Abstract
As a consequence of improved quality of abdominal imaging techniques in the last decades, discovery of pancreatic cystic lesions has become more common. The clinical significance of these lesions is often unclear and poses a diagnostic dilemma. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a subject of debate regarding its role in the diagnostic evaluation of pancreatic masses and cysts. Although risks associated with the procedure are low, consequences can be serious and even life-threatening. We report a case of a previously healthy 59-year-old woman who suffered severe acute pancreatitis after EUS-FNA of a pancreatic cyst, requiring admission to the intensive care unit (ICU). Development of infected pancreatic necrosis and, successively, bowel ischaemia, led to multiple organ failure. Despite maximal antibiotic and surgical treatment the patient succumbed to refractory septic shock. The fatal outcome of this case illustrates the importance of balanced decision-making in the diagnostic approach of pancreatic cystic lesions.
Background
Pancreatic cystic neoplasms (PCNs) account for up to 10% of pancreatic cystic lesions, which also comprise inflammatory fluid collections and non-neoplastic pancreatic cysts.1 Most of these lesions are encountered incidentally when cross-sectional imaging is used. The frequency of detection may be up to 2% in patients who undergo CT or MRI for unrelated reasons.2 The malignant potential of PCNs makes it important to distinguish them from non-neoplastic cystic lesions, as surgical removal has to be considered in case of premalignant lesions.
EUS-FNA can be used to obtain detailed imaging information as well as tissue and cyst fluid for analysis. Morphological characteristics associated with higher risk of malignancy are cyst size and growth of the cyst during follow-up, dilation of the main pancreatic duct, mural nodules and septations with calcification.3 4 It is common practice to analyse cyst fluid for carcinoembryonic antigen (CEA), amylase and cytology. However, it is underdiscussion whether EUS-FNA enhances diagnostic yield due to its limited sensitivity and specificity.5
Although EUS-FNA is generally considered safe, there is associated morbidity and even mortality. Complications most frequently include postprocedural pain and pancreatitis, and, to a lesser extent, include haemorrhage, febrile episodes following aspiration of cystic lesions, infection, endoscope-induced perforation and seeding of the needle track with tumour cells.6 The reported incidence of complications ranges from 0% to 5%, of which none were reported beyond 30 days.7–11 The incidence of pancreatitis after EUS-FNA of the pancreas ranges from under 1% to 2%.6 7 12 13 Alternatives for EUS-FNA, depending on morphology of the pancreatic cyst, symptoms and patient comorbidity, consist of watchful waiting using repeat EUS or other imaging modalities, or primary resection of the lesion.
Case presentation
A 59-year-old previously healthy woman presented to our outpatient clinic with abdominal symptoms, for which she had undergone extensive diagnostic testing elsewhere. Although no cause for her symptoms was found, CT of the abdomen revealed a pancreatic cyst, suggesting intraductal papillary mucinous neoplasm (IPMN; figure 1). She underwent an EUS-FNA, as a solid nodule was present in the cystic wall (figure 2A). Amylase and CEA in the fluid were, respectively, <10 U/L and 45.0 µg/L (reference value <5.0 µg/L). Cytology showed no malignancy. Follow-up EUS-FNA was repeated 4 months later under antibiotic prophylaxis as the nodule was still present, and showed a slight increase in diameter (figure 2B). Amylase and CEA levels in the fluid were 271 U/L and 7.1 µg/L, respectively. Again, no malignant cells were found during analysis. The same day the patient collapsed and was admitted to the intensive care unit (ICU) with systemic inflammatory response syndrome based on biochemical and CT-proven acute pancreatitis (figure 3A).
Figure 1.
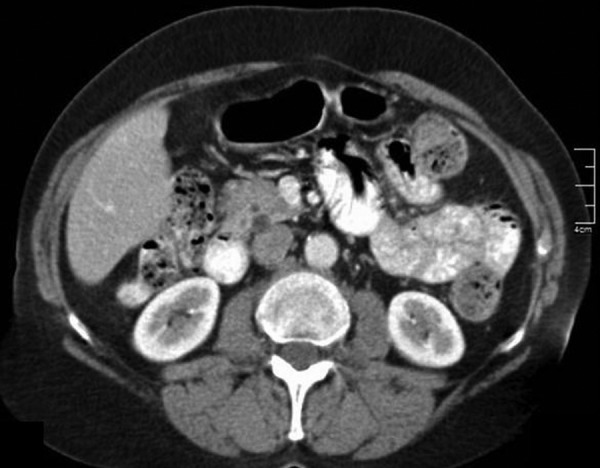
CT of the abdomen: cross-section showing a cystic process dorsally located in the head of the pancreas. The differential diagnosis of side branch intraductal papillary mucinous neoplasm comprises non-neoplastic pancreatic cyst, pseudocyst (as seen after pancreatitis), or other types of pancreatic cystic neoplasms.
Figure 2.

