Abstract
Objective:
The aim of this study was to compare the QEEG of adolescents affected by bipolar II disorder with age and gender matched healthy controls, and to extract the characteristics of the alpha frequency band to better understand this disorder.
Methods:
Twenty one adolescents affected by acute episodes of bipolar II disorder (BMD II), both hypomanic and depressive episodes, were selected via convenience sampling based on DSM IV criteria and child and adolescent psychiatrist diagnosis. Eleven patients were going through a hypomanic episode and 10 patients were going through a depression episode. Of the participants, 18 who were matched with the patient group participated in this study as a normal group. Any major comorbidities and intellectual disabilities were excluded through applying K-SADS-PL and Raven’s IQ test for all the patients and the healthy participants. Electroencephalogram signals were obtained according to 10–20 international system by 21 electrodes from participants in open and closed eyes in a resting state. We selected 40 seconds length segments from each recorded EEG signals that had minimal noise and artifacts. Power spectrum density (PSD) was estimated for each segment and extracted alpha band frequency. We used only referential (unipolar) montage for comparison. Eventually, data were analyzed by independent Mann-Whitney test and independent t test.
Results:
We observed significant differences in the alpha frequency band in some brain regions. Alpha power increased in the fronto-central region and right parietal lobe in the patients (P < 0.05). In the patients with BMD II, entropy of alpha oscillations was larger than the normal participants in the central region and in the F3, F4 and P4 channels. Also, there were differences in the variance of alpha oscillations in these regions between the two groups (P < 0.05). In the occipital lobe, alpha wave had different skewness between the two groups (P < 0.05).
Conclusion:
Thalamus as a generator and modulator of at least a part of alpha oscillations may be involved in this disorder and hence this explains the major symptoms like distractibility and inattention in both hypomanic and depressive episodes of bipolar II disorder.
Keywords: Bipolar II Disorder (BMD II), Quantitative EEG (QEEG), Alpha Oscillations, Power Spectra, Thalamus, Attention
Bipolar disorder is one of the most severe and chronic psychiatry disorders with mood involvement appearing in a form of euphoric or elevated mood episodes as seen in mania or hypomania and in a form of low mood episodes as seen in depressive episode (1). This disorder is often considered to be in the spectrum in which the one end is the classic bipolar of bipolar type I disorder and in the other end is the sub-threshold mania or depression (2, 3). Bipolar II disorder is generally considered as a milder form of bipolar I disorder and according to DSM IV criteria, the number of criteria described for the both disorders is the same (1, 4, 5). The diagnostic criteria for these disorders are only based on the severity of symptoms; in the BMD II there is no significant losses of function in various domains of life, but in the BMD I the patient will have psychosis or marked loss of functions in various fields of her/his life (5). However, both disorders have significant morbidity and mortality (6). Bipolar II disorder has more lifetime prevalence than bipolar I disorder (1, 5). Although the first onset of bipolar II disorder often occurs within late adolescence and early adulthood, it is frequently seen in children and adolescents (7, 8). Although bipolar disorder appear in late adolescence and early adulthood with more clear and definite symptoms as episodic form, the early onset of the disorder is often presented with atypical and chronic symptoms without episodic features (7, 8). Also, it might be that the child or adolescent with bipolar II disorder experiences several episodes of elation or depression during a day; and it is possible that he/she lives with these symptoms without treatment for a few years and that the disorder severely impacts his/her educational and interpersonal performance and interfere with his/her personal and social development (8).
Accordingly, this disorder has a diagnosis difficulty in childhood and adolescence. Due to the mentioned above issues, under-diagnosis and mis-diagnosis in this disorder is common (6, 9). One of the most important symptoms in BMD II is distractibility and decreased ability of concentration in both hypomanic and depressive episodes (1).
