Abstract
Background
The treatment of locally recurrent endometrial cancer is based on limited evidence. The standard treatment is radiotherapy (RT) which is effective for local control and the effect has been documented in prospective studies. Investigations of surgical treatment (ST) of recurrences are few and limited to previously irradiated patients or patients with advanced disease. Investigation of surgical treatment for isolated vaginal vault recurrence is practically nonexistent. The aim of this study is to evaluate the efficacy of RT and ST in a non-irradiated group with recurrent endometrial cancer limited to the vaginal vault.
Methods
Patients treated for recurrent endometrial cancer at Odense University Hospital, Denmark between 2003 and 2012 were identified, n = 118. Thirty-three patients had an isolated vaginal vault recurrence and were treated with either RT, ST or both.
Re-recurrence rates and survival rates were calculated at 2 year follow-up using Fishers exact test.
Results
Twenty-six patients were treated with RT, 5 with ST, 2 with both. The mean (SD) follow-up-time was 4.4 years (2.99) (RT) and 3.9 years (0.90) (ST). Two year re-recurrence rates were 40% (RT) (95 CI 9.2–48%) and 0% (ST) (95 CI 0–60%). Two-year survival rates were 83% (RT) (95 CI 71–100%) and 100% (ST) (95 CI 40–100%) ST had one re-recurrence at 2.3 years.
Conclusion
This study indicates that ST is an appropriate treatment for locally recurrent endometrial cancer. Our study involves a limited number of patients and is made retrospectively, therefore prospective and ideally randomized trials evaluating both survival and complications are warranted.
Keywords: Endometrial, Cancer, Vault, Recurrence, Radiotherapy, Surgery
Highlights
-
•
Investigate radiotherapy and surgical treatment of vaginal vault recurrences of endometrial cancer.
-
•
All patients were naïve to radiotherapy and 5 were treated with surgery and 26 with radiotherapy.
-
•
Rates of survival and re-recurrence were equivalent though in favor of surgery.
Introduction
Worldwide endometrial cancer is the fifth most common cancer in women. Furthermore, it is the most common gynecologic cancer in developed countries (Cancer W.I.A.F.R.O., 2012). The continuing rise in incidence is most likely explained by increased fat consumption and obesity in developed countries and previous use of unopposed estrogens. Both are well recognized risk factors for endometrial cancer (Amant et al., 2005).
Endometrial cancer is often detected in early stage because of abnormal uterine bleeding which is the most frequent symptom. Around 5–10% of women with this symptom are diagnosed with endometrial cancer and the risk increases with age and other additional risk factors (Gredmark et al., 1995).
Women diagnosed with endometrial cancer generally have a favorable prognosis. Seventy-five percent are diagnosed in FIGO stage I and have a 5-year survival of 85%. Women diagnosed in FIGO stage II have a 5-year survival of 75%, 40% for FIGO stage III and 20% for FIGO stage IV (Amant et al., 2005, Danish Gynecological Cancer Group D, 2010). Approximately 6–13% of all patients with endometrial cancer will develop recurrent disease. The majority of the recurrences occur during the first 3 years after a primary disease and most of these are located in the vaginal vault (Creutzberg et al., 2011, Huh et al., 2007).
In Denmark primary disease is surgically treated according to national guidelines with total hysterectomy with bilateral salpingo-oophorectomy including cytological examination of the peritoneal fluid. In grades 1 and 2 endometrioid adenocarcinomas (EAC) peroperative evaluation of myometrial invasion determines if lymph node excision is performed (performed if invasion exceeds 50%). In grade 3 EAC and type 2 histology (serous, clear cell, undifferentiated carcinomas and carcinosarcomas) it is performed without evaluation of myometrial invasion. In stage II radical hysterectomy is performed. Patients with type 2 histology furthermore have the omentum removed. FIGO stages III–IV are generally treated with adjuvant chemotherapy (Danish Gynecological Cancer Group D, 2010). The patients in our department are offered follow-up three times/year during the first two years, then twice a year on the third year after treatment for low risk primary cancer and for further 3 years in other groups (Danish Gynecological Cancer Group D, 2010).
