Abstract
Endothelial thrombomodulin (TM) plays a critical role in hemostasis as a cofactor for thrombin-dependent formation of activated protein C, a potent anticoagulant. Chloramine T, H2O2, or hypochlorous acid generated from H2O2 by myeloperoxidase rapidly destroy 75-90% of TM cofactor activity. Activated PMN, the primary in vivo source of biological oxidants, also rapidly inactivate TM. Oxidation of TM by PMN is inhibited by diphenylene iodonium, an inhibitor of NADPH oxidase. Both Met291 and Met388 in the six epidermal growth factor-like repeat domain are oxidized; however, only substitutions of Met388 lead to TM analogues that resist oxidative inactivation. We suggest that in inflamed tissues activated PMN may inactivate TM and demonstrate further evidence of the interaction between the inflammatory process and induction of thrombotic potential.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Edgington T. S. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J Biol Chem. 1988 May 25;263(15):7007–7015. [PubMed] [Google Scholar]
- Altieri D. C., Etingin O. R., Fair D. S., Brunck T. K., Geltosky J. E., Hajjar D. P., Edgington T. S. Structurally homologous ligand binding of integrin Mac-1 and viral glycoprotein C receptors. Science. 1991 Nov 22;254(5035):1200–1202. doi: 10.1126/science.1957171. [DOI] [PubMed] [Google Scholar]
- Altieri D. C., Morrissey J. H., Edgington T. S. Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation protease cascade. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7462–7466. doi: 10.1073/pnas.85.20.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Szot S., Venkatasubramanian K., Schiffmann E. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980 Apr;124(4):2020–2026. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. M., Rosenberg R. D. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988 Dec;8(12):5588–5592. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R. The inhibitory effects of some iodonium compounds on the superoxide generating system of neutrophils and their failure to inhibit diaphorase activity. Biochem Pharmacol. 1987 Feb 15;36(4):489–493. doi: 10.1016/0006-2952(87)90356-x. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991 Jul 12;66(1):49–61. [PubMed] [Google Scholar]
- Dittman W. A., Majerus P. W. Sequence of a cDNA for mouse thrombomodulin and comparison of the predicted mouse and human amino acid sequences. Nucleic Acids Res. 1989 Jan 25;17(2):802–802. doi: 10.1093/nar/17.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon N. L., Carroll R. C., Esmon C. T. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983 Oct 25;258(20):12238–12242. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Friedman H. M., Hajjar D. P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990 May 18;61(4):657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Glaser C. B., Karic L., Fallat R. Isolation and characterization of alpha-1-antitrypsin from the Cohn fraction IV-I of human plasma. Prep Biochem. 1975;5(4):333–348. doi: 10.1080/00327487508061581. [DOI] [PubMed] [Google Scholar]
