Abstract
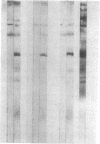
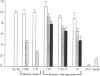
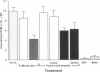
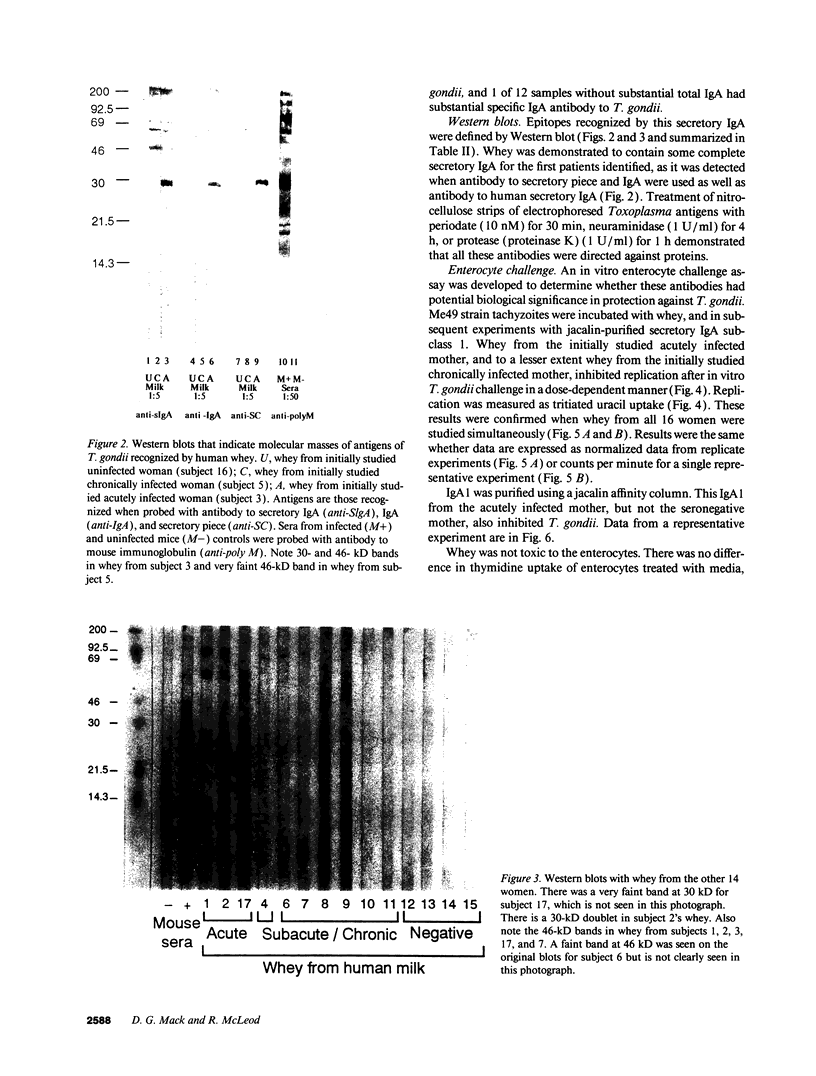
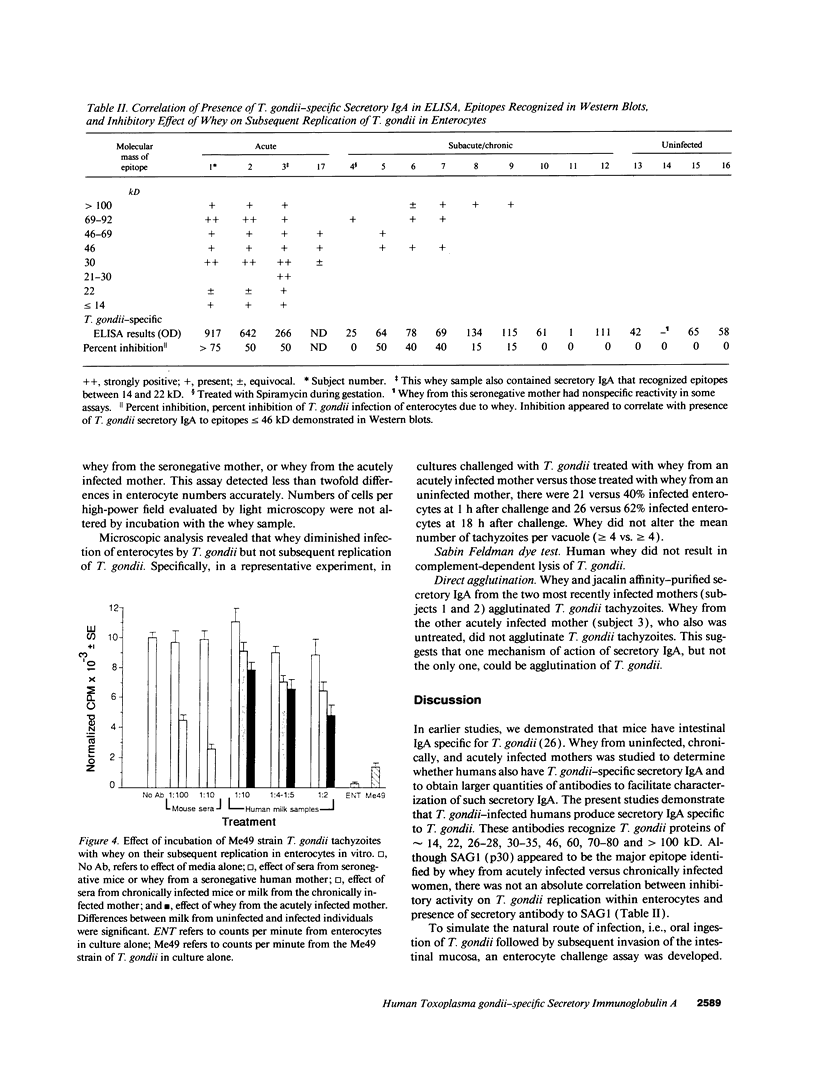
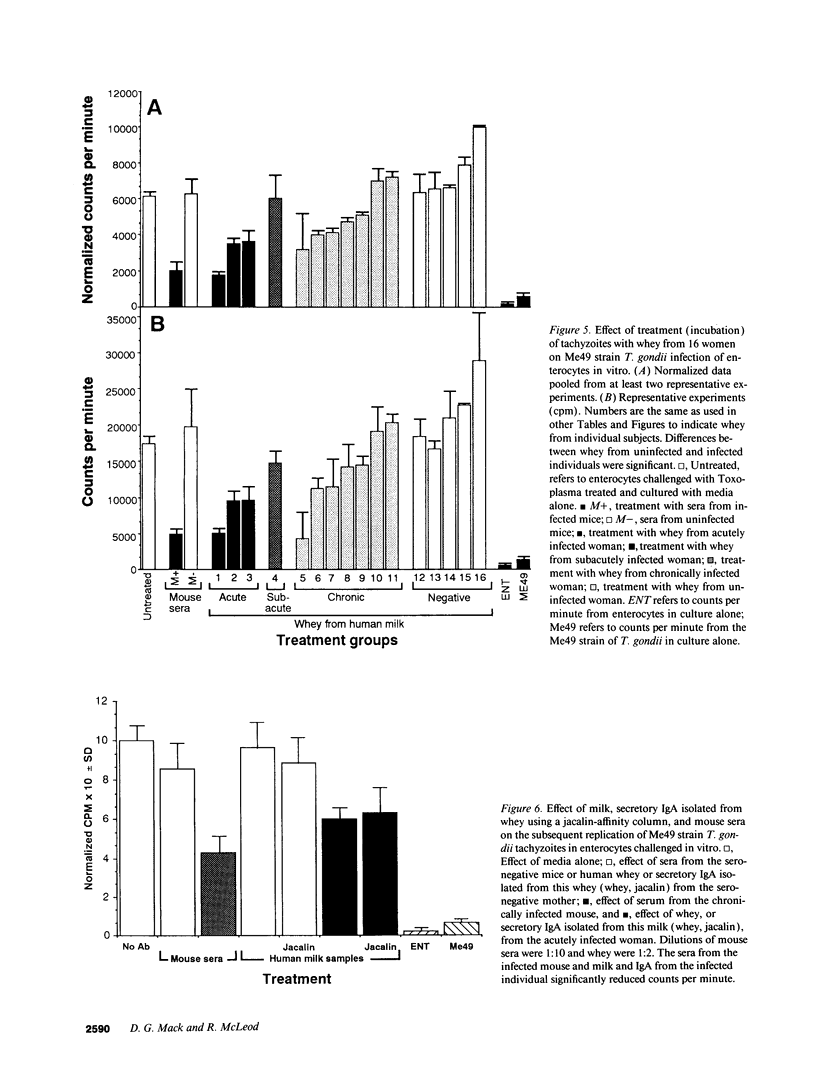
Whey from 17 women (four acutely infected with Toxoplasma gondii, eight chronically infected, and five uninfected) was studied. T. gondii-specific secretory IgA antibodies were demonstrated by ELISA in whey from acutely infected and one of eight chronically infected women. Such antibodies to tachyzoite proteins of < or = 14, 22, 26-28, 30, 46, 60, 70-80, and > 100 kD (eliminated by protease but not periodate or neuraminidase treatment) were demonstrated in whey from acutely infected subjects when Western blots were probed with their whey and antibodies to human secretory IgA or IgA or secretory piece. Secretory IgA from four of eight chronically infected women recognized the 46- and 69-kD epitopes. Other whey samples were negative. Incubation of T. gondii tachyzoites with whey or purified secretory IgA from acutely infected (but not seronegative) women caused 50-75% reduction in infection of enterocytes in vitro. Whey reactive with the 46-kD epitope from three of six chronically infected women caused less (> or = 40%) inhibition. Whey and purified secretory IgA from two of three acutely infected women agglutinated tachyzoites. Whey did not result in complement-dependent lysis of T. gondii. These results indicate that it may be possible to produce human secretory IgA to T. gondii capable of reducing initial infection of enterocytes, as such IgA is present during natural infection. They also demonstrate candidate epitopes for such protection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clancy R. L., Cripps A. W., Husband A. J., Buckley D. Specific immune response in the respiratory tract after administration of an oral polyvalent bacterial vaccine. Infect Immun. 1983 Feb;39(2):491–496. doi: 10.1128/iai.39.2.491-496.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL J. K., JACOBS L. Ocular toxoplasmosis; pathogenesis, diagnosis and treatment. AMA Arch Ophthalmol. 1958 Feb;59(2):260–279. [PubMed] [Google Scholar]