Alpha frequency band is defined in 8 to 13 Hz range and it has an inverse correlation with attention and concentration particularly selective attention (10). Thalamus as a gate of sensory inputs generates and modulates the EEG alpha oscillation. Also, it plays a critical role in attention and concentration (the roll of alpha band) (10, 11). The increase of thalamic metabolism associated with alpha power decreases in the resting state EEG. Previous studies revealed that attention and concentration are related to increased metabolism in thalamus and reduced activity or power in alpha oscillation in the relevant regions of the human brain (10).
There are almost limited electrophysiology studies on bipolar disorder in both acute and euthymic states, particularly on BMD II. In a previous study, EEG has been recorded in different states of 202 patients with BMD I and it was reported that left-sided abnormalities are more common than the right (12). Increase in the relative power in low the frequency EEG bands (delta and theta bands), results in a decrease in relative alpha power and reduction of the dominant alpha wave (13). Basar et al. (14) examined EEG alpha activity in euthymic bipolar disorder patients and compared it with healthy subjects. The results showed that the the alpha power reduced up to 70% in patients. Ozerdem et al. (15) evaluated brain oscillatory responses in bipolar patients going through a manic episode before and after valproate treatment. They reported that alpha pre-treatment response in patients is lower than healthy subjects in occipital lobe, and after six weeks of treatment with valproate the occipital alpha response decreased more compared to the before treatment and normal subjects. The study conducted by O’Donnell et al. (16) showed a reduction in P300 amplitude and prolonged latency in patients with BMD.
Considering distractibility and inattention as the important symptoms of bipolar disorder, the thalamus key role in attention and its association with alpha activity, we hypothesized that the thalamus is involve in bipolar disorder and that the alpha power can reflect thalamus disturbance.
Material and Methods
Participants
Twenty one patients with BMD II (11 females and 10 males) with the age range of 12 to 18 years (16.1±1.51) and 18 healthy participants (9 females and 9 males) with the age range of 12 to 18 years (16.3±1.32) were recruited for the study. Eleven patients were going through the hypomanic episode and 10 patients were going through depression episode. Handedness of all participants was examined by the Edinburgh test and only the right-handed persons were entered into the study. The patients were diagnosed based on DSM-IV criteria and this was verified by interviews conducted by two child and adolescent psychiatrists. Any major psychiatry comorbidities and intellectual disabilities were excluded through applying K-SADS-PL (Kiddie Schedule for Schizophrenia and Affective Disorders Present and Lifetime versions) and Raven’s IQ test for all patients and healthy participants. Also, any neurological diseases (e.g., history of seizure disorder and severe head injury) were ruled out in all participants. The severity of the hypomanic and depressive episodes of the disorder was assessed through applying YMRS (Young Mania Rating Scale) and BDI-II (Beck Depression Inventory-II) in the patient group. All participants were in a drug free state. Informed consent was obtained from all participants and their families prior to the start of the study. Table 1 demonstrates the demographics information of the participants.
Table 1:
Demographics Information of Adolescents with Bipolar II Disorder and Healthy Adolescents
| Parameters | BMD II (n = 21) | HC (n = 18) | P-value | t (df) |
|---|---|---|---|---|
| Age | 16.1±1.51 | 16.3±1.32 | 0.234 | 1.069 (37) |
| Gender (male, female) | 10, 11 | 9, 9 | 0.473 | −0.451 (37) |
| Current Episode | Hypomanic (11), Depressive (10) | N/A | ||
| YMRS Score | 17.20 ± 2.46 | N/A | ||
| BDI-II Score | 26.31 ± 4.81 | N/A | ||
| Handedness | Right (21) | Right (18) |
Experiment
Signals were recorded from each participant at the psychiatry and psychology research center in Roozbeh hospital. The EEG recording was performed in a noiseless room at a resting state in open and closed eyes conditions for 10 minutes. 10–20 international system was used to place 19 electrodes on the scalp and these electrodes were located on : Fz, Cz, Pz, C3, T3, C4, T4, Fp1, Fp2, F3, F4, F7, F8, P3, P4, T5, T6, O1, O2, A1, A2. A1 and A2 channels were used as references. The signals were recorded between 0.1 and 70 Hz, and the sampling frequency was set to 256 Hz. After preprocessing to remove the noises with a FIR band-pass filter (order 7) between 0.3 and 70 Hz and a notch filter at 50 Hz and to reduce various artifacts, a segment with 40 seconds length was selected for each subject. This segment selection was performed by an experienced neurologist such that minimized artifacts were further analyzed. Short Time Fourier Transform (STFT) was utilized to obtain the alpha wave in the frequency range between 8 to 13 Hz and to estimate the power spectral density (PSD) of alpha oscillations. The idea of the STFT originates from the Fourier transform (FFT). In STFT, FFT is restricted to a fixed time interval to cover the FFT defect. STFT provides a two-dimensional map in terms of time-frequency space, and it is defined as (17):
Where w (t) is a window function with a short time duration and * marks the complex conjugate.