Recurrent disease is most often treated by radiotherapy which is in accordance with recommendations in the international literature (van Wijk et al., 2009). Surgical extirpation is in addition a valid and well recognized treatment albeit not as common (van Wijk et al., 2009). To the best of our knowledge publications describing the evidence for surgical treatment of isolated vaginal vault recurrences of endometrial cancer in non-irradiated patients do not exist.
At our department, the Department of Gynecology and Obstetrics at Odense University Hospital (OUH), the treatment of recurrent disease has been either surgery or radiotherapy at the discretion of the treating gynae-oncologist.
In order to investigate potential differences in the outcome of radiotherapy versus surgical treatment, we conducted a retrospective cohort study based on patients treated for recurrent disease in the vaginal vault.
Materials and method
All the patients treated for endometrial cancer at Odense University Hospital, Odense, Denmark between January 1st 2003 and December 31st 2012 were identified in the electronic patient data system FPAS, by searching for the diagnosis code DC549 cancer corporis uteri (n = 896).
Patient records and pathology reports were examined to identify those with recurrence of endometrial cancer.
Records from patients found to have recurrent disease were examined further and the following data recorded; date of birth, date of death (or alive at follow up), date of primary cancer diagnosis, histological type and grade of primary cancer, primary and adjuvant treatment of primary cancer, FIGO stage (2009 revision), degree of myometrial invasion, number and location of malignant and non-malignant lymph nodes, and other metastases at the time of diagnosis. The date which the recurrence was diagnosed, location of recurrence, treatment plus neo-adjuvant and adjuvant treatments were recorded as were dates of re-recurrences along with their size and location. Furthermore, data regarding tumor size for the recurrences were collected.
For all included patients' dates of primary cancer diagnosis, recurrence and re-recurrence were verified by histological samples and pathology reports. Follow up was conducted on April 1st, 2014.
The patients included were selected by these criteria 1) recurrence of endometrial cancer in the period from January 1st 2003 to December 31st 2012 2) recurrence was local (vaginal vault or directly connected) and 3) treatment was started with curative intent, either surgically or with radiotherapy. Patients with distant metastases or recurrence on the pelvic sidewall were excluded.
The radiotherapy treatment consisted primarily of external beam radiation with a dosage of 50 Gy in 27 fractions and pulse dose rate brachytherapy with a dosage of 15 Gy in 3 treatments. Surgical treatment consisted of excision of tumor along with a border of tumor free tissue. There were no cases in which bladder or bowel resection was performed.
The palliative treatment chosen for the re-recurrences (n = 7) and not cured first recurrence (n = 2) in the radiotherapy group (all re-recurrences including re-recurrences at 2 year follow-op) consisted in three cases of endocrine treatment (aromatase inhibitors), one patient was in a too poor condition to receive any treatment, one patient declined any treatment, three patients received chemotherapy with Taxol and carboplatin, and in one case a patient received additional radiotherapy against lung metastasis. The single re-recurrence in the surgical treatment group was treated with Caelyx, a cytostatica of the topoisomerase inhibitor group.
For comparison of our results regarding re-recurrence rate and survival after surgical treatment of locally recurrent endometrial cancer, we conducted a PubMed search in September 2014 using these terms: endometrial cancer, endometrial carcinoma, uterine cancer, recurrence, vaginal vault, vaginal recurrence, pelvic exenteration, surgical treatment, and surgical resection.
The study was approved by the Danish Data Protection Agency.
Statistical analysis
As the number of included patients is only 33 with 5 in one group (surgery), 26 in the other group (radiotherapy) and 2 patients receiving combined treatment in a third group, we decided against elaborate survival analysis such as cox proportional hazards regression. The limited number of data would not be able to give statistically meaningful and valid results.
We conducted univariate analysis of risk factors using logrank test and Cox regression for continuous variables.
We decided on a descriptive approach presenting the data using Fisher's exact test to calculate re-recurrence and survival rates.
Re-recurrence rate and survival rates were calculated at 2 years of follow-up.
