis was noted in a tissue specimen taken during the third PIPAC. Fig. 2 demonstrates histopathological specimens taken during PIPAC #1 and PIPAC #3 confirming PMP. Before the first PIPAC, histology showed peritoneal infiltration by a poorly differentiated adenocarcinoma with extracellular mucus deposits with isolated tumor cells in 4/5 biopsies. 2/3 biopsies taken after therapy were tumor free, showing fibrosis with acute and chronic inflammation. The third biopsy revealed localized peritoneal infiltration by a highly regressive mucinous adenocarcinoma. The PCI was 24 (1st PIPAC), 21 (2nd PIPAC), and 22 (3rd PIPAC). The treatment was well tolerated. CTCAE events grade 1 (nausea) and grade 2 (abdominal pain) were noted within 72 h after the first, second, and third PIPACs. No CTCAE event grade ≥ 3 was observed. There was no hematologic toxicity noted on red and white blood cell counts performed 7 days after each PIPAC. With a follow-up of 6 months, the patient is well and alive and required no further treatment so far. CA 125 was down to 34 U/ml.
Fig. 1.
Intraoperative findings (macroscopy) before PIPAC therapy (panels a1, a2) and 6 weeks after PIPAC #2 (panels b1, b2). After therapy, mucus had largely disappeared from the abdomen, and ascites had disappeared. Nodular sclerosis of peritoneal nodules was observed, as well as reticular scarring of the visceral and the parietal peritoneum. Total peritoneal carcinomatosis index (PCI) remained constant, since quantitative parameters (the number and the size of tumor nodes) did not change significantly, although the qualitative aspect of tumor nodes was modified after therapy.
Fig. 2.
Intraoperative findings (microscopy) before PIPAC #1 (panel a1) and 6 weeks after PIPAC #2 (panel a2) confirming pseudomyxoma peritonei (PMP). Before PIPAC, histology showed peritoneal infiltration by a poorly differentiated adenocarcinoma with extracellular mucus deposits with isolated tumor cells in 4/5 biopsies. After therapy, 2/3 biopsies were tumor free, showing fibrosis with acute and chronic inflammation. The third biopsy revealed localized peritoneal infiltration by a highly regressive mucinous adenocarcinoma (panel a2, inset *).
Comment
PMP is a rare disease, which is difficult to treat due to its protracted course requiring repeated interventions (Smeenk et al., 2008, Buell-Gutbrod and Gwin, 2013). Cytoreductive surgery and IPC with or without hyperthermia are the mainstay of treatment (McBride et al., 2013, Saxena et al., 2010). In an effort to improve the efficacy of IPC, PIPAC has been developed and tested in experimental models (Reymond et al., 2000, Solass et al., 2012). Preliminary data in patients with peritoneal carcinomatosis demonstrated good tolerability and objective tumor response (Solass et al., 2014, Tempfer et al., 2014). PIPAC can be applied repeatedly, potentially complementing cytoreductive surgery and minimizing systemic side effects of chemotherapy. These properties make PIPAC an attractive therapeutic tool in patients with PMP, because repeated treatments are typically required due to the protracted course of this disease. In addition, reducing the morbidity associated with cytoreductive surgery and IPC may be a way to improve the quality of life of patients with PMP. We present the case of a woman with PMP and a history of repeated surgical interventions. She was treated with three courses q 28–42 days of PIPAC with cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 at 12 mm Hg and 37 °C for 30 min. Major objective tumor response was noted, defined as tumor regression on histology and decline in CA 125. The treatment was well tolerated. With a follow-up of 6 months, the patient is well and alive and required no further treatment.
The choice of the cytotoxic drugs and the dosage of these drugs was empirical, based on previous clinical experience in patients with ovarian cancer (Solass et al., 2014, Tempfer et al., 2014) rather than a formal phase I dose escalation trial. A phase I trial exploring higher dosages of PIPAC with cisplatin and doxorubicin is under way and will define the maximum tolerable dose of this combination of chemotherapeutic agents. This trial is registered with EudraCT, number 2014-001034-28.