Statistical features are used as typical features since they are easy to implement, have a lower computational burden and show significant properties of EEG signals. Therefore, seven features were extracted from alpha PSD including mean, power, variance, skewness, entropy and threshold mean (18). This threshold was set to 0.7 multiplied by the maximum of the alpha spectrum (17).
Statistical analysis
After feature extraction from the alpha wave, statistical analysis was performed by SPSS version 21 to determine the significant differences between patients and healthy subjects. Based on the normality of the data (according to kolmograph-smirnov test), independent t-test and mannMann-whitney Whitney were applied. The significance level was set at 0.05.
Results
Due to the induction of stress and anxiety, and excessive head movement during the EEG signal recording in the closed eyes state, the analysis was only done on the recorded signals in the open eyes state. Analysis was performed between the patient and control groups and also between those patients going through the hypomanic episode and depressive episode. Analysis of alpha frequency band in the QEEG of the participants revealed that power of the alpha band increases significantly in fashion of bihemispheric fronto-central and temporal, and right parietal regions in F7, F3, FZ, F4, F8, T3, C3, C4, T4 and P4 channels compared with the control group (P < 0.05) (Figure 1). Also, entropy, mean and variance of alpha activity increased significantly in the same fashion in the patient group except in locations of F7, FZ, and F8 compared with healthy subjects (P < 0.05). Figure 2 shows the entropy of alpha oscillations in all channels. The differences were significant only in seven channels but in general the entropy of the alpha wave increased in all channels for patients with BMD II compared with the normal group. Skewness of alpha band frequency increased in patients’ occipital lobes in locations of O1 and O2 compared with the healthy group (P < 0.05). There were no significant differences in the alpha power between the patients with hypomanic and depressive episodes.
Figure 1:
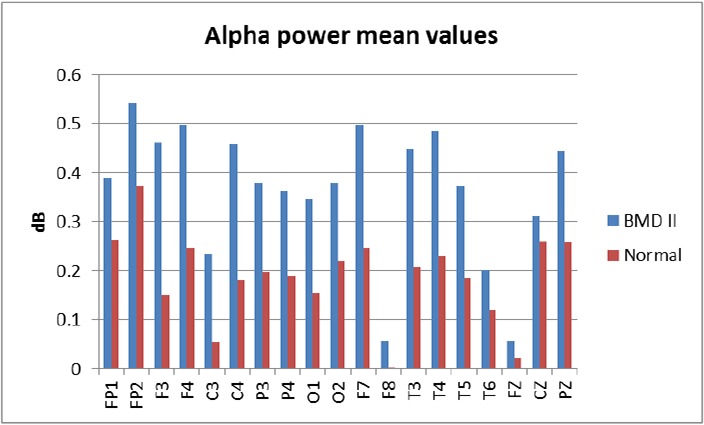
Alpha Wave Power in 19 Channels for Patients with BMD II and Normal Subjects Based on statistical analysis, differences in F7, F3, FZ, F4, F8, T3, C3, C4, T4 and P4 channels were significant (P < 0.05).