Results
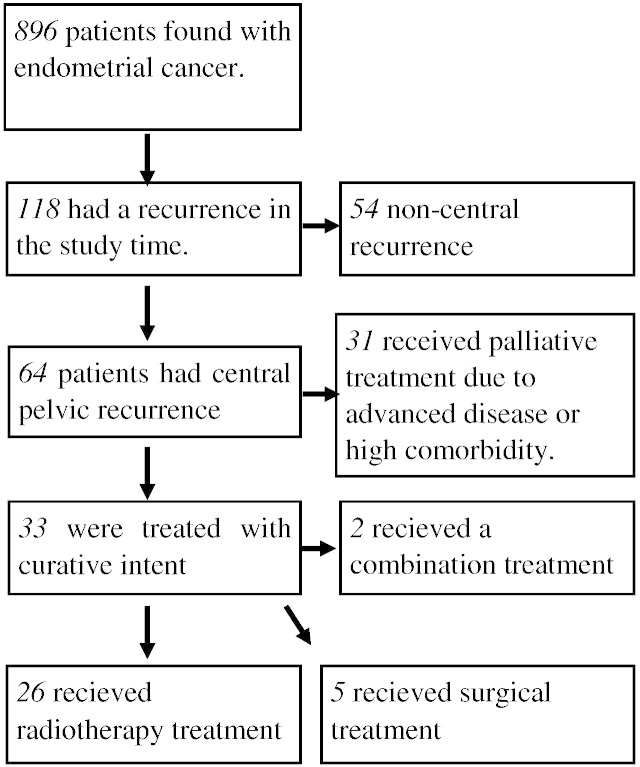
Fig. 1 presents the process of identifying the 33 patients included in the analysis. All the patients were treated with curative intent; 26 received radiotherapy, 5 received surgical treatment and 2 received a combination of treatment modalities.
Fig. 1.
Flow chart describing inclusion process.
Table 1 shows the number of all recurrences per year in the collected material, and in which year the included patients had a recurrence.
Table 1.
Number of recurrences treated at our hospital each year in the study period.
| Year | Recurrences (included in our study) |
|---|---|
| 2003 | 2 (2) |
| 2004 | 5 (1) |
| 2005 | 5 (1) |
| 2006 | 7 (4) |
| 2007 | 8 (1) |
| 2008 | 4 (1) |
| 2009 | 10 (3) |
| 2010 | 22 (5) |
| 2011 | 25 (7) |
| 2012 | 30 (8) |
Table 2 presents descriptive data on all 33 patients included in our study. Two year follow-up data was available for all but five patients in the radiotherapy group. These 5 patients are not included in the two year rates. At the two year follow-up the 21 patients in the radiotherapy group presented with the following outcomes; 15 patients had no re-recurrence and 4 patients had re-recurrence of which 2 died within the 2 year follow-up. Two patients never became free of disease, one of which died within the 2 year follow-up. Of the two patients in the combined group, one has been without re-recurrence while the other had incurable metastatic disease after 3.9 years.
Table 2.
Data set of included patient characteristics.
| Patient number | Follow-up time | Re-recurrence eventa | Death eventb | BMI | Differentation gradec | Myometrial invasiond | FIGO stagee | Tumor sizef | Ageg | Histological typeh |
|---|---|---|---|---|---|---|---|---|---|---|
| Radiotherapy treatment | ||||||||||
| 1 | 10.31 | 6.19 | 8.94 | 21.23 | 1 | − | 1A | + | 72 | ED |
| 2 | 9.35 | 3.11 | 7.23 | 27.51 | 2 | − | 1A | − | 80 | ED |
| 3 | 8.41 | – | – | n/a | 2 | + | 1B | − | 58 | ED |
| 4 | 7.76 | 0.88 | 2.79 | 27.14 | 2 | + | 1B | − | 79 | ED |
| 5 | 7.59 | – | 2.24 | 23.96 | 3 | + | 1B | − | 82 | ED |
| 6 | 11.09 | 2.97 | 3.08 | n/a | 1 | − | 1A | − | 78 | ED |
| 7 | 3.59 | – | – | 28.53 | 1 | + | 1B | − | 78 | ED |
| 8 | 4.51 | – | – | 22.66 | 2 | + | 1B | − | 77 | ED |
| 9 | 6.23 | 0.79 | 0.89 | n/a | 2 | + | 1B | + | 61 | ED |
| 10 | 7.7 | 2.11 | 7.11 | 27.95 | 1 | − | 1A | + | 78 | ED |
