Abstract
The association of idiopathic intracranial hypertension (IIH) with stenosis or narrowing of the transverse sinuses (TSs) is well known. However, there is debate as to whether the stenosis is a cause or consequence. Here we describe a case of IIH and narrowing of the TSs, with four relapses and recoveries after repeated CSF diversions with lumbar puncture (LP) over 2 months. Subsequently, implantation of a lumboperitoneal shunt (LPrS) ensured recovery. MR venography 20 months after LPrS showed normally calibrated TSs. We show repeated MR venography findings before and after the LPs, and discuss the pathogenesis of IIH in terms of the cause and effect relationship between IIH and sinus collapse.
Keywords: Intracranial Pressure, Stenosis, Magnetic Resonance Angiography
Background
Debate about the pathogenesis of idiopathic intracranial hypertension (IIH) is focused on the increase in pressure of the intracranial venous sinuses. Previous studies have reported stenosis of the transverse sinuses (TSs) without thrombosis in IIH.1 2 Nevertheless, the cause and effect relationship between these two entities is not clear.
Here we describe a patient with IIH who underwent MR venography (MRV) both before and after multiple lumbar punctures (LPs).
Case presentation
A 32-year-old man was admitted to our hospital with a history of headaches over the past 6 months and visual impairment over the past 2 months. His headaches were located in the right frontal area, with a band-like tightness or pressure across the head. He had blurred vision and a sensation of dark spots. Fundoscopic examination showed papilledema. Other neurological findings were normal. Lumbar CSF opening pressure was 290 mm H2O. CSF (50 mL) was drained, which eliminated the headache. Acetazolamide treatment was started, and the patient was discharged home with a diagnosis of IIH.
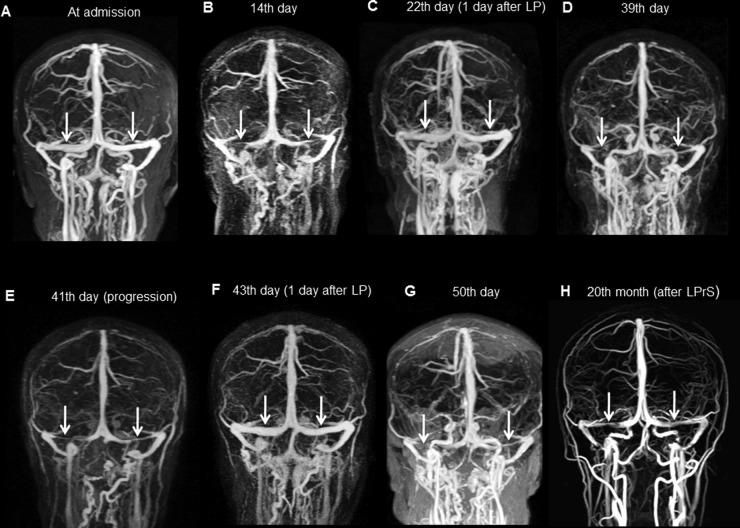
After 2 weeks, he was readmitted to our center with severe headache. Fundus examination showed worsening of the papilledema. Cranial MRV showed thinning/stenosis of the bilateral TSs (figure 1B). LP revealed a high opening pressure. CSF (40 mL) was drained, and an intravenous heparin infusion was started for the possibility of sinus thrombosis. Markers for vasculitis syndromes, a thrombophilic polymorphism panel, and HLA-B51 test were negative. After LP, the patient’s headache improved. MRV, performed 24 h after LP, showed TSs with a normal caliber (figure 1C), and therefore the heparin infusion was stopped.
Figure 1.
(A) MR venography (MRV), performed at the first admission, showing open sinuses. (B) MRV, at the second attack, showing bilateral transverse sinus (TS) stenosis (arrows). (C) After intravenous heparinization and lumbar puncture (LP), follow-up screening showed open sinuses. (D) Fourth attack under warfarin treatment: stenotic TSs (arrows). (E) Stenosis showing progression (arrows). (F) After LP, reopened sinuses. (G) Fifth attack: TSs. (H) Twenty months after lumboperitoneal shunt–MRV, normal calibrated TSs.
Ten days later, the headaches returned. CT venography showed re-collapse of the bilateral TSs. LP opening pressure was 350 mm H2O and 30 mL of CSF were drained off. Intravenous heparin infusion and warfarin were started. He was discharged home with warfarin.
