Sympatric speciation was long assumed to be a rare, if not impossible, phenomenon. Although theoreticians have identified the conditions and evolutionary processes under which sympatric speciation may be possible, empirical support of divergence that initiated and proceeded in sympatry has been limited to studies of monophyletic, geographically isolated ‘island' populations, such as found in small crater lakes or on small remote oceanic islands, where other historical biogeographical scenarios can be effectively ruled out (see Bolnick and Fitzpatrick, 2007 for a review). However, in a recent study published in Heredity, Moura et al. (2014) claim their phylogenomic analysis of historical biogeography indicates killer whale ecotypes found in the largest ocean basin, the North Pacific, diverged in sympatry. We contend that Moura et al.'s inference of divergence within the Pacific Ocean do not equate to divergence in sympatry, but given that the criteria for robustly establishing sympatric divergence have already been much debated and are well established (Coyne and Orr, 2004; Bolnick and Fitzpatrick, 2007), we focus here on questioning the robustness of their biogeographical inference at the ocean basin level. We question whether their data can exclude alternative biogeographical scenarios, and argue that gene flow upon secondary contact could have resulted in the changes in topology of their nuclear phylogeny that ultimately led to the Bayesian binary model (BBM) analysis inferring that divergence of the North Pacific ecotypes had occurred in situ.
Killer whales are a globally distributed, highly mobile predator, but distinct ecotypes are found in sympatry in a number of locations including the North Pacific. A previous study by Foote et al. (2011) interpreted complete mitochondrial (mt) genome phylogenies as evidence that three North Pacific killer whale ecotypes did not meet the criteria for having diverged in sympatry (Coyne and Orr, 2004), as they were not sister taxa or a monophyletic endemic species flock. Two North Pacific ecotypes (‘resident' and ‘offshore') shared a more recent ancestor with two North Atlantic clades than they did with the third North Pacific ‘transient' ecotype. The branching order, supported by tests of alternative topologies, suggested a series of dispersal events from the Pacific to the Atlantic and back again, additionally supported by stepwise reductions in genetic diversity with each putative dispersal and founding event. Foote et al. (2011) inferred from these results that the matrilineal history of North Pacific ecotypes included a period of allopatry, but acknowledged mtDNA may not fully reflect the underlying pattern of divergence and lineage formation.
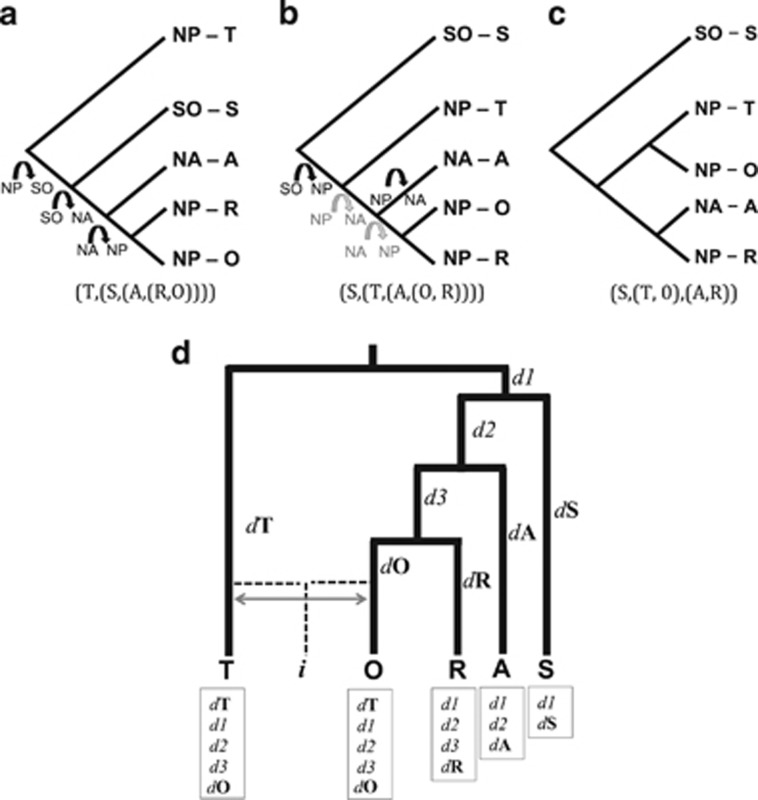
The phylogeographic reconstruction by Moura et al. using nuclear (nu) DNA phylogenies inferred that divergence at the specified node most probably occurred in the North Pacific. In Figure 1b we show how their nu phylogeny is also compatible with the matrilineal historical biogeographical scenario suggested by Foote et al. (2011). The positioning of taxa within the in-group for which the biogeographical history is under debate (i.e., North Atlantic, North Pacific Resident and North Pacific Offshore) does not change between the mt and nu phylogenies in a way that would affect the inference of ancestral distribution (Figures 1a and b). However, the positioning of the in-group has changed, so that it is now a sister taxa to the North Pacific transients, rather than the Southern Ocean population. The BBM analysis has in this example selected an evolutionary history that minimises the changes between different ancestral states as the most likely model, given the data. However, the difference in branch ordering (and consequently the biogeographical reconstruction) between the mt and nu phylogenies could be due to the influence of recombination and gene flow on the nuclear genome upon secondary contact.