Figure 2:
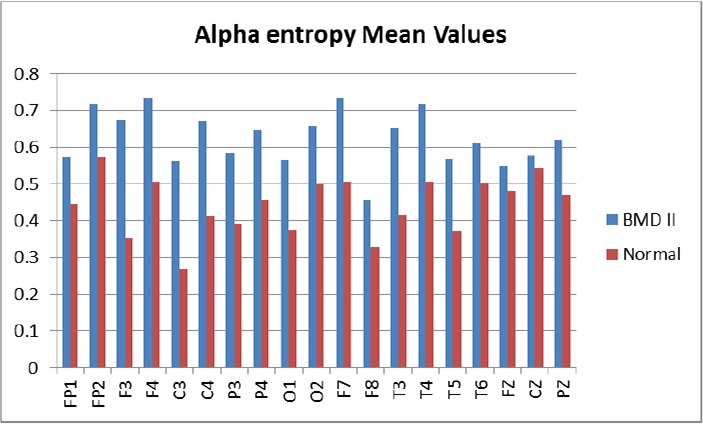
Alpha Wave Entropy in 19 Channels for Patients with BMD II and Normal Participants Based on statistical analysis, differences in F3, C3, T3, F4, C4, T4 and P4 channels were significant (P < 0.05).
Discussion
According to our results, there were significant increases in power, mean, variance and entropy of the alpha band frequency in fronto-central, bilateral temporal and right parietal regions in patients with BMD II in comparison to the healthy group.
Several studies (15, 19–22) reported a decrease in the alpha power in the patients with bipolar disorder which is opposite to our observation, but there are two reasons for the accuracy of our results. First, most of these studies were performed in euthymic state without considering type of the disorder. However our participants were patients with bipolar II disorder with acute episodes (hypomania and depression) and they did not receive any psychotropic drugs. Second, based on our hypothesis, increase of the alpha power corresponds to a decrease in the thalamic metabolism that leads to diminished attention; and this is consistent with the symptoms in the bipolar disorder.
The results obtained from the comparison of hypomanic and depressive episodes of the disorder showed no significant differences between them in the EEG alpha activity (these results are not reported here). Therefore, we can conclude that increased alpha activity and thus decreased thalamic metabolism is independent from the both episodes of the disorder.
Limitations
Sample size and lack of evaluation of alpha band interaction with other frequency bands from EEG are the limitations of this study.
Conclusion
In this study, we evaluated the EEG’s alpha oscillation as an important and controversial component in electrical activity of the brain of adolescents with acute BMD II. In the literature, alpha activity is often regarded as a normal oscillation and it is important to assess the variations of alpha activity in different regions of the brain. In general, according to the obtained results and increased alpha activity observation, we can conclude that thalamus as a generator and modulator of at least a part of alpha oscillations may be involved in this disorder and hence this explains the major symptoms like distractibility and inattention in both hypomanic and depressive episodes of bipolar II disorder.
In the future, dividing the alpha frequency band (i.e. 8–13 Hz) into smaller sub-bands can provide useful information with more detailed. Also, this study needs to be replicated with task for assessment of response QEEG and also could be performed concurrently with PET or fMRI study.
Acknowledgement
The authors wish to thanks who are helped us to conduct this project, especially Dr. Sheikhani and Dr. Nasrabadi and Mr. Parvaneh for cooperation in signal recording and their great advices.