| 11 | 2.39 | – | – | 18.99 | 3 | + | 1B | + | 68 | ED |
| 12 | 1.53 | – | – | 23.62 | 2 | + | 1B | − | 69 | ED |
| 13 | 4.06 | 0a | 3.16 | 26.44 | 2 | + | 3C | − | 67 | ED |
| 14 | 1.93 | – | – | 57.16 | 1 | − | 1A | + | 58 | ED |
| 15 | 3.99 | 1.69 | 2.56 | 22.41 | 3 | + | 1B | − | 69 | CS |
| 16 | 2.46 | – | – | 25.31 | 1 | − | 1A | − | 62 | ED |
| 17 | 4.14 | – | – | 34.19 | 2 | − | 1A | + | 60 | ED |
| 18 | 2.4 | – | – | 22.32 | 3 | + | 1B | − | 60 | ED |
| 19 | 2.02 | – | – | 27.47 | 1 | + | 2 | + | 63 | ED |
| 20 | 2.31 | – | – | 36.73 | 3 | + | 3A | + | 81 | ED |
| 21 | 2.41 | – | – | n/a | 2 | − | 1A | − | 52 | ED |
| 22 | 1.62 | 0.79 | 1.62 | 25.64 | 1 | − | 2 | − | 73 | ED |
| 23 | 1.39 | – | – | 31.86 | 2 | − | 1A | − | 63 | ED |
| 24 | 2.07 | 0.91 | 1.89 | 35.16 | 3 | − | 1A | + | 72 | CC |
| 25 | 1.57 | 0.08 | – | 16 | 1 | − | 1A | − | 79 | ED |
| 26 | 2.22 | 0i | 1.11 | n/a | 3 | − | 1A | − | 68 | SA |
| Combined treatment | ||||||||||
| 1 | 3.93 | – | – | n/a | 2 | + | 1C | + | 60 | ED |
| 2 | 6.78 | 3.93 | – | n/a | 3 | + | 3A | + | 59 | ED |
| Surgical treatment | ||||||||||
| 1 | 4.61 | – | – | 22.22 | 2 | + | 1B | − | 62 | ED |
| 2 | 4.16 | – | – | n/a | 3 | + | 3C | − | 84 | CS |
| 3 | 4.89 | – | – | 22.77 | 2 | − | 1A | + | 80 | ED |
| 4 | 3.25 | 2.29 | – | 22.23 | 2 | + | 1B | + | 72 | ED |
| 5 | 2.47 | – | – | n/a | 2 | − | 1A | − | 80 | ED |
Re-recurrence in years after recurrence.
Death in years after recurrence, surviving patients as –.
Differentiation grade is histological differentiation of endometrioid adenocarcinoma grade 1 as high differentiation to grade 3 low differentiation.
At + invasion is through 50% of the wall, − less than.
FIGO stage follows the 2009 revision
Tumor size above 2 cm as +. Below 2 cm as −.
Age at the time of primary cancer diagnosis.
ED = endometrioid adenocarcinoma. CS = carcinosarcoma. CC = clear cell carcinoma. SA = serous adenocarcinoma.
Were never cured of their recurrence.
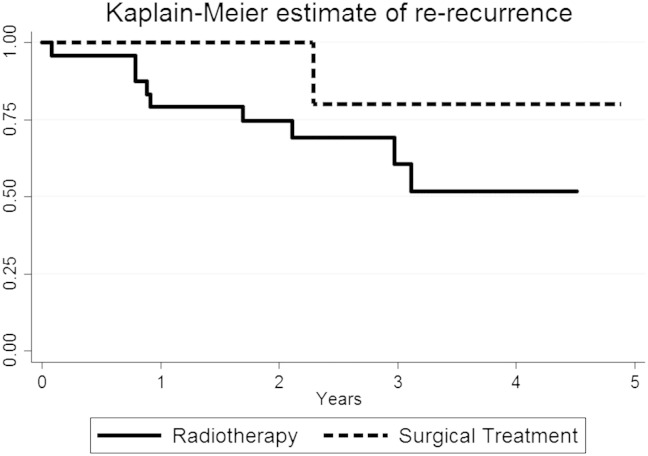
All death events were related to re-recurrence except for one patient who died from unrelated causes 2.2 years after successful treatment of recurrent endometrial cancer. None of the patients in the surgical group experienced re-recurrence or death during the two year follow-up, but a single re-recurrence was seen after 2.3 years. This includes deaths outside the 2 year follow-up.