- Fayer R., Perryman L. E., Riggs M. W. Hyperimmune bovine colostrum neutralizes Cryptosporidium sporozoites and protects mice against oocyst challenge. J Parasitol. 1989 Feb;75(1):151–153. [PubMed] [Google Scholar]
- Glass R. I., Svennerholm A. M., Stoll B. J., Khan M. R., Hossain K. M., Huq M. I., Holmgren J. Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med. 1983 Jun 9;308(23):1389–1392. doi: 10.1056/NEJM198306093082304. [DOI] [PubMed] [Google Scholar]
- Gregory R. L., Filler S. J. Protective secretory immunoglobulin A antibodies in humans following oral immunization with Streptococcus mutans. Infect Immun. 1987 Oct;55(10):2409–2415. doi: 10.1128/iai.55.10.2409-2415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood J., Smith J. E. Toxoplasma gondii: the role of a 30-kDa surface protein in host cell invasion. Exp Parasitol. 1992 Feb;74(1):106–111. doi: 10.1016/0014-4894(92)90144-y. [DOI] [PubMed] [Google Scholar]
- Hilpert H., Brüssow H., Mietens C., Sidoti J., Lerner L., Werchau H. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. J Infect Dis. 1987 Jul;156(1):158–166. doi: 10.1093/infdis/156.1.158. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Hoover D. L., Bergquist E. J., Hornick R. B., Young C. R. Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979 Mar;23(3):729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S., Soulsby E. J. The role of IgA immunoglobulins in the passive transfer of protection to Taenia taeniaeformis in the mouse. Immunology. 1978 May;34(5):939–945. [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Estes R. G., Mack D. G., Cohen H. Immune response of mice to ingested Toxoplasma gondii: a model of toxoplasma infection acquired by ingestion. J Infect Dis. 1984 Feb;149(2):234–244. doi: 10.1093/infdis/149.2.234. [DOI] [PubMed] [Google Scholar]