References
- 1.Association AP Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR). City: American Psychiatric Association; 2000. [Google Scholar]
- 2.Bauer M, Pfennig A. Epidemiology of bipolar disorders. Epilepsia 2005; 46: 8–13. [DOI] [PubMed] [Google Scholar]
- 3.Pini S, de Queiroz V, Pagnin D, Pezawas L, Angst J, Cassano GB, et al. Prevalence and burden of bipolar disorders in European countries. European Neuropsychopharmacology 2005; 15: 425–434. [DOI] [PubMed] [Google Scholar]
- 4.Hadjipavlou G, Bond DJ, Yatham LN. Bipolar II disorder in context: a review of epidemiology disability and economic burden. Bipolar II disorder: Modelling, measuring and managing 2012: 46. [Google Scholar]
- 5.Sadock BJ, Sadock VA. Synopsis of psychiatry. 2003. [Google Scholar]
- 6.Vieta E, Gasto C, Otero A, Nieto E, Vallejo J. Differential features between bipolar I and bipolar II disorder. Comprehensive psychiatry 1997; 38: 98–101. [DOI] [PubMed] [Google Scholar]
- 7.Ghaemi S, Ko JY, Goodwin FK. “Cade’s disease” and beyond: misdiagnosis, antidepressant use, and a proposed definition for bipolar spectrum disorder. Canadian journal of psychiatry. Revue canadienne de psychiatrie 2002; 47: 125–134. [DOI] [PubMed] [Google Scholar]
- 8.Yatham LN. Diagnosis and management of patients with bipolar II disorder. The Journal of clinical psychiatry 2004; 66: 13–17. [PubMed] [Google Scholar]
- 9.Angst J. In Debate. Canadian journal of psychiatry 2006; 51: 3–5. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren KA, Larson CL, Schaefer SM, Abercrombie HC, Ward RT, Oakes TR, et al. Thalamic metabolic rate predicts EEG alpha power in healthy control subjects but not in depressed patients. Biological psychiatry 1999; 45: 943–952. [DOI] [PubMed] [Google Scholar]
- 11.Schürmann M, Başar E. Functional aspects of alpha oscillations in the EEG. International Journal of Psychophysiology 2001; 39: 151–158. [DOI] [PubMed] [Google Scholar]
- 12.Small JG, Milstein V, Malloy FW, Medlock CE, Klapper MH. Clinical and quantitative EEG studies of mania. Journal of affective disorders 1999; 53: 217–224. [DOI] [PubMed] [Google Scholar]
- 13.Schulz C, Mavrogiorgou P, Schröter A, Hegerl U, Juckel G. Lithium-induced EEG changes in patients with affective disorders. Neuropsychobiology 2000; 42: 33–37. [DOI] [PubMed] [Google Scholar]
- 14.Başar E, Güntekin B, Atagün I, Gölbaşi BT, Tülay E, Özerdem A. Brain’s alpha activity is highly reduced in euthymic bipolar disorder patients. Cognitive neurodynamics 2012; 6: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Özerdem A, Güntekin B, Tunca Z, Başar E. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain research 2008; 1235: 98–108. [DOI] [PubMed] [Google Scholar]
- 16.O’donnell B, Vohs J, Hetrick W, Carroll C, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology 2004; 53: 45–55. [DOI] [PubMed] [Google Scholar]
- 17.Sheikhani A, Behnam H, Noroozian M, Mohammadi MR, Mohammadi M. Abnormalities of quantitative electroencephalography in children with Asperger disorder in various conditions. Research in Autism Spectrum Disorders 2009; 3: 538–546. [Google Scholar]
- 18.Gudmundsson S, Runarsson TP, Sigurdsson S, Eiriksdottir G, Johnsen K. Reliability of quantitative EEG features. Clinical Neurophysiology 2007; 118: 2162–2171. [DOI] [PubMed] [Google Scholar]
- 19.Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology 1994; 31: 486–494. [DOI] [PubMed] [Google Scholar]
- 20.Gerez M, Tello A. Clinical significance of focal topographic changes in the electroencephalogram (EEG) and evoked potentials (EP) of psychiatric patients. Brain topography 1992; 5: 3–10. [DOI] [PubMed] [Google Scholar]
- 21.Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, et al. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological psychiatry 2008; 63: 693–698. [DOI] [PubMed] [Google Scholar]
- 22.Kano K, Nakamura M, Matsuoka T, Iida H, Nakajima T. The topographical features of EEGs in patients with affective disorders. Electroencephalography and Clinical Neurophysiology 1992; 83: 124–129. [DOI] [PubMed] [Google Scholar]