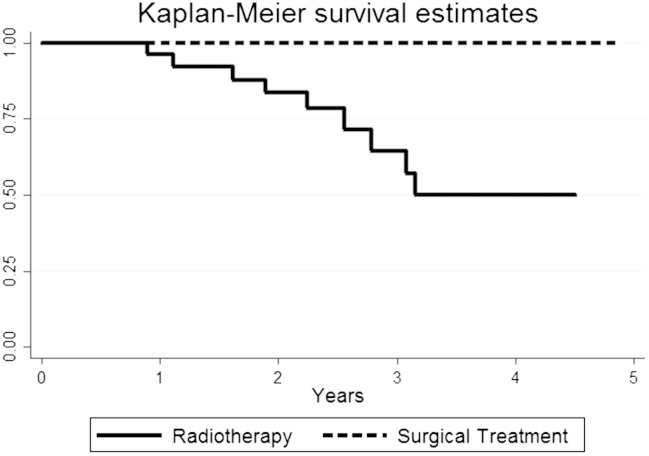
Fig. 2 shows a Kaplan–Meier estimate for re-recurrence and Fig. 3 shows a Kaplan–Meier estimate for survival.
Fig. 2.
Kaplan Meier estimate of re-recurrence-free rate of all included patients by treatment modality.
Fig. 3.
Kaplan Meier overall survival curves of all included patients by treatment modality.
Table 3 shows the descriptive statistics conducted, 2 year re-recurrence and survival rates and mean rates for recorded variables. Patient age in the different treatment groups is comparable. Body mass index, which is known for 21 of the patients in the radiotherapy group and 3 of the patients in the surgical group, was higher in the radiotherapy group. None of the patients in the surgical group received adjuvant chemotherapy treatment for the primary cancer. Three of the patients in the radiotherapy group had received adjuvant chemotherapy after surgery for the primary cancer.
Table 3.
Comparison of treatment modalities.
| Radiotherapy group | Surgical group | |
|---|---|---|
| 2 Year re-recurrence ratea | 40% (95% CI 9.2%–48%) | 0% (95% CI 0%–60%)b |
| P value = 0.2981. 95 | ||
| 2 Year survival ratea | 83% (95% CI 71%–100%) | 100% (95% CI 40%–100%)b |
| P value = 1.00 | ||
| Mean (SD) | Mean (SD) | |
| Follow up time | 4.42 years (2.99) | 3.88 years (0.90) |
| BMI | 27.7 (8.29) | 22.4 (0.26) |
| Age | 69.5 years (8.44) | 69.5 years (7.84) |
Calculated using fishers exact test, the two-sided P value estimated using the method of summing small P values.
Estimated using rule of three.
Univariate analysis of risk factors was calculated for both re-recurrence and survival rate as outcome and results were BMI with p-values of 0.56 (re-recurrence) and 0.57 (survival). Age p = 0.33 and 0.50, FIGO p = 0.60 and 0.63, Grade p = 0.41 and 0.13, myometrial invasion p = 0.20 and 0.71, tumor size p = 0.51 and 0.48.
In our PubMed search we found no articles that dealt with the specific topic, survival and re-recurrence rate after resection of local recurrences of endometrial cancer in non-eradicated patients.
Discussion
This study is to our knowledge the first to investigate and describe differences in outcome in non-irradiated patients with central recurrence of endometrial cancer treated with either radiotherapy or surgery. The study describes the treatment and outcomes for 33 patients with vaginal vault recurrences and the results indicate that surgical removal of the recurrence is an effective treatment.
Our description of a cohort that is naive to radiotherapy makes the study unique and very relevant regarding future choice of treatment.
Firstly, the use of adjuvant radiotherapy in the primary treatment of endometrial cancer has been scaled down in many countries in the recent years, so in the future the majority of patients with recurrent disease will be non-irradiated.
Furthermore it is important to focus on research regarding treatment of specifically vaginal vault recurrences, as this is the most prevalent type of recurrence in a non-irradiated population (Creutzberg et al., 2011, Group et al., 2009, Keys et al., 2004).