- McLeod R., Mack D. G. Secretory IgA specific for Toxoplasma gondii. J Immunol. 1986 Apr 1;136(7):2640–2643. [PubMed] [Google Scholar]
- Mietens C., Keinhorst H., Hilpert H., Gerber H., Amster H., Pahud J. J. Treatment of infantile E. coli gastroenteritis with specific bovine anti-E. coli milk immunoglobulins. Eur J Pediatr. 1979;132(4):239–252. doi: 10.1007/BF00496847. [DOI] [PubMed] [Google Scholar]
- Nayak N., Ganguly N. K., Walia B. N., Wahi V., Kanwar S. S., Mahajan R. C. Specific secretory IgA in the milk of Giardia lamblia-infected and uninfected women. J Infect Dis. 1987 Apr;155(4):724–727. doi: 10.1093/infdis/155.4.724. [DOI] [PubMed] [Google Scholar]
- Ogra P. L. Mucosal immune response to poliovirus vaccines in childhood. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S361–S368. doi: 10.1093/clinids/6.supplement_2.s361. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque-Barreira M. C., Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985 Mar;134(3):1740–1743. [PubMed] [Google Scholar]
- Tagliabue A., Villa L., De Magistris M. T., Romano M., Silvestri S., Boraschi D., Nencioni L. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J Immunol. 1986 Sep 1;137(5):1504–1510. [PubMed] [Google Scholar]
- Taylor H. P., Dimmock N. J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985 Jan 1;161(1):198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Roberton D., Chapman C. Remission of diarrhoea due to cryptosporidiosis in an immunodeficient child treated with hyperimmune bovine colostrum. Br Med J (Clin Res Ed) 1986 Nov 15;293(6557):1276–1277. doi: 10.1136/bmj.293.6557.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Stone J., Lazzell V., Bergmann K. C., Khakoo R., Jacknowitz A., Howard S., Rose C. Oral route as method for immunizing against mucosal pathogens. Ann N Y Acad Sci. 1983 Jun 30;409:510–516. doi: 10.1111/j.1749-6632.1983.tb26895.x. [DOI] [PubMed] [Google Scholar]