Based on this experience, we propose to further explore PIPAC as a new and potential additional treatment modality in patients with PMP. Due to the rarity of PMP, we invite colleagues to join a multicenter effort to assess PIPAC in a prospective phase II efficacy study. In summary, PIPAC is a new form of IPC, which can be applied repeatedly and may become a complementary or alternative treatment to repeated surgery and IPC in patients with PMP.
Conflict of Interest Statement
One of the authors (MAR) discloses that he is holding a patent of the high-pressure device used to deliver the intraperitoneal chemotherapy described in this paper and that he has received royalties from Reger Medizintechnik GmbH, Rottweil, Germany. The other authors have no conflicts of interest.
References
- Baratti D., Kusamura S., Nonaka D., Langer M., Andreola S., Favaro M., Gavazzi C., Laterza B., Deraco M. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann. Surg. Oncol. 2008;15(2):526–534. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- Buell-Gutbrod R., Gwin K. Pathologic diagnosis, origin, and natural history of pseudomyxoma peritonei. Am. Soc. Clin. Oncol. Educ. Book. 2013;221–5 doi: 10.14694/EdBook_AM.2013.33.221. [DOI] [PubMed] [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) version 4.0; published: May 28, 2009 (v4.03: June 14, 2010); U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute
- Dedrick R.L., Flessner M.F. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J. Natl. Cancer Inst. 1997;89:480–487. doi: 10.1093/jnci/89.7.480. [DOI] [PubMed] [Google Scholar]
- Leen S.L., Singh N. Pathology of primary and metastatic mucinous ovarian neoplasms. J. Clin. Pathol. 2012;65(7):591–595. doi: 10.1136/jclinpath-2011-200162. [DOI] [PubMed] [Google Scholar]
- Mazzei M.A., Khader L., Cirigliano A., Cioffi Squitieri N., Guerrini S., Forzoni B., Marrelli D., Roviello F., Mazzei F.G., Volterrani L. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC) Abdom. Imaging. 2013;38(6):1422–1430. doi: 10.1007/s00261-013-0013-9. [DOI] [PubMed] [Google Scholar]
- McBride K., McFadden D., Osler T. Improved survival of patients with pseudomyxoma peritonei receiving intraperitoneal chemotherapy with cytoreductive surgery: a systematic review and meta-analysis. J. Surg. Res. 2013;183(1):246–252. doi: 10.1016/j.jss.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Reymond M.A., Hu B., Garcia A. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg. Endosc. 2000;14:51–55. doi: 10.1007/s004649900010. [DOI] [PubMed] [Google Scholar]
- Saxena A., Yan T.D., Chua T.C., Morris D.L. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann. Surg. Oncol. 2010;17(5):1291–1301. doi: 10.1245/s10434-009-0875-9. [DOI] [PubMed] [Google Scholar]
- Smeenk R.M., van Velthuysen M.L., Verwaal V.J., Zoetmulder F.A. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur. J. Surg. Oncol. 2008;34(2):196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Solass W., Herbette A., Schwarz T., Hetzel A., Sun J.S., Dutreix M., Reymond M.A. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg. Endosc. 2012;26(3):847–852. doi: 10.1007/s00464-011-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solass W., Giger-Pabst U., Zieren J., Reymond M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann. Surg. Oncol. 2013;20(11):3504–3511. doi: 10.1245/s10434-013-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solass W., Kerb R., Muerdter T., Giger U., Strumberg D., Tempfer C., Zieren J., Schwab M., Reymond Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann. Surg. Oncol. 2014;21(2):553–559. doi: 10.1245/s10434-013-3213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempfer C.B., Celik I., Solass W., Buerkle B., Pabst U.G., Zieren J., Strumberg D., Reymond M.A. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol. Oncol. 2014;132(2):307–311. doi: 10.1016/j.ygyno.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Varona J.F., Guerra J.M., Salamanca J., Colina F., Lopez G., Morales M. Pseudomyxoma peritonei: a clinicopathologic analysis and follow-up of 21 patients. Hepatogastroenterology. 2005;52(63):812–816. [PubMed] [Google Scholar]