Studies on surgical treatment
Previous studies regarding surgical treatment of recurrent endometrial cancer are few and differ from the studies that describe recurrences treated with radiotherapy. We assume it is mainly because radiotherapy has been the first line of treatment for locally recurrent disease for the last decades (Creutzberg et al., 2011, Huh et al., 2007, Jhingran et al., 2003). Surgery has mostly been used when the extent of the disease or other factors indicated that radiotherapy could not be curative (Huh et al., 2007, Keys et al., 2004, Awtrey et al., 2006, Barakat et al., 1999, Khoury-Collado et al., 2012, Berek et al., 2005, Bristow et al., 2006, Campagnutta et al., 2004). Therefore, the studies reviewing surgical treatments are historically focused on total pelvic exenteration as a potentially curative treatment for advanced recurrent disease in the pelvis (Barakat et al., 1999). More recently less radical surgical approaches to the same disease have been studied by Awtrey et al. (2006) In both investigations most of the study population are irradiated patients, in which case radiotherapy is known to be less effective and with a much higher complication rate, but patients are also selected due to local metastasis into neighboring organs or large tumor mass. Unsurprisingly the survival rate for patients with advanced disease is lower than in patients treated for isolated local recurrences, with a 5 year survival rate of 20–45% (Barakat et al., 1999, Morris et al., 1996).
Therefore, these studies are not directly comparable to the results for patients treated surgically in our study. However they present findings that we have used to design this study. One is that the location of the recurrence is an important prognostic factor, where vaginal vault recurrences have a significantly better outcome than recurrences located elsewhere (Awtrey et al., 2006, Bristow et al., 2006, Campagnutta et al., 2004). Another is that tumor diameter is of importance, as a smaller tumor size indicates better outcome (van Wijk et al., 2009). It has been found that tumor size below 2 cm3 indicates a better outcome, which prompted us to use the same demarcation to stratify for tumor size in this study. We did however not find any significant difference based on tumor size.
Studies on radiotherapy
Radiotherapy used as adjuvant treatment for primary endometrial cancer and also as a primary treatment of local recurrent endometrial cancer has a long history (van Wijk et al., 2009). In particular the use of adjuvant radiotherapy is common in many countries for certain stages and histologic types/grades of endometrial cancer (Creutzberg et al., 2011, van Wijk et al., 2009, Group et al., 2009, Keys et al., 2004, Ackerman et al., 1996). However, in the last two decades several large randomized studies examining the effects of adjuvant radiotherapy in the treatment endometrial cancer, partially prompted by findings by Ackerman et al. (1996) have been conducted. In a retrospective study they found no survival benefit for adjuvant radiotherapy despite reduction in recurrence rate. This led to the PORTEC trial, ASTEC trial, GOG trial and the NCIC GTC EN5 trials that convincingly showed that long term survival was not improved by adjuvant radiotherapy in low to intermediary risk endometrial cancers (Creutzberg et al., 2011, Group et al., 2009, Keys et al., 2004). One reason is that complete cure of locally recurrent disease was achieved in a larger percentage in the non-irradiated population (in the PORTEC study 5 year survival rates after vaginal recurrence were 70% in the non-irradiated group versus 38% in the irradiated group) (Creutzberg et al., 2011). Another reason is that recurrent disease in irradiated patients was more like to be non-local (Creutzberg et al., 2011, Group et al., 2009, Keys et al., 2004).
The findings in our radiotherapy group show a survival rate comparable to what is found in the previous studies on survival after radiotherapy treatment of recurrent endometrial cancer, with 83% after 2 years. (Creutzberg et al., 2011, Group et al., 2009, Keys et al., 2004, Ackerman et al., 1996).
Our study has a relatively short mean follow-up time. This is because most of the patients diagnosed with a recurrent disease at OUH were diagnosed in the years 2010–2012, and fewer in the preceding years. The main reason is that within the study period gynecologic cancer treatment has been centralized from many smaller hospitals to five highly specialized centers in Denmark, one of which is OUH, with the effect that many more patients were treated at the center per year after 2010. The number of patients with recurrent disease treated at OUH per year can be seen in Table 1.
There are limiting factors regarding the nature of retrospective studies like this, and we cannot completely account for a possible selection bias that can have influenced which patients received radiotherapy and which received surgical treatment. We found that the mean BMI was higher in the radiotherapy group by 5 points, which indicates that patients with higher BMI were selected for radiotherapy rather than surgical treatment, but the difference was not statistically significant. We also found that there were no statistically significant differences in tumor size, age, differentiation grade or FIGO stage.