Figure 1.
Schematic diagrams of the topology of (a) the mitochondrial phylogeny, illustrating the dispersal events of maternal lineages from the North Pacific to the North Atlantic via the Southern Ocean and dispersal back to the North Pacific as inferred by Foote et al. (2011); (b) the nuclear phylogeny based on the full data set partitioned for GC content, illustrating the dispersal events inferred by the BBM analysis of Moura et al. (in black) and an alternative biogeographical scenario that is consistent with the inferences from the mt tree, but which requires an additional dispersal event (in grey) and is less well supported under the BBM model; (c) the nuclear phylogeny based on the AT-rich (low recombination) contigs produced by Moura et al. (d) A hypothetical scenario in which the mitochondrial phylogeny represents the true species tree and historical biogeography, but in which gene flow upon secondary contact could result in the clustering of the offshore and transient ecotypes in a nuclear phylogeny. Tip labels indicate lineage (Transient, Offshore, Resident, Atlantic and Southern Ocean). d1, d2, d3 and so on, are derived alleles/shifts in allele frequency that have occurred along different branches of the phylogeny. If upon secondary contact, gene flow occurred between the offshore and transient ecotypes, either directly or via an intermediate population (i), then some alleles derived following sequential splits from the Southern Ocean, North Atlantic and resident lineages would be shared with the transients. In addition, the alleles derived in the transients would be shared with the offshores, but not the other lineages. Allele frequencies at shared ancestral polymorphisms would also become more correlated between the offshore and transient lineages. The relative phylogenetic signal from introgressed versus shared ancestral alleles would depend upon the demographic history and the level of gene flow and would likely vary between genomic regions with different GC content owing to the differences in recombination rates, consistent with the pattern of different topologies being observed in the different nuclear phylogenies generated by Moura et al.
Moura et al. reason that ‘the nuclear phylogeny could not be explained by male-mediated gene flow following secondary contact' as ‘secondary contact could not explain why the Southern Ocean ecotype branches from the most basal node in the nuclear phylogeny, or why offshores and residents show greater divergence at nuclear loci'. However, if gene flow upon secondary contact was primarily between the transient and offshore ecotypes (as suggested by Pilot et al., 2010), this would result in the offshores and transients sharing derived alleles and the correlation of allele frequencies at shared ancestral polymorphisms (Figure 1d), potentially changing the basal position of the transients.
We conclude that the author's analyses are equivocal and could be explained by divergence within the same ocean basin and dispersal into the Atlantic, or by gene flow, directly or indirectly, among North Pacific ecotypes upon secondary contact. The differences in topology of their nu phylogenies including (Figure 1b) and excluding GC-rich (high recombination) regions (Figure 1c) suggest incomplete lineage sorting and that different gene trees reflect different population histories. Further, the conclusions drawn by Moura et al. from this single analytical method are based on inferring the ancestral state at a single node from a phylogeny with incomplete taxon sampling; lacking sister taxa that would be likely to influence biogeographical inference by the BBM. Clearly, both the mt and nu phylogenies are compatible with several biogeographical histories of varying complexity, and the most likely history under one evolutionary model may not necessarily be the true history. The data produced by the authors will allow further investigation of some of this uncertainty and the comparison of different models including those that consider gene flow upon secondary contact, providing further insight into the diversification process of these highly mobile and social marine predators.
Acknowledgments
We thank Mike Ford, Sebastián Duchene, the editor and an anonymous reviewer for helpful comments on this letter and Yan Yu for helpful discussions on the BBM analysis.
The authors declare no conflict of interest.
References
- Bolnick DI, Fitzpatrick BM. Sympatric speciation: models and empirical evidence. Annu Rev Ecol Evol Syst. 2007;38:459–487. [Google Scholar]
- Coyne J, Orr H. Speciation. Sinauer Associates: Sunderland, MA, USA; 2004. [Google Scholar]
- Foote AD, Morin PA, Durban JW, Willerslev E, Orlando L, Gilbert MTP. Out of the Pacific and back again: insights into the matrilineal history of Pacific killer whale ecotypes. PLoS ONE. 2011;6:e24980. doi: 10.1371/journal.pone.0024980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura AE, Kenny JG, Chaudhuri RR, Hughes MA, Reisinger RR, de Bruyn PJ, et al. Phylogenomics of the killer whale indicates ecotype divergence in sympatry. Heredity. 2014;114:48–55. doi: 10.1038/hdy.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot M, Dahlheim ME, Hoelzel AR. Social cohesion among kin, gene flow without dispersal and the evolution of population genetic structure in the killer whale (Orcinus orca. J Evol Biol. 2010;23:20–31. doi: 10.1111/j.1420-9101.2009.01887.x. [DOI] [PubMed] [Google Scholar]