(A) Image of first endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) 6 months prior showing a pancreatic cyst with a solid nodule in the wall. (B) Image of EUS-FNA after which pancreatitis developed, showing an increase in diameter of the mural nodule.
Figure 3.
(A) CT of the abdomen (day 1, day of endoscopic ultrasound-guided fine-needle aspiration): image of a swollen pancreas with induration and fluid infiltration of the surrounding mesenteral fat, and free fluid in the left paracolic groove. (B) CT of the abdomen (day 9): image of extensive necrotising pancreatitis in which the complete body and the majority of the head and tail of the pancreas appear necrotic. (C) CT of the abdomen (day 22): cross-section reflecting progression of pancreatic necrosis fluid in the omental bursa, and infiltration and induration of the retroperitoneal space. (D) CT of the abdomen (day 22): cross-section showing absent contrast enhancement of the right colonic wall, suggesting ischaemic bowel disease.
Treatment
The patient was resuscitated with fluids and vasopressor therapy, and invasive mechanical ventilation was required. The next week a CT scan was performed, showing extensive necrotising pancreatitis with free fluid in the omental bursa (figure 3B). Blood cultures were positive for Bacteroides fragilis, for which treatment with antibiotics was started. After an initial improvement in the first 20 days, another phase of haemodynamic and respiratory deterioration followed, based on extensive pancreatic necrosis and bowel ischaemia, as shown by CT (figure 3C, D). Laparotomy revealed an ischaemic ascending colon, for which a right-sided hemicolectomy was performed.
Outcome and follow-up
Despite maximal efforts, the patient sustained a refractory septic shock with multiple organ failure. She died within 48 h after surgery.
Discussion
We report of a fatal case of pancreatitis directly related to EUS-FNA of a pancreatic cyst in a previously healthy 59-year-old woman. As illustrated above, the incidental discovery of pancreatic cystic lesions in patients who undergo abdominal imaging poses a diagnostic dilemma. Up to 10% of pancreatic cystic lesions are neoplastic. Their subsequent relative malignant potential differs between subtypes, depending on their morphological appearance and results of tissue or cyst fluid analysis. EUS-FNA permits detailed imaging and fluid analysis.
In our patient, abdominal imaging revealed a pancreatic cyst that was interpreted as a possible side branch IPMN. IPMNs are mucin-producing papillary neoplasms of the pancreatic ductal system that exhibit variable cellular atypia and cause dilation of the pancreatic ducts.14 The relative malignant potential of IPMNs of the main duct is considered to be high, as opposed to that of an IPMN of the branch duct, which is low to moderate. A solid component in the cyst wall may suggest malignancy, as might growth of the lesion. CEA levels typically may be above 200 ng/mL in approximately 75% of lesions.4
As initial EUS in our case revealed a solid nodule in the cystic wall, FNA was performed showing low levels of CEA and no malignant cells in cytological analysis. The solid nodule was confirmed at follow-up EUS with a slight increase in diameter, therefore FNA was repeated.
The literature reports low complication rates from EUS-FNA.6 7 11 15 EUS-FNA of cystic lesions appears to have higher complication rates than that of solid lesions. Wiersema and colleagues reported a high complication rate of 14% from EUS-FNA of cystic lesions, but studies by O'Toole and Lee suggested a lower complication rate of only 3.5% and 2.2%, respectively.15–17 No patient or cyst characteristics appear to be predictive of adverse events.15 The risk of acute pancreatitis was estimated in a pooled analysis of 19 centres, involving a total of almost 5000 EUS-FNAs, however, concerning only pancreatic masses.13 In this cohort, pancreatitis occurred after 14 procedures, which corresponds with an incidence of 0.29% of the cases. Where 10 cases concerned mild pancreatitis, only in 1 of these 14 patients was the pancreatitis classified as severe. In another study, with a small number of patients, the complication rate from EUS-FNA of the pancreas was 2%.18 Gress et al19 noted that acute pancreatitis occurred within 2–4 h of EUS-FNA of the pancreas in 2 of 121 patients (1.2%). Both patients had a history of a recent episode of acute pancreatitis that had resolved before EUS-FNA. They were treated with supportive measures and recovered uneventfully.
Our patient's pancreatitis after EUS-FNA was treated in accordance with guidelines of the Dutch Pancreatitis Study Group. We suspect that bowel ischaemia developed either due to a low-flow state during septic shock, or from vascular erosion by the infected pancreatic necrotic mass. Before bowel ischaemia occurred, there was no indication for invasive necrosectomy, since the patient was clinically improving. It is impossible to say whether, for example, endoscopic transluminal necrosectomy or video assisted retroperitoneal debridement could have prevented the fatal outcome. To the best of our knowledge, a fatal case of pancreatitis as a complication of EUS-FNA of a pancreatic cyst has not been reported in the literature before.
Patient's perspective.
