Abstract
We describe a diagnostic dilemma in a middle-aged man presenting with dyspnoea and bilateral pedal oedema who had been diagnosed with right heart failure based on clinical evidence. The evaluation for aetiology eventually led to discovery of an unusual extrathoracic cause, a left-to-right communication in the renal vasculature. Renal arteriovenous fistulae are rare and can be congenital, acquired or idiopathic. A left-to-right shunt typically presents with high-output cardiac failure involving the left and right sides of the heart. An atypical feature of this case was the finding of overt right heart failure in the setting of a normal left heart. Such a presentation has only been described in a few isolated case reports. Diagnostic approaches include CT angiography and cardiac catheterisation for haemodynamic measurements. The primary treatment options for arteriovenous fistulae are medical management, arterial embolisation and surgical repair.
Background
Dyspnoea, a commonly encountered presenting symptom in clinical practice, can often represent a diagnostic challenge due to the myriad possible aetiologies. This case describes an unusual aetiology with an atypical presentation that was diagnosed after serial testing ruled out all of the common possibilities.
Case presentation
A middle-aged man presented with dyspnoea on exertion. Dyspnoea had been present for 5 months and had progressed to the point of interfering with activities of daily living. On review of systems, the patient also reported abdominal distention and lower extremity swelling. The patient denied chest pain, dyspnoea at rest, palpitations, orthopnoea, paroxysmal nocturnal dyspnoea, cough and constitutional symptoms. His medical history was significant for controlled long-standing hypertension, non-insulin-dependent diabetes mellitus and hyperlipidemia. Surgical history was positive for an L5 laminectomy 18 years prior to presentation. He had never smoked and denied alcohol abuse. He had no history of coronary disease, pneumonia or airway disease. Family history was positive for hypertension, heart disease and cerebrovascular accidents. Medications at the time of presentation were losartan, amlodipine, atenolol, glipizide and pravastatin.
On physical examination, the patient was obese with a body mass index of 35.44 kg/m2. His pulse was 65 bpm, respiratory rate 14 min, blood pressure 125/58 mm Hg and resting room air oxygen saturation 96%. There was jugular venous distention to the angle of the mandible. Heart rate and rhythm were regular, with a loud second heart sound and grade III/VI systolic murmur best heard in the left third parasternal space at the end of inspiration. Lung fields were clear to auscultation. The abdomen appeared distended. It was soft to palpation with normal bowel sounds, no clinically detectable ascites, and a liver margin palpable 2 cm below the costal margin. A bruit was heard in the right mid abdomen. Grade 2 oedema was present. There was no cyanosis or digital clubbing.
Investigations
Initial laboratory testing revealed a normal complete blood count with 4200 white cells /µL, haemoglobin 14.2 g/dL and 250 000 platelets/µL. Renal function tests were notable for a blood urea nitrogen of 19 mg/dL, creatine of 1.7 mg/dL and estimated glomerular filtration rate of 49 mL/min/1.73 m2. Brain natriuretic peptide was 126 pg/mL (normal range 0–100), serum albumin 3.7 g/dL and thyroid-stimulating hormone 1.67 µIU/mL. Urinalysis revealed 1+ haematuria without proteinuria. Chest radiography was unremarkable. Pulmonary function testing demonstrated normal spirometric volumes, unremarkable plethysmographic volumes and a normal diffusing capacity or transfer factor of the lung for carbon monoxide (DLCO). Transthoracic echocardiogram revealed normal left ventricular function, dilated right heart chambers and mild tricuspid regurgitation. Estimated pulmonary artery (PA) systolic pressure was >56 mm Hg. A bubble study did not show evidence of intracardiac shunting. Lower extremity Doppler studies were negative for clotting and a lung ventilation-perfusion scan did not demonstrate any segmental perfusion defects.
Right heart catheterisation (RHC) was performed to document right heart pressures and exclude left heart dysfunction. Findings on RHC: right ventricular pressure was 39/4 mm Hg, PA pressure was 36/15 mm Hg with a mean of 23 mm Hg and pulmonary capillary wedge pressure was 10 mm Hg. The calculated pulmonary/systemic (Qp/Qs) flow ratio was 1.6, consistent with a moderate left-to-right shunt. The estimated cardiac output was 6.49 L/min with a pulmonary vascular resistance of 122 dyn s/cm5 (normal range 150–250). Systemic blood pressure was 155/63 mm Hg with a systemic vascular resistance of 1195 dyn s/cm5 (normal range 900–1400). The angiographer also noted high PA saturations, and on further investigation identified a large stepup between superior vena caval and inferior vena caval (IVC) oxygen saturations (table 1). Subsequent contrast venography revealed minimal opacification of the IVC, indicating high flow from below the diaphragm.
