Abstract
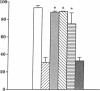
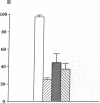
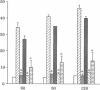
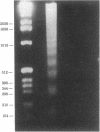
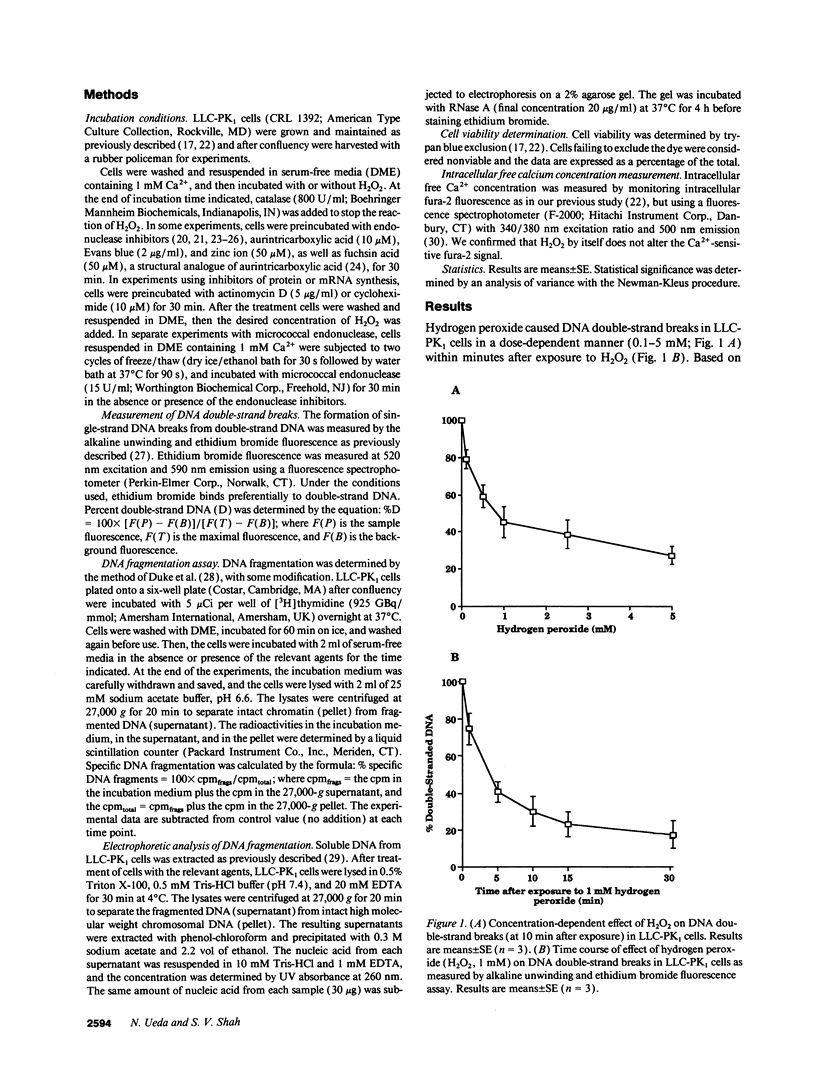
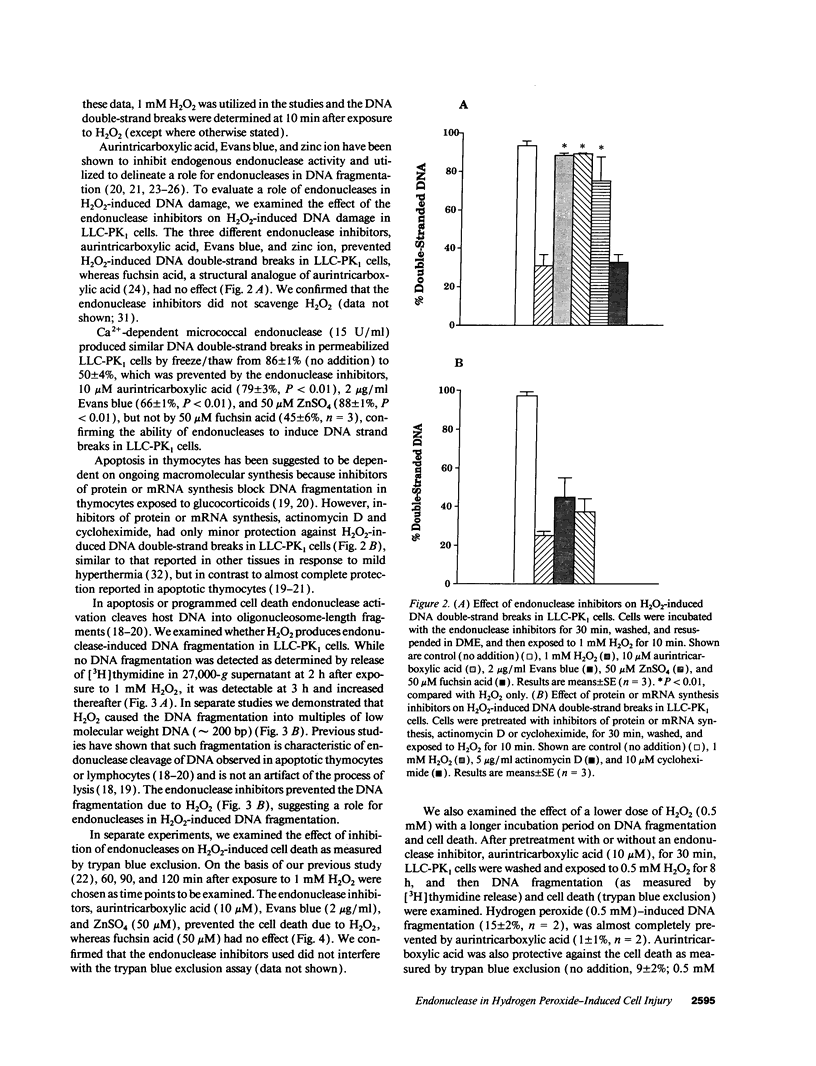
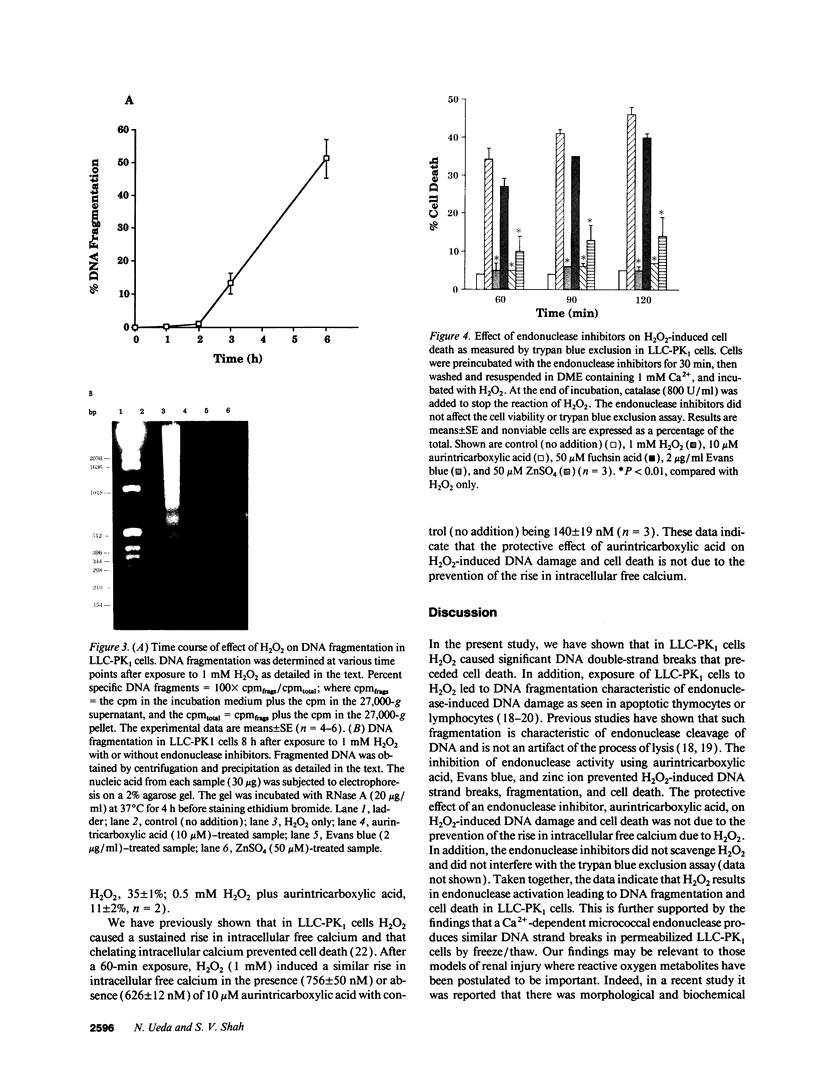
Hydrogen peroxide (H2O2)-induced DNA damage and cell death have been attributed to the direct cytotoxicity of H2O2 and other oxidant species generated from H2O2. We examined the possibility that oxidants activate endonucleases leading to DNA damage and cell death in renal tubular epithelial cells, similar to that described for apoptosis. Within minutes, H2O2 caused DNA strand breaks in a dose-dependent manner, followed by cell death. DNA fragmentation was demonstrated both by the release of [3H]thymidine in 27,000-g supernatant as well as the occurrence of low molecular weight DNA fragments on agarose gel electrophoresis, characteristic of endonuclease cleavage. Endonuclease inhibitors, aurintricarboxylic acid, Evans blue, and zinc ion prevented H2O2-induced DNA strand breaks, fragmentation, and cell death. Inhibitors of protein or mRNA synthesis had only minor protection against H2O2-induced DNA damage in contrast to complete protection reported in apoptotic thymocytes. Micrococcal endonuclease induced similar DNA strand breaks in LLC-PK1 cells, and the endonuclease inhibitors prevented the events confirming the ability of endonucleases to induce DNA damage. The protective