(Provided by the daughter of the patient)
Since our mother was a previously healthy woman we were completely overwhelmed by her admission to the ICU and the critical period that followed. Her gastroenterologist informed us about the relation with the procedure she had undergone, but it appeared that the pancreatitis was much more severe than expected. After initially improving, her condition worsened again leading to the surgery and finally her passing away. It has been an incomprehensible series of events that left us with intense grief.
Learning points.
Detection of pancreatic cystic lesions is increasing due to more frequent use and improved quality of abdominal imaging techniques.
Pancreatic cysts are often of unclear clinical significance, however, they pose a diagnostic dilemma since a minority is neoplastic. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) permits detailed imaging and fluid analysis of pancreatic cysts and thus can be very helpful in distinguishing neoplastic pancreatic cystic lesions.
Complication rates after EUS-FNA of cystic lesions have been reported to be significantly higher than those of solid lesions. Although complication rates are still low, consequences can be serious. One of the known complications is mild acute pancreatitis, with an incidence of 0–2%.
Therefore, when dealing with pancreatic cystic lesions, it is essential to make a balanced decision based on endosonographic criteria combined with patient history and comorbidity.
Footnotes
Contributors: EFJ and LEMH drafted the case and manuscript, and obtained consent from the patient's next of kin to publish. BACvT critically revised the manuscript and was the gastroenterologist responsible for the patient. FBMS revised the manuscript and reviewed the figure subscripts, as the radiologist involved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Warshaw AL, Compton CC, Lewandrowski K et al. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg 1990;212:432–43. 10.1097/00000658-199010000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffan TA, Horton KM, Klein AP et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008;191:802–7. 10.2214/AJR.07.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu BU, Sampath K, Berberian CE et al. Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol 2014;109:121–9. 10.1038/ajg.2013.334 [DOI] [PubMed] [Google Scholar]
- 4.Khalid A, Brugge WR. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49. 10.1111/j.1572-0241.2007.01516.x [DOI] [PubMed] [Google Scholar]
- 5.Nakai Y, Isayama H, Itoi T et al. Role of endoscopic ultrasonography in pancreatic cystic neoplasms: where do we stand and where will we go? Dig Endosc 2014;26:135–43. 10.1111/den.12202 [DOI] [PubMed] [Google Scholar]
- 6.Wang KX, Ben QW, Jin ZD et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc 2011;73:283–90. 10.1016/j.gie.2010.10.045 [DOI] [PubMed] [Google Scholar]
- 7.Gress F, Michael H, Gelrud D et al. EUS-guided fine-needle aspiration of the pancreas: evaluation of pancreatitis as a complication. Gastrointest Endosc 2002;56:864–7. 10.1016/S0016-5107(02)70361-1 [DOI] [PubMed] [Google Scholar]
- 8.Faigel DO, Ginsberg GG, Bentz JS et al. Endoscopic ultrasound-guided real-time fine needle aspiration biopsy of the pancreas in cancer patients with pancreatic lesions. J Clin Oncol 1997;15:1439–43. [DOI] [PubMed] [Google Scholar]
- 9.Chang KG, Katz KD, Durbin TE et al. Endoscopic ultrasound guided fine-needle aspiration. Gastrointest Endosc 1994;40:694–6. [PubMed] [Google Scholar]
- 10.Voss M, Hammel P, Molas G et al. Value of endoscopic ultrasound-guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut 2000;46:244–9. 10.1136/gut.46.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eloubeidi MA, Chen VK, Eltoum IA et al. Endoscopic ultrasoundguided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute 30-day complications. Am J Gastroenterol 2003;98:2663–8. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Tamhane A, Varadarajulu S et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006;63:622–9. 10.1016/j.gie.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Gress FG, Savides TJ et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc 2004;60:385–9. 10.1016/S0016-5107(04)01714-6 [DOI] [PubMed] [Google Scholar]
- 14.D'Angelica M, Brennan MF, Suriawinata AA et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg 2004;239:400–8. 10.1097/01.sla.0000114132.47816.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LS, Saltzman JR, Bounds BC et al. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol 2005;3:231–6. 10.1016/S1542-3565(04)00618-4 [DOI] [PubMed] [Google Scholar]
- 16.Wiersema MJ, Vilmann P, Giovannini M et al. Endosonographyguided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087–95. 10.1016/S0016-5085(97)70164-1 [DOI] [PubMed] [Google Scholar]
- 17.O'Toole D, Palazzo L, Arotcarena R et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 2001; 53:470–4. 10.1067/mge.2001.112839 [DOI] [PubMed] [Google Scholar]
- 18.Chang KJ, Nguyen P, Erickson RA et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc 1997;45:387–93. 10.1016/S0016-5107(97)70149-4 [DOI] [PubMed] [Google Scholar]
- 19.Gress FG, Hawes RH, Savides TJ et al. Endoscopic ultrasound-guided fine needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc 1997;45:243–50. 10.1016/S0016-5107(97)70266-9 [DOI] [PubMed] [Google Scholar]