Table 1.
Oxygen saturations (%) during right heart catheterisation
| Recorded saturations (%) | |
|---|---|
| RA | 76.3 |
| High RA | 74.2 |
| Low RA | 84.8 |
| SVC | 68.9 |
| IVC (distal, before bifurcation) | 88.1 |
| High IVC (above hepatic vein) | 88.9 |
| Low IVC (below hepatic vein) | 85.7 |
| Right ventricle | 85.4 |
| Pulmonary artery | 83.7 |
IVC, inferior vena cava; RA, right atrium; SVC, superior vena cava.
Aortography performed for further delineation demonstrated an aberrant right renal artery connected to the IVC through a large and tortuous arteriovenous fistulae. Subsequent CT angiographic evaluation of the renal vasculature showed that the aberrant right renal artery originated from the aorta just below the oesophageal hiatus, was mildly aneurysmal at its origin, measuring about 1.1 cm in diameter, and fed a large right renal arteriovenous malformation (figure 1). The central nidus of this malformation measured 4.2×3.7 cm and emptied into the right renal vein just proximal to IVC through a lumen 2.2 cm in diameter (figure 2).
Figure 1.
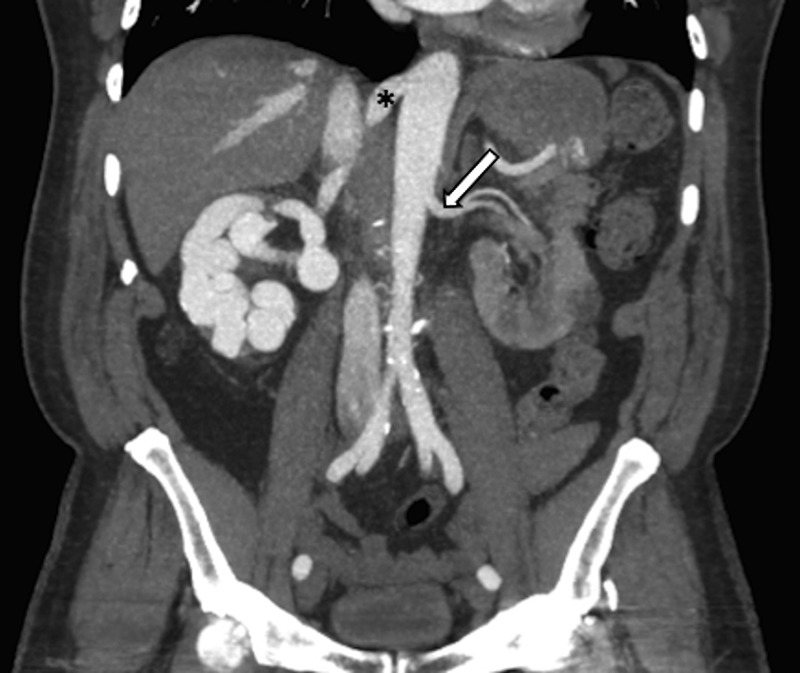
Coronal CT angiogram of the abdomen and pelvis. * Indicates aneurysmal right renal supplying or feeding artery. White arrow indicates left renal artery, comparison for the normal calibre of the renal arteries.
Figure 2.