effect of aurintricarboxylic acid was not due to the prevention of the rise in intracellular free calcium. We conclude that endonuclease activation occurs as an early event leading to DNA damage and cell death in renal tubular epithelial cells exposed to oxidant stress and, in contrast to apoptotic thymocytes, does not require macromolecular synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Baba M., Schols D., Pauwels R., Balzarini J., De Clercq E. Fuchsin acid selectively inhibits human immunodeficiency virus (HIV) replication in vitro. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1404–1411. doi: 10.1016/s0006-291x(88)81297-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Rao G., Halliwell B., Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch Biochem Biophys. 1991 Mar;285(2):317–324. doi: 10.1016/0003-9861(91)90366-q. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guidet B., Shah S. V. Enhanced in vivo H2O2 generation by rat kidney in glycerol-induced renal failure. Am J Physiol. 1989 Sep;257(3 Pt 2):F440–F445. doi: 10.1152/ajprenal.1989.257.3.F440. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Razandi M., Sugimoto T., Ivey K. J. Role for iron in reactive oxygen species-mediated cytotoxicity to cultured rat gastric mucosal cells. Am J Physiol. 1991 Apr;260(4 Pt 1):G556–G563. doi: 10.1152/ajpgi.1991.260.4.G556. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Oxygen free radicals linked to many diseases. Science. 