Two days after discharge, he was admitted with the same symptoms, and MRV again showed bilateral collapsed TSs (figure 1D). Despite a heparin infusion, the headaches continued to increase in severity. Repeated MRV showed progression of bilateral TS stenosis (figure 1E). After normalization of his coagulation parameters, LP (for the fourth time) again improved the patient's headache (figure 1F). The accuracy of the diagnosis was re-evaluated at this time. The clinical course and radiological findings indicated TS collapse due to IIH. Although the patient's symptoms improved after LP, permanent recovery could not be obtained (table 1).
Table 1.
Clinical course of the patient
| Day | Admission | Day 14 | Day 22 | Day 30 | Day 39 | Day 41 | Day 43 | Day 50 | 24th month |
|---|---|---|---|---|---|---|---|---|---|
| Symptoms (before LP) | Headache visual loss | Headache | No | Headache | Headache | Headache | No | Headache | No |
| Papilledema | Present | Present | Reduced | Present | Present | Present | Reduced | Present | No |
| Opening pressure | 290 | 300 | 160 | 350 | Absent | 330 | No | 340 | No |
| Drainage volume (mL) | 50 | 30 | 0 | 30 | 0 | 30 | 0 | 40 | No |
| MRV | N | TS col | N | TS col (CTA) | TS col | TS col | N | TS col | N (at 20th month) |
| Anticoagulation | – | Hep started | Hep stopped | Hep, warfarin started | Warfarin continued*, hep started | Hep stop | None | LPrS | No |
| Recovery | Yes | Yes | – | Yes | No | Yes | Yes | Yes | Yes |
*International normalized ratio efficient.
CTA, computerised tomography angiography; Hep, heparin; LP, lumber puncture; LPrS, lumboperitoneal shunt; MRV, MR venography; N, normal; TSs col, transverse sinus collapse.
Finally, a lumboperitoneal shunt (LPrS) was inserted. MRV recorded at the 20 month follow-up showed TSs with a normal caliber (figure 1H). At the 24 month follow-up, he was free of symptoms (figure 2).
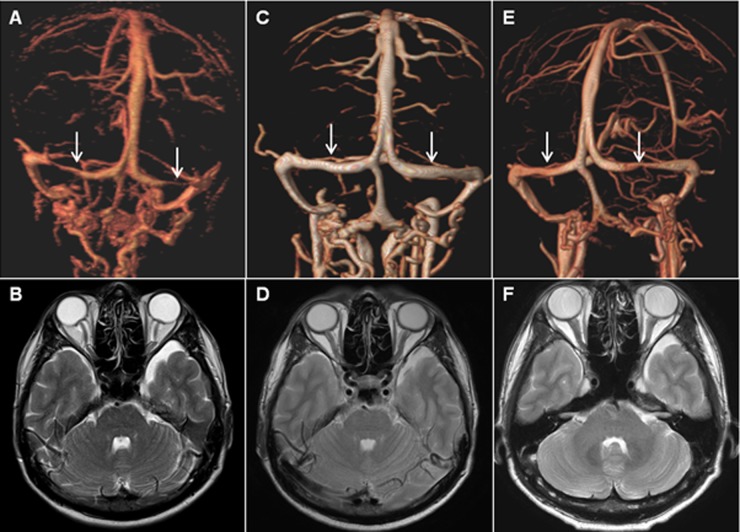
Figure 2.
Volume rendering MR venography images showing alterations in the bilateral transverse sinuses: at the moment of headache (A), 1 day after lumbar puncture (C), and follow-up image 20 months after lumboperitoneal shunt (E). Axial T2 weighted images showing posterior sclera flattening, vertical tortuosity of the optic nerves, and prominent CSF space around the optic nerves at the moment of headache (B). Images 1 day after lumbar puncture (D) and follow-up 20 months after lumboperitoneal shunt (F), demonstrate an improvement in the abnormal orbital findings. Arrows indicate transverse sinus collapse.
Discussion
The association of IIH with narrowing of the TSs is well known. TS stenosis was reported in more than 90% of IIH patients.1 In fact, narrowing of the TSs on MRV can result from various causes, such as sinus thrombosis, true stenosis, compression from increased intracranial pressure (ICP), and congenital/structural thinness.