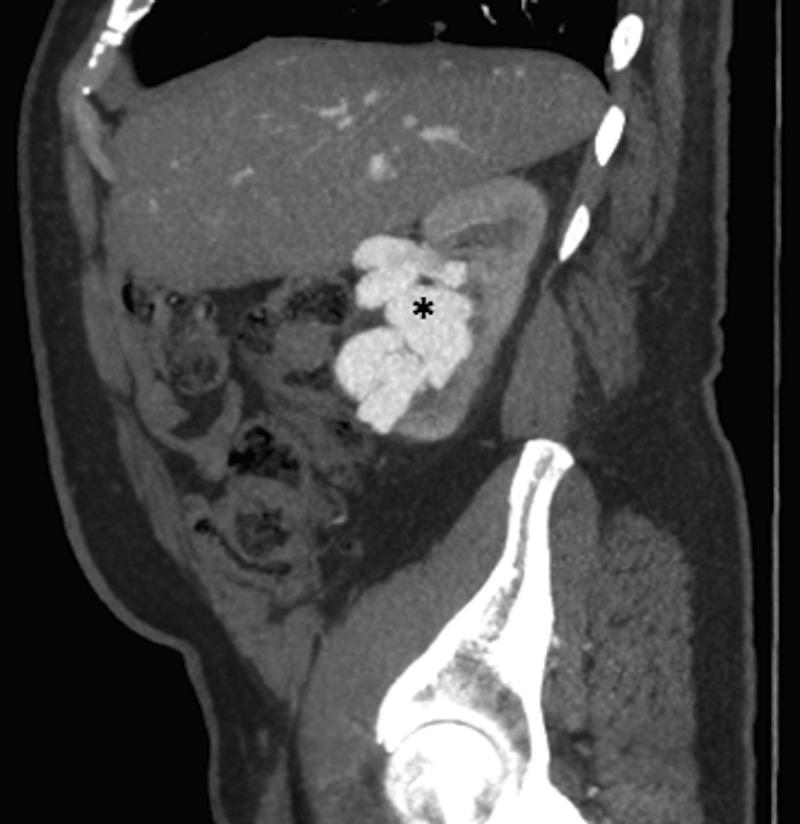
Sagittal CT angiogram of the abdomen and pelvis through the right kidney. * Indicates tortuous enlarged vessels throughout the right kidney.
Differential diagnosis
Right heart failure secondary to left heart failure. Echocardiography and intracardiac pressure as well as pulmonary capillary wedge pressure measurements helped rule out left heart dysfunction.
Pulmonary parenchymal disease. Pulmonary function tests and CT imaging of the lung helped rule out parenchymal disease.
Idiopathic pulmonary hypertension. The pulmonary vascular resistance on this was presumptive evidence against idiopathic pulmonary hypertension and the documentation of the large left-to-right shunt conclusively excluded it.
Pulmonary hypertension due to chronic venous thromboembolism. Lung ventilation perfusion scan helped to rule out chronic venous thromboembolic disease.
Intracardiac shunting due to an atrial septal defect or patent foramen ovale. Echocardiography with agitated saline injection was performed to rule out an intracardiac shunt.
Treatment
Based on our patient's clinical deterioration and the size of his shunt, he was advised of possible treatment options, which included transarterial embolisation and surgical ligation. He declined all invasive interventions. Treatment was supportive, including a low salt diet, afterload reduction, judicious diuretics and compressive stockings.
Outcome and follow-up
At 2-year follow-up, the patient had no evidence of progression of his right heart failure or worsening of dyspnoea. We presumed that effective pharmacological manipulation of volume and peripheral vascular resistance had prevented the shunt from increasing in flow and physiological impact.
The patient was diagnosed with an aggressively metastasising sarcoma around the time of his last visit with us. He refused cancer therapy and chose hospice care. He succumbed to the cancer within 6 months of diagnosis.
Discussion
Renal arteriovenous fistulae are extra-renal artery-to-vein connections without an intermediary capillary bed. They are rare and can be congenital, acquired or idiopathic in aetiology. Congenital arteriovenous (AV) fistulae represent 14–24% of total, while acquired fistulae represent 70–80% of total, and result from trauma or are iatrogenic following invasive diagnostic or surgical procedures.1 2 The idiopathic type, due to erosion of an artery into a vein without preceding trauma, is the least common at approximately 2.8% of total.1–3 The aetiology in this case is not clear. The patient had a history of lumbar surgery in the region of the fistulae, an argument for iatrogenic, while the tortuous nature of the fistulae made a congenital aetiology seem more likely.