1987 Jan 30;235(4788):529–531. doi: 10.1126/science.3810154. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H., Balzarini J., De Clercq E., Ono K. Differential inhibition of various deoxyribonucleic acid polymerases by Evans blue and aurintricarboxylic acid. Eur J Biochem. 1988 Oct 15;177(1):91–96. doi: 10.1111/j.1432-1033.1988.tb14348.x. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M., Colombel M. C., Sawczuk I. S., Gobé G., Connor J., O'Toole K. M., Olsson C. A., Wise G. J., Buttyan R. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992 Apr;140(4):831–838. [PMC free article] [PubMed] [Google Scholar]
- Shah S. V. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989 May;35(5):1093–1106. doi: 10.1038/ki.1989.96. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Takano Y. S., Harmon B. V., Kerr J. F. Apoptosis induced by mild hyperthermia in human and murine tumour cell lines: a study using electron microscopy and DNA gel electrophoresis. J Pathol. 1991 Apr;163(4):329–336. doi: 10.1002/path.1711630410. [DOI] [PubMed] [Google Scholar]
- Ueda N., Shah S. V. Role of intracellular calcium in hydrogen peroxide-induced renal tubular cell injury. Am J Physiol. 1992 Aug;263(2 Pt 2):F214–F221. doi: 10.1152/ajprenal.1992.263.2.F214. [DOI] [PubMed] [Google Scholar]
- Walker P. D., Shah S. V. Hydrogen peroxide cytotoxicity in LLC-PK1 cells: a role for iron. Kidney Int. 1991 Nov;40(5):891–898. doi: 10.1038/ki.1991.290. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]