Abstract
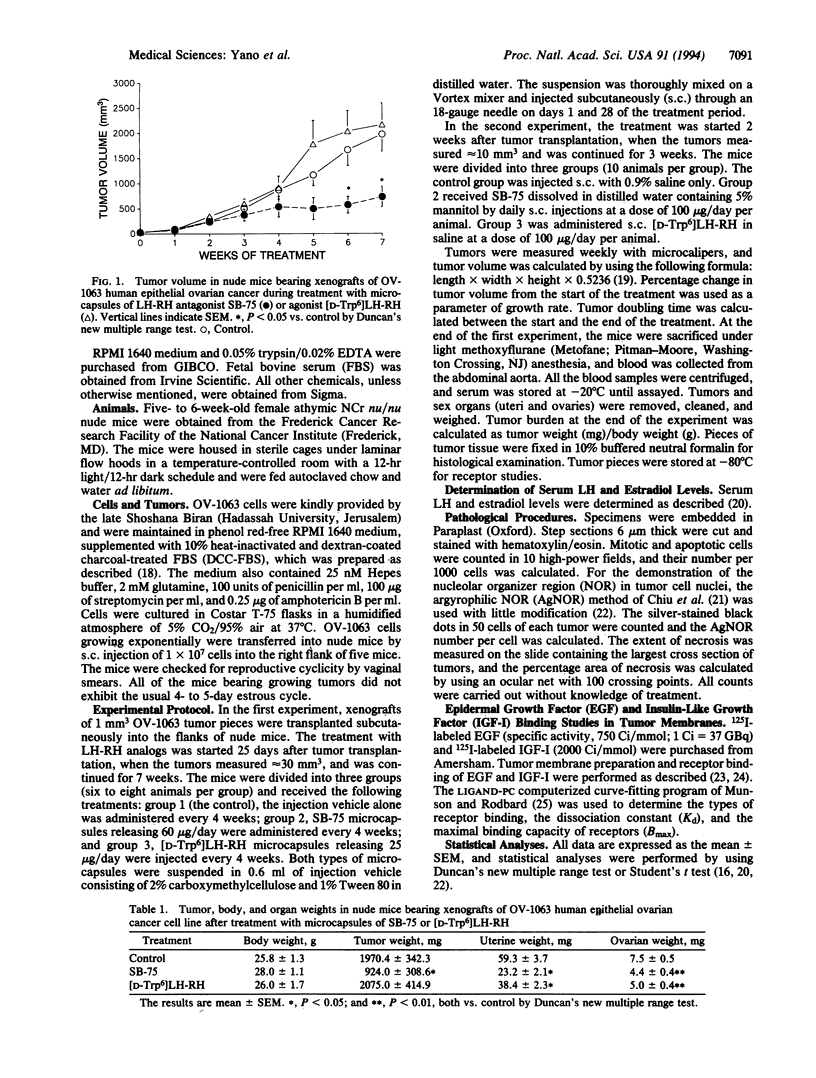
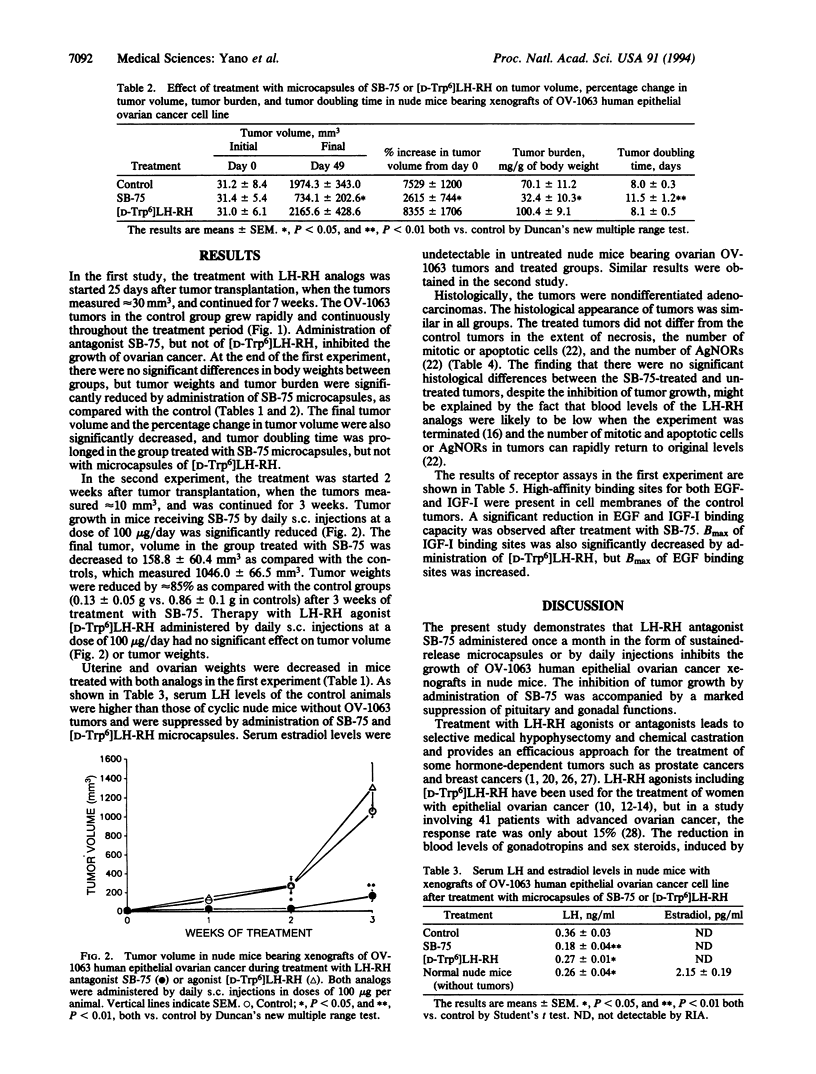
Female athymic nude mice bearing xenografts of OV-1063 human epithelial ovarian cancer cell line were treated with potent luteinizing hormone (LH)-releasing hormone (LH-RH) antagonist SB-75 (Cetrorelix; [Ac-D-Nal(2)1, D-Phe(4 CI)2, D-Pal(3)3, D-Cit6, D-Ala10]LH-RH in which Ac-D-Nal(2) = N-acetyl-3-(2-naphthyl)-D-alanine, D-Phe(4CI) = 4-chloro-D-phenylalanine, D-Pal(3) = 3-(3-pyridyl)-D-alanine, and D-Cit = D-Citrulline) or with the agonist [D-Trp6]LH-RH. In the first experiment, SB-75 and [D-Trp6]LH-RH were administered in the form of microcapsules releasing 60 and 25 micrograms/day, respectively. In the second study, the analogs were given by daily s.c. injections in doses of 100 micrograms/day. In both experiments, tumor growth, as measured by reduction in tumor volume, percentage change in tumor volume, tumor burden, and increase in tumor doubling time, was significantly inhibited by treatment with SB-75 but not with [D-Trp6]LH-RH. Uterine and ovarian weights were reduced and serum LH levels decreased by administration of either analog. Chronic treatment with SB-75 greatly reduced the concentration of receptors for epidermal growth factor and insulin-like growth factor I in tumor cell membranes, a phenomenon that might be related to tumor growth inhibition. It is possible that the antitumoral effects of SB-75 on OV-1063 ovarian cancers are exerted not only through the suppression of the pituitary-gonadal axis, but also directly. In view of its strong inhibitory effect on the growth of OV-1063 ovarian cancers in vivo, the potent LH-RH antagonist SB-75 might be considered for possible hormonal therapy of advanced epithelial ovarian carcinoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz S., Csernus V. J., Janaky T., Bokser L., Fekete M., Schally A. V. New antagonists of LHRH. II. Inhibition and potentiation of LHRH by closely related analogues. Int J Pept Protein Res. 1988 Dec;32(6):425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Rodriguez G. C., Kamel A., Dodge R. K., Soper J. T., Clarke-Pearson D. L., Bast R. C., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am J Obstet Gynecol. 1991 Feb;164(2):669–674. doi: 10.1016/s0002-9378(11)80044-x. [DOI] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1992. CA Cancer J Clin. 1992 Jan-Feb;42(1):19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- Bruckner H. W., Motwani B. T. Treatment of advanced refractory ovarian carcinoma with a gonadotropin-releasing hormone analogue. Am J Obstet Gynecol. 1989 Nov;161(5):1216–1218. doi: 10.1016/0002-9378(89)90669-8. [DOI] [PubMed] [Google Scholar]