About 80% of all endometrial carcinomas are of the endometrioid type, which generally have a more favorable prognosis than tumors with type 2 histology (Danish Gynecological Cancer Group D, 2010). With 23 endometrioid adenocarcinomas and 3 non-endometrioid carcinomas (see exact type in Table 2) in the radiotherapy group and 4 endometrioid adenocarcinomas and 1 carcinosarcoma in the surgical treatment group, we found no statistically significant difference in histology in the two groups. Those treated with a combination treatment both had endometrioid adenocarcinoma.
With no deaths during the follow-up in the surgical group and just one re-recurrence in a woman 2.3 years after the primary recurrence, the results of surgery-only are encouraging. The small sample size and relatively short follow up period limit the statistical significance of the results and makes it impossible to do weighted analysis of the data. Nevertheless, the results indicate that the surgical treatment of locally recurrent endometrial cancer is a valid and effective treatment method and worth further investigations. It will be important to reexamine patient data in a few years, when more follow-up time has elapsed.
Having more than one documented effective treatment for locally recurrent endometrial cancer is of course highly beneficial and it should warrant larger studies on the efficacy of surgical treatment versus radiotherapy, preferably in randomized trials. If surgical treatment is comparable to radiotherapy in survival, other outcome factors such as complications and quality of life need to be evaluated further.
Conflict of interest statement
The authors declare no competing interests.
References
- Ackerman I. Endometrial carcinoma — relative effectiveness of adjuvant irradiation vs therapy reserved for relapse. Gynecol. Oncol. 1996;60(2):177–183. doi: 10.1006/gyno.1996.0022. [DOI] [PubMed] [Google Scholar]
- Amant F. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Awtrey C.S. Surgical resection of recurrent endometrial carcinoma. Gynecol. Oncol. 2006;102(3):480–488. doi: 10.1016/j.ygyno.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Barakat R.R. Pelvic exenteration for recurrent endometrial cancer. Gynecol. Oncol. 1999;75(1):99–102. doi: 10.1006/gyno.1999.5536. [DOI] [PubMed] [Google Scholar]
- Berek J.S. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol. Oncol. 2005;99(1):153–159. doi: 10.1016/j.ygyno.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Bristow R.E. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol. Oncol. 2006;103(1):281–287. doi: 10.1016/j.ygyno.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Campagnutta E. Surgical treatment of recurrent endometrial carcinoma. Cancer. 2004;100(1):89–96. doi: 10.1002/cncr.11868. [DOI] [PubMed] [Google Scholar]
- Cancer W.I.A.F.R.O. 2012. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Google Scholar]
- Creutzberg C.L. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(4):e631–e638. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Danish Gynecological Cancer Group D . 2010. Guidelines for visitation, diagnostics, treatment and control of cancer corporis uteri. [Google Scholar]
- Gredmark T. Histopathological findings in women with postmenopausal bleeding. Br. J. Obstet. Gynaecol. 1995;102(2):133–136. doi: 10.1111/j.1471-0528.1995.tb09066.x. [DOI] [PubMed] [Google Scholar]
- Group A.E.S. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W.K. Salvage of isolated vaginal recurrences in women with surgical stage I endometrial cancer: a multiinstitutional experience. Int. J. Gynecol. Cancer. 2007;17(4):886–889. doi: 10.1111/j.1525-1438.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Jhingran A., Burke T.W., Eifel P.J. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int. J. Radiat. Oncol. Biol. Phys. 2003;56(5):1366–1372. doi: 10.1016/s0360-3016(03)00414-0. [DOI] [PubMed] [Google Scholar]
- Keys H.M. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2004;92(3):744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Khoury-Collado F. Pelvic exenteration with curative intent for recurrent uterine malignancies. Gynecol. Oncol. 2012;124(1):42–47. doi: 10.1016/j.ygyno.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Morris M. Treatment of recurrent adenocarcinoma of the endometrium with pelvic exenteration. Gynecol. Oncol. 1996;60(2):288–291. doi: 10.1006/gyno.1996.0040. [DOI] [PubMed] [Google Scholar]
- van Wijk F.H. Management of recurrent endometrioid endometrial carcinoma: an overview. Int. J. Gynecol. Cancer. 2009;19(3):314–320. doi: 10.1111/IGC.0b013e3181a7f71e. [DOI] [PubMed] [Google Scholar]