In particular, the association of IIH with stenosis or narrowing of the TSs is well known.1–4 However, it is not clear whether narrowing of the sinuses is a cause or consequence of IIH. IIH itself can cause stenosis, but there are conflicting reports about IHH pathophysiology. Several studies have addressed this association, and stent placement trials have been conducted. One report showed no evidence for the use of endovascular treatment4 whereas in another study, four patients with IIH resistant to alternative treatments responded to an endovascular stent method.5 In a recent case series, it was reported that in 49 of 52 patients, who were diagnosed with IIH and showed stenosis either at the sagittal sinuses or TSs bilaterally or one dominant were cured of all IIH symptoms after stent placement in the TSs. In this study, sagittal sinus pressures and pressure gradients across the stenosis were measured before and after the procedure, which provided excellent documentation of the improvement in the underlying pathology with stent placement.6
There are some reports of secondary sinus stenosis due to ICP. These studies reported treatment of sinus stenosis with diversion methods, such as repeated LP or LPrS.4 5 7 8 King et al defined a subgroup of IIH patients where a smooth tapering compression of the TSs appeared. They reported that this narrowing resolved when CSF pressure was lowered. As a result, they suggested compression as a consequence of the pressure increase but also a contributing factor to this cycle.2
The clinical and radiological course of our case suggested that raised ICP, above a threshold, led to compression of the TSs which resulted in a vicious cycle that developed more easily every time due to possible mechanical damage at the TSs. In contrast, Bono et al demonstrated persistent TS stenosis in their 14 patients over 6 years of follow-up; nine were medically controlled, and normalized CSF pressure at follow-up LPs was found. As a result, they demonstrated no relation between sinus stenosis and ICP and, in addition, suggested that other coincidental factors may play a role, together with TSs.9 On the other hand, Rohr et al8 showed reversal of bilateral TSs after treatment in 47% of their IIH patients showing TSs.
We suggest that sinus collapse, shown here in our patient, may be a different subgroup of the entity ‘stenotic sinus’ in which clinical contributions and correlations may be more significant. Also, collapse of the TSs should be kept in mind in treatment resistant patients, as in our case, avoiding useless and risky anticoagulation. A venous sinus collapse cycle in these patients can be an important laboratory criterion to predict poor prognosis, and may be useful in determining the treatment management. For further treatment options, such as LPrS, repeat MRV may provide crucial clues about the response to this surgical option as well as the selection criteria for surgery. Future studies, in large case series, must be conducted to prove this hypothesis.
Learning points.
Clinicians should be aware of the association between IIH and dural sinus stenosis.
Sinus collapse can be considered a distinct subgroup of ‘stenotic sinus’.
A venous sinus collapse cycle may be an important criterion to predict poor prognosis, and may be useful in determining treatment management.
Repeat MR venography may provide crucial clues about the prognosis of IIH and the utility of further treatment options, such as LPrS.
Footnotes
Contributors: HO made substantial contributions to the acquisition and interpretation of the data, and was involved in drafting the manuscript. RG and YGO made substantial contributions to the interpretation of the data, were involved in drafting the manuscript or revising it critically for important intellectual content, and gave final approval of the version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Farb RI, Vanek I, Scott JN et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003;60:1418–24. 10.1212/01.WNL.0000066683.34093.E2 [DOI] [PubMed] [Google Scholar]
- 2.King JO, Mitchell PJ, Thomson KR et al. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002;58:26–30. 10.1212/WNL.58.1.26 [DOI] [PubMed] [Google Scholar]
- 3.Higgins JNP, Cousins C, Owler BK et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003;74:1662–6. 10.1136/jnnp.74.12.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins JNP, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004;62:1907–8. 10.1212/01.WNL.0000125285.44539.D7 [DOI] [PubMed] [Google Scholar]
- 5.Owler BK, Parker G, Halmagyi M et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 2003;98:1045–55. 10.3171/jns.2003.98.5.1045 [DOI] [PubMed] [Google Scholar]
- 6.Ahmed RM, Wilkinson M, Parker GD et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol 2011;32:1408–14. 10.3174/ajnr.A2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004;62:1445–6. 10.1212/01.WNL.0000120750.40453.64 [DOI] [PubMed] [Google Scholar]
- 8.Rohr A, Dorner L, Stingele R et al. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2007;28:656–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Bono F, Giliberto C, Mastrandrea C et al. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology 2005;65:1090–3. 10.1212/01.wnl.0000178889.63571.e5 [DOI] [PubMed] [Google Scholar]