- Chiu K. Y., Loke S. L., Wong K. K. Improved silver technique for showing nucleolar organiser regions in paraffin wax sections. J Clin Pathol. 1989 Sep;42(9):992–994. doi: 10.1136/jcp.42.9.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernus V. J., Szende B., Groot K., Redding T. W., Schally A. V. Development of radioimmunoassay for a potent luteinizing hormone-releasing hormone antagonist. Evaluation of serum levels after injection of [Ac-3-(2-naphthyl)-D-Ala1, D-Phe(pCl)2, 3-(3-pyridyl)-D-Ala3, D-Cit6, D-Ala10] LHRH. Arzneimittelforschung. 1990 Feb;40(2 Pt 1):111–118. [PubMed] [Google Scholar]
- Eidne K. A., Flanagan C. A., Harris N. S., Millar R. P. Gonadotropin-releasing hormone (GnRH)-binding sites in human breast cancer cell lines and inhibitory effects of GnRH antagonists. J Clin Endocrinol Metab. 1987 Mar;64(3):425–432. doi: 10.1210/jcem-64-3-425. [DOI] [PubMed] [Google Scholar]
- Emons G., Ortmann O., Becker M., Irmer G., Springer B., Laun R., Hölzel F., Schulz K. D., Schally A. V. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res. 1993 Nov 15;53(22):5439–5446. [PubMed] [Google Scholar]
- Emons G., Pahwa G. S., Brack C., Sturm R., Oberheuser F., Knuppen R. Gonadotropin releasing hormone binding sites in human epithelial ovarian carcinomata. Eur J Cancer Clin Oncol. 1989 Feb;25(2):215–221. doi: 10.1016/0277-5379(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Emons G., Schally A. V. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum Reprod. 1994 Jul;9(7):1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- Fekete M., Wittliff J. L., Schally A. V. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J Clin Lab Anal. 1989;3(3):137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- Foekens J. A., van Putten W. L., Portengen H., Rodenburg C. J., Reubi J. C., Berns P. M., Henzen-Logmans S. C., van der Burg M. E., Alexieva-Figusch J., Klijn J. G. Prognostic value of pS2 protein and receptors for epidermal growth factor (EGF-R), insulin-like growth factor-1 (IGF-1-R) and somatostatin (SS-R) in patients with breast and ovarian cancer. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):815–821. doi: 10.1016/0960-0760(90)90425-k. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hershkovitz E., Marbach M., Bosin E., Levy J., Roberts C. T., Jr, LeRoith D., Schally A. V., Sharoni Y. Luteinizing hormone-releasing hormone antagonists interfere with autocrine and paracrine growth stimulation of MCF-7 mammary cancer cells by insulin-like growth factors. J Clin Endocrinol Metab. 1993 Oct;77(4):963–968. doi: 10.1210/jcem.77.4.8408472. [DOI] [PubMed] [Google Scholar]
- Jäger W., Wildt L., Lang N. Some observations on the effect of a GnRH analog in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1989 Aug;32(2):137–148. doi: 10.1016/0028-2243(89)90195-0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nash J. D., Ozols R. F., Smyth J. F., Hamilton T. C. Estrogen and anti-estrogen effects on the growth of human epithelial ovarian cancer in vitro. Obstet Gynecol. 1989 Jun;73(6):1009–1016. doi: 10.1097/00006250-198906000-00021. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. Chemotherapy of ovarian cancer. Semin Oncol. 1984 Sep;11(3):251–263. [PubMed] [Google Scholar]