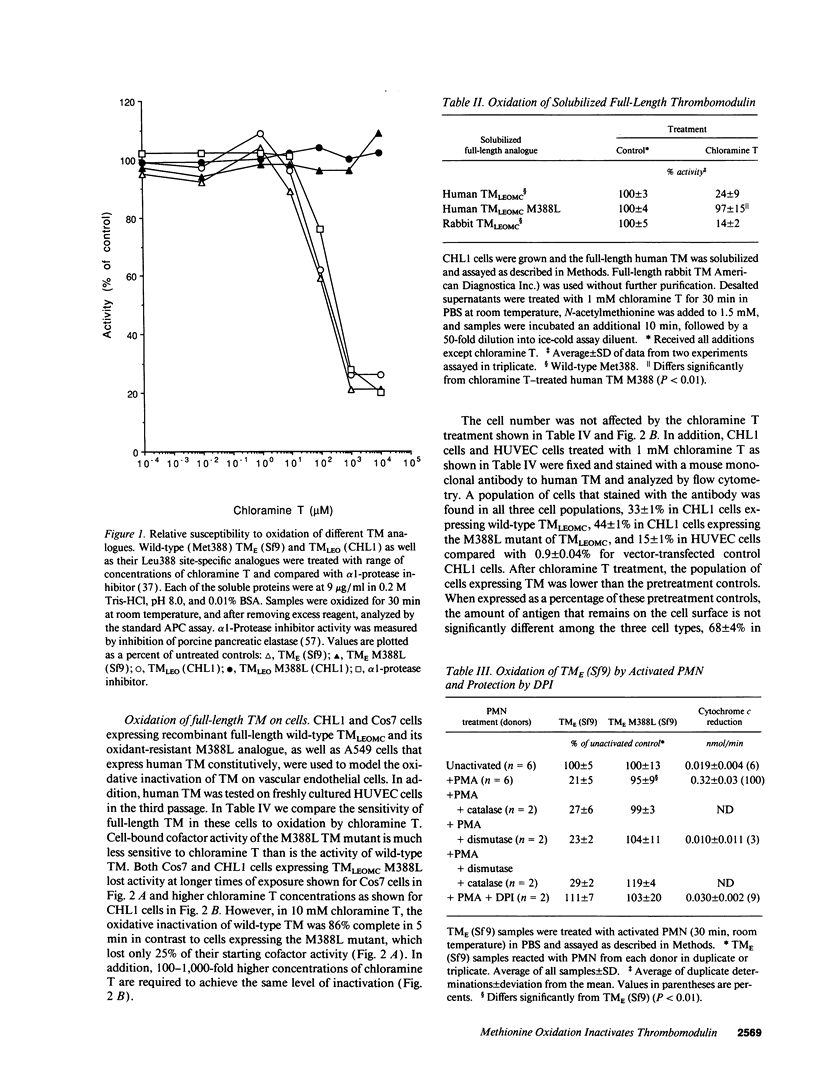
- Glaser C. B., Li C. H. Reaction of bovine growth hormone with hydrogen peroxide. Biochemistry. 1974 Feb 26;13(5):1044–1047. doi: 10.1021/bi00702a033. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hancock W. W., Tanaka K., Salem H. H., Tilney N. L., Atkins R. C., Kupiec-Weglinski J. W. TNF as a mediator of cardiac transplant rejection, including effects on the intragraft protein C/protein S/thrombomodulin pathway. Transplant Proc. 1991 Feb;23(1 Pt 1):235–237. [PubMed] [Google Scholar]
- Harvath L., Aksamit R. R. Oxidized N-formylmethionyl-leucyl-phenylalanine: effect on the activation of human monocyte and neutrophil chemotaxis and superoxide production. J Immunol. 1984 Sep;133(3):1471–1476. [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., Fritze L., Soff G., Rosenberg R. D. Human thrombomodulin gene is intron depleted: nucleic acid sequences of the cDNA and gene predict protein structure and suggest sites of regulatory control. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6425–6429. doi: 10.1073/pnas.84.18.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., VanDeWater L., Rosenberg R. D. Characterization of a thrombomodulin cDNA reveals structural similarity to the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8834–8838. doi: 10.1073/pnas.83.23.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
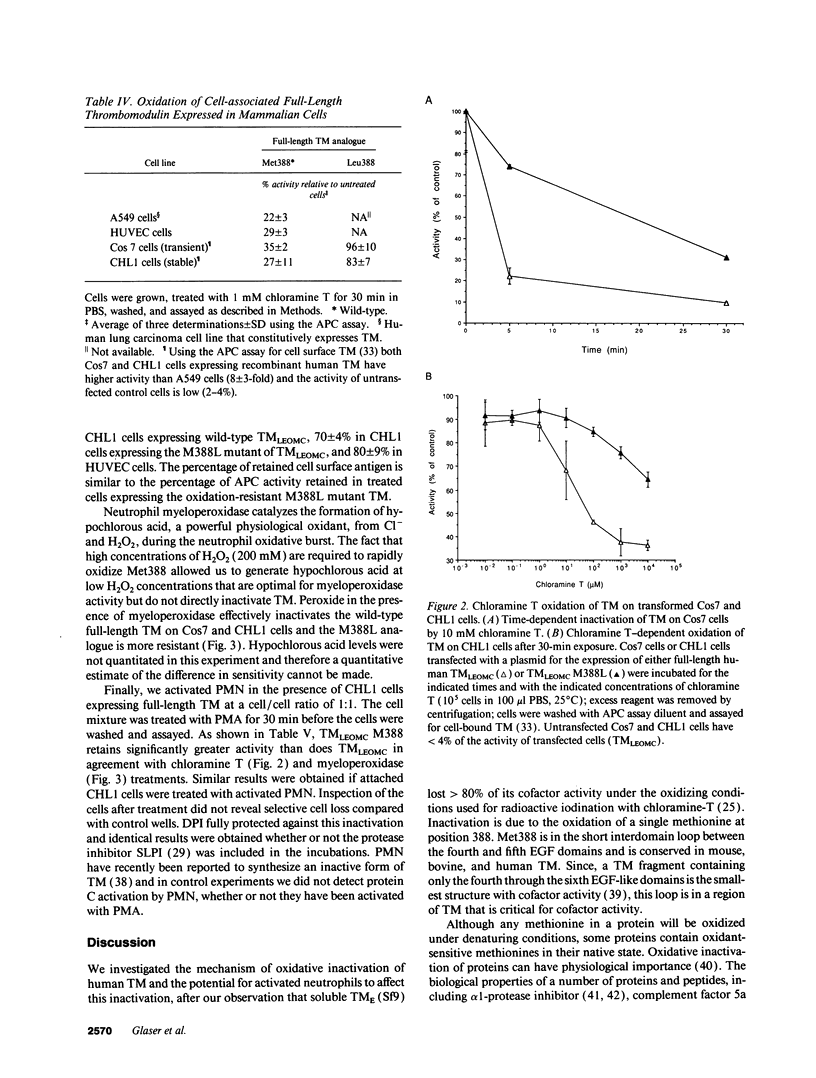
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Loskutoff D. J. Inactivation of plasminogen activator inhibitor by oxidants. Biochemistry. 1986 Oct 21;25(21):6351–6355. doi: 10.1021/bi00369a001. [DOI] [PubMed] [Google Scholar]
- Lentz S. R., Tsiang M., Sadler J. E. Regulation of thrombomodulin by tumor necrosis factor-alpha: comparison of transcriptional and posttranscriptional mechanisms. Blood. 1991 Feb 1;77(3):542–550. [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Maruyama I., Majerus P. W. The turnover of thrombin-thrombomodulin complex in cultured human umbilical vein endothelial cells and A549 lung cancer cells. Endocytosis and degradation of thrombin. J Biol Chem. 1985 Dec 15;260(29):15432–15438. [PubMed] [Google Scholar]