AV fistulae between systemic arterial and venous systems are a form of left-to-right shunt. The most common cardiopulmonary complication from a large left-to-right shunt is high-output cardiac failure.4 The high-output state leads initially to volume overload and subsequently to pressure overload, resulting in left heart dysfunction. Right heart dysfunction, when present, is almost always felt to be secondary. Our patient had isolated right heart dysfunction secondary to pulmonary hypertension. His measured pulmonary pressures were only mildly elevated, but he presented with overt right heart failure. Left heart function was normal as evidenced by normal left heart measurements on echocardiography and normal pulmonary capillary wedge pressure. Such a presentation is atypical, but has been reported in isolated case reports.5
A mechanism for isolated pulmonary complications has been proposed. AV fistulae act as a conduit between the high-pressure arterial system and the low-pressure venous system, thus transmitting higher pressures to the pulmonary circulation. Owing to the pliable nature of this vascular bed, an initial increase in pressure leads to reflex vasodilation, but it has been postulated that persistence of high pressures causes mechanical strain and vascular remodelling, in the form of endothelial and smooth muscle proliferation, likely mediated by endothelin.6 This process can result in pulmonary arterial hypertension and right heart failure. Similar reports of development of pulmonary hypertension in patients with an extracardiac arteriovenous connection are seen in patients with AV fistulae for dialysis in end-stage renal disease and hereditary haemorrhagic telangiectasia.7 8
Diagnosis requires a high level of suspicion, and is based on imaging modalities such as ultrasound with Doppler studies, CT, MRI and arteriography/venography.2 Management involves destruction of the shunt channel, with several options; obliteration of the fistulous connection by trans-arterial embolisation, by surgical ligation of the feeding vessels, or by nephrectomy. Reports indicate reversal of pulmonary hypertension and resolution of symptoms with appropriate treatment.9
In summary, we have described an unusual case in which dyspnoea led to a diagnosis of right heart failure and eventually to an extrathoracic process, a left-to-right shunt via a renal arteriovenous fistulae.
Learning points.
Arteriovenous (AV) fistulae are rare and should not be forgotten in the differential diagnoses of pulmonary hypertension, right heart failure and dyspnoea.
Isolated right heart impact of a left-to-right shunt is unusual but has been described in isolated case reports.
Renal AV fistulae can be congenital, acquired or idiopathic. The acquired type is the most common, following trauma or iatrogenic invasive diagnostic or surgical procedures such as spine surgery and renal biopsy.
Acknowledgments
Jennifer McCarty, MD Deptartment of Radiology, University of Arkansas for Medical Sciences.
Footnotes
Competing interests: None declared.
Patient consent: Not Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Maldonado JE, Sheps SG. Renal arteriovenous fistula. Postgrad Med 1966;40:263–9. [DOI] [PubMed] [Google Scholar]
- 2.Crotty KL, Orihuela E, Warren MM. Recent advances in the diagnosis and treatment of renal arteriovenous malformations and fistulas. J Urol 1993;150(5 Pt 1):1355–9. [DOI] [PubMed] [Google Scholar]
- 3.Nagahara A, Nishimura K, Okuyama A. A giant idiopathic renal arteriovenous fistula associated with high-output heart failure. Int J Urol 2009;16:648–9. 10.1111/j.1442-2042.2009.02318.x [DOI] [PubMed] [Google Scholar]
- 4.MacRae JM, Pandeya S, Humen DP et al. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis 2004;43:e17–22. 10.1053/j.ajkd.2004.01.016 [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Morrison JF, Bower TC et al. Pulmonary hypertension in the setting of acquired systemic arteriovenous fistulas. Mayo Clin Proc 2003;78:908–12. 10.4065/78.7.908 [DOI] [PubMed] [Google Scholar]
- 6.Nakhoul F, Yigla M, Gilman R et al. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol Dial Transplant 2005;20:1686–92. 10.1093/ndt/gfh840 [DOI] [PubMed] [Google Scholar]
- 7.Kosmadakis G, Aguilera D, Carceles O et al. Pulmonary hypertension in dialysis patients. Ren Fail 2013;35:514–20. 10.3109/0886022X.2013.766559 [DOI] [PubMed] [Google Scholar]
- 8.Peacock AJ. Commentary: unusual causes of pulmonary hypertension. Thorax 1997;52:1013 10.1136/thx.52.11.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson MR, Giblin L, Brown A et al. Reversal of pulmonary hypertension after ligation of a brachiocephalic arteriovenous fistula. Am J Kidney Dis 2002;40:E8 10.1053/ajkd.2002.34932 [DOI] [PubMed] [Google Scholar]