- Parmar H., Nicoll J., Stockdale A., Cassoni A., Phillips R. H., Lightman S. L., Schally A. V. Advanced ovarian carcinoma: response to the agonist D-Trp-6-LHRH. Cancer Treat Rep. 1985 Nov;69(11):1341–1342. [PubMed] [Google Scholar]
- Parmar H., Phillips R. H., Rustin G., Lightman S. L., Schally A. V. Therapy of advanced ovarian cancer with D-Trp-6-LH-RH (decapeptyl) microcapsules. Biomed Pharmacother. 1988;42(8):531–538. [PubMed] [Google Scholar]
- Parmar H., Rustin G., Lightman S. L., Phillips R. H., Hanham I. W., Schally A. V. Response to D-Trp-6-luteinising hormone releasing hormone (Decapeptyl) microcapsules in advanced ovarian cancer. Br Med J (Clin Res Ed) 1988 Apr 30;296(6631):1229–1229. doi: 10.1136/bmj.296.6631.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. M., Zimniski S. J. A long-acting gonadotropin-releasing hormone agonist inhibits the growth of a human ovarian epithelial carcinoma (BG-1) heterotransplanted in the nude mouse. Obstet Gynecol. 1990 Aug;76(2):264–267. [PubMed] [Google Scholar]
- Rao B. R., Slotman B. J. Endocrine factors in common epithelial ovarian cancer. Endocr Rev. 1991 Feb;12(1):14–26. doi: 10.1210/edrv-12-1-14. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V., Radulovic S., Milovanovic S., Szepeshazi K., Isaacs J. T. Sustained release formulations of luteinizing hormone-releasing hormone antagonist SB-75 inhibit proliferation and enhance apoptotic cell death of human prostate carcinoma (PC-82) in male nude mice. Cancer Res. 1992 May 1;52(9):2538–2544. [PubMed] [Google Scholar]
- Rodriguez G. C., Berchuck A., Whitaker R. S., Schlossman D., Clarke-Pearson D. L., Bast R. C., Jr Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. II. Relationship between receptor expression and response to epidermal growth factor. Am J Obstet Gynecol. 1991 Mar;164(3):745–750. doi: 10.1016/0002-9378(91)90508-o. [DOI] [PubMed] [Google Scholar]
- Segal-Abramson T., Kitroser H., Levy J., Schally A. V., Sharoni Y. Direct effects of luteinizing hormone-releasing hormone agonists and antagonists on MCF-7 mammary cancer cells. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2336–2339. doi: 10.1073/pnas.89.6.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon W. E., Albrecht M., Hänsel M., Dietel M., Hölzel F. Cell lines derived from human ovarian carcinomas: growth stimulation by gonadotropic and steroid hormones. J Natl Cancer Inst. 1983 May;70(5):839–845. [PubMed] [Google Scholar]
- Srkalovic G., Szende B., Redding T. W., Groot K., Schally A. V. Receptors for D-Trp6-luteinizing hormone-releasing hormone, somatostatin, and insulin-like growth factor I in MXT mouse mammary carcinoma. Proc Soc Exp Biol Med. 1989 Dec;192(3):209–218. doi: 10.3181/00379727-192-42987. [DOI] [PubMed] [Google Scholar]