- McCachren S. S., Diggs J., Weinberg J. B., Dittman W. A. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood. 1991 Dec 15;78(12):3128–3132. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987 May 15;46(7):2402–2406. [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates A. M., Salem H. H. The binding and regulation of protein S by neutrophils. Blood Coagul Fibrinolysis. 1991 Oct;2(5):601–607. doi: 10.1097/00001721-199110000-00003. [DOI] [PubMed] [Google Scholar]
- Okajima K., Yang W. P., Okabe H., Inoue M., Takatsuki K. Role of leukocytes in the activation of intravascular coagulation in patients with septicemia. Am J Hematol. 1991 Apr;36(4):265–271. doi: 10.1002/ajh.2830360408. [DOI] [PubMed] [Google Scholar]
- Parkinson J. F., Grinnell B. W., Moore R. E., Hoskins J., Vlahos C. J., Bang N. U. Stable expression of a secretable deletion mutant of recombinant human thrombomodulin in mammalian cells. J Biol Chem. 1990 Jul 25;265(21):12602–12610. [PubMed] [Google Scholar]
- Parkinson J. F., Nagashima M., Kuhn I., Leonard J., Morser J. Structure-function studies of the epidermal growth factor domains of human thrombomodulin. Biochem Biophys Res Commun. 1992 Jun 15;185(2):567–576. doi: 10.1016/0006-291x(92)91662-a. [DOI] [PubMed] [Google Scholar]
- Rinder H. M., Bonan J. L., Rinder C. S., Ault K. A., Smith B. R. Activated and unactivated platelet adhesion to monocytes and neutrophils. Blood. 1991 Oct 1;78(7):1760–1769. [PubMed] [Google Scholar]
- Stief T. W., Aab A., Heimburger N. Oxidative inactivation of purified human alpha-2-antiplasmin, antithrombin III, and C1-inhibitor. Thromb Res. 1988 Mar 15;49(6):581–589. doi: 10.1016/0049-3848(88)90255-1. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Fasehun O. A., Kwon N. S., Gross S. S., Gonzalez J. A., Levi R., Nathan C. F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991 Jan;5(1):98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Stenflo J., Dahlbäck B., Teodorsson B. Inactivation of human coagulation factor V by activated protein C. J Biol Chem. 1983 Feb 10;258(3):1914–1920. [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wen D. Z., Dittman W. A., Ye R. D., Deaven L. L., Majerus P. W., Sadler J. E. Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry. 1987 Jul 14;26(14):4350–4357. doi: 10.1021/bi00388a025. [DOI] [PubMed] [Google Scholar]
- Zushi M., Gomi K., Honda G., Kondo S., Yamamoto S., Hayashi T., Suzuki K. Aspartic acid 349 in the fourth epidermal growth factor-like structure of human thrombomodulin plays a role in its Ca(2+)-mediated binding to protein C. J Biol Chem. 1991 Oct 25;266(30):19886–19889. [PubMed] [Google Scholar]
- Zushi M., Gomi K., Yamamoto S., Maruyama I., Hayashi T., Suzuki K. The last three consecutive epidermal growth factor-like structures of human thrombomodulin comprise the minimum functional domain for protein C-activating cofactor activity and anticoagulant activity. J Biol Chem. 1989 Jun 25;264(18):10351–10353. [PubMed] [Google Scholar]