- Szende B., Srkalovic G., Groot K., Lapis K., Schally A. V. Growth inhibition of mouse MXT mammary tumor by the luteinizing hormone-releasing hormone antagonist SB-75. J Natl Cancer Inst. 1990 Mar 21;82(6):513–517. doi: 10.1093/jnci/82.6.513. [DOI] [PubMed] [Google Scholar]
- Szepeshazi K., Korkut E., Schally A. V. Decrease in the AgNOR number in Dunning R3327 prostate cancers after treatment with an agonist and antagonist of luteinizing hormone-releasing hormone. Am J Pathol. 1991 May;138(5):1273–1277. [PMC free article] [PubMed] [Google Scholar]
- Thompson M. A., Adelson M. D., Kaufman L. M. Lupron retards proliferation of ovarian epithelial tumor cells cultured in serum-free medium. J Clin Endocrinol Metab. 1991 May;72(5):1036–1041. doi: 10.1210/jcem-72-5-1036. [DOI] [PubMed] [Google Scholar]
- Tomayko M. M., Reynolds C. P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- Wimalasena J., Dostal R., Meehan D. Gonadotropins, estradiol, and growth factors regulate epithelial ovarian cancer cell growth. Gynecol Oncol. 1992 Sep;46(3):345–350. doi: 10.1016/0090-8258(92)90230-g. [DOI] [PubMed] [Google Scholar]
- Yano T., Korkut E., Pinski J., Szepeshazi K., Milovanovic S., Groot K., Clarke R., Comaru-Schally A. M., Schally A. V. Inhibition of growth of MCF-7 MIII human breast carcinoma in nude mice by treatment with agonists or antagonists of LH-RH. Breast Cancer Res Treat. 1992;21(1):35–45. doi: 10.1007/BF01811962. [DOI] [PubMed] [Google Scholar]
- Yano T., Pinski J., Groot K., Schally A. V. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 1992 Aug 15;52(16):4545–4547. [PubMed] [Google Scholar]
- Yano T., Pinski J., Radulovic S., Schally A. V. Inhibition of human epithelial ovarian cancer cell growth in vitro by agonistic and antagonistic analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1701–1705. doi: 10.1073/pnas.91.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T., Pinski J., Szepeshazi K., Milovanovic S. R., Groot K., Schally A. V. Effect of microcapsules of luteinizing hormone-releasing hormone antagonist SB-75 and somatostatin analog RC-160 on endocrine status and tumor growth in the Dunning R-3327H rat prostate cancer model. Prostate. 1992;20(4):297–310. doi: 10.1002/pros.2990200405. [DOI] [PubMed] [Google Scholar]
- Yee D., Morales F. R., Hamilton T. C., Von Hoff D. D. Expression of insulin-like growth factor I, its binding proteins, and its receptor in ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5107–5112. [PubMed] [Google Scholar]
- Zimniski S. J., Garola R. E., Fendl K., Peterson C. M. Endocrine characterization of a human ovarian carcinoma (BG-1) established in nude mice. Steroids. 1989 Dec;54(6):593–606. doi: 10.1016/0039-128x(89)90083-4. [DOI] [PubMed] [Google Scholar]


