Abstract
Background:
Poliomyelitis is still an endemic disease in many areas of the world including Africa and South Asia. Iran is polio free since 2001. However, due to endemicity of polio in neighboring countries of Iran, the risk of polio importation and re-emergence of wild polio virus is high. Case definition through surveillance system is a well-defined method for maintenance of polio eradication in polio free countries.
Methods:
In a cross-sectional survey from 2007 to 2013, we reviewed all the records of under 15 years old patients reported to Acute Flaccid Paralysis Committee (AFPC) in Isfahan province, Iran. All cases were visited by members of the AFPC. Three stool samples were collected from each reported case within 2 weeks of onset of paralysis and sent to National Polio Laboratory in Tehran, Iran, for poliovirus isolation. Data were analyzed by SSPS software (version 22). Student's t-test and Chi-square was used to compare variables. Statistical significance level was set at P < 0.05.
Results:
In this 6-year period 85 cases were analyzed, 54 patients were male (63.5%) and 31 were female (36.5%). The mean age of patients was 5.7 ± 3.9 years. The most common cause of paralysis among these patients was Guillian–Barré syndrome (83.5%). We did not found any poliomyelitis caused by wild polio virus. Only one case of vaccine associated poliomyelitis was reported.
Conclusion:
Since 1992, Iran has a routine and high percent coverage of polio vaccination program for infants (>94%), with six doses of oral polio vaccine (OPV). Accurate surveillance for poliomyelitis is essential for continuing eradication.
Keywords: Acute flaccid paralysis, poliomyelitis, surveillance
INTRODUCTION
Acute flaccid paralysis (AFP) or a floppy paralysis is defined as any case of new onset of hypotonic weakness or complete paralysis in a child aged <15 years old.[1] AFP is a complex clinical syndrome with a broad range of potential etiologies.[2] This includes possible illness due to Poliomyelitis (Polio), Guillian–Barré syndrome (GBS), Transverse Myelitis (TM), Trauma-related anterior horn cell disease, infections caused by non-polio Enteroviruses, and toxins. Polio is a highly infectious disease caused by polioviruses. Polio has been considered a candidate for eradication.[3] The global effort to eradicate polio has become the largest public health initiative in history spearheaded by the World Health Organization (WHO).[4,5] The introduction of Salk inactivated polio vaccine (IPV) in 1995 led to an immediate and the dramatic reduction in both epidemic and endemic polio.[6] Polio has been eradicated in many parts of the world by IPV and trivalent OPV (oral polio vaccine).[7] Polio has been controlled worldwide but still is a threat in a few areas in sub-Saharan Africa, Indian subcontinent and areas with low socioeconomic and involving in war issues.[7,8] As of June 1998, WHO reported two foci of known wild polio virus transmission in Turkey, northern Iraq, Tajikistan, Pakistan, and Afghanistan. In Iran stopping the transmission of polio virus has been pursued through a combination of routine immunization and supplementary immunization campaigns, which are guided by high-quality surveillance.[3] WHO adopted several strategies to control and ultimately eradicate polio from most regions of the world such as routine childhood immunization, supplementary immunization, intensified surveillance and rapid response to identified outbreaks. Surveillance for polio is essential for eradication. Surveillance systems for polio have been developed under guidance of the global polio eradication initiative.[7,9] The existence of sensitive surveillance system and high coverage of routine immunization by six doses of oral polio vaccine (OPV3) for 6 years after birth has led to the eradication of polio in IRAN.[3] Primary care system based on health houses, health posts, and rural and urban health centers are cornerstones for achievement and maintenance of polio eradication.
Surveillance of cases of AFP among children <15 years old is a key component for a well-functioning polio surveillance system. The aim of this study was to detect wild polio virus through a surveillance system from cases of AFP.
METHODS
In a cross-sectional descriptive study, 85 cases of AFP during 2007–2013 were evaluated. We reviewed all the medical records of cases in urban health care center, Isfahan, Iran (Isfahan is located in the center region of Iran). As a rule, all cases of AFP are reported by physicians to neighboring health centers. Cases are reviewed by a committee including a pediatric neurologist, an infectious diseases specialist, and a virologist. Three samples of stool from each patient were transferred to National Polio Laboratory in Tehran, Iran, to rule out polio. AFP is defined as any case of acute hypotonic weakness or complete paralysis in a child aged <15 years old. Transient weakness (e.g., postictal weakness) and acute spastic paralysis were excluded from this survey. Diagnosis of GBS was based on guidelines provided by the National Institute of Neurological Disorders and Stroke. In order to rule out other causes of paralysis physical examination by an expert pediatric neurologist, magnetic resonance imaging (MRI), electromyography, Nerve Conduction Velocity and toxin assay were employed. Patient demographics including age, sex, and final diagnosis were extracted from medical records. Data were analyzed by SPSS software version 16. Statistical analysis was done by Student's t-test and Chi-square test. Level of statistical significance was set at P < 0.05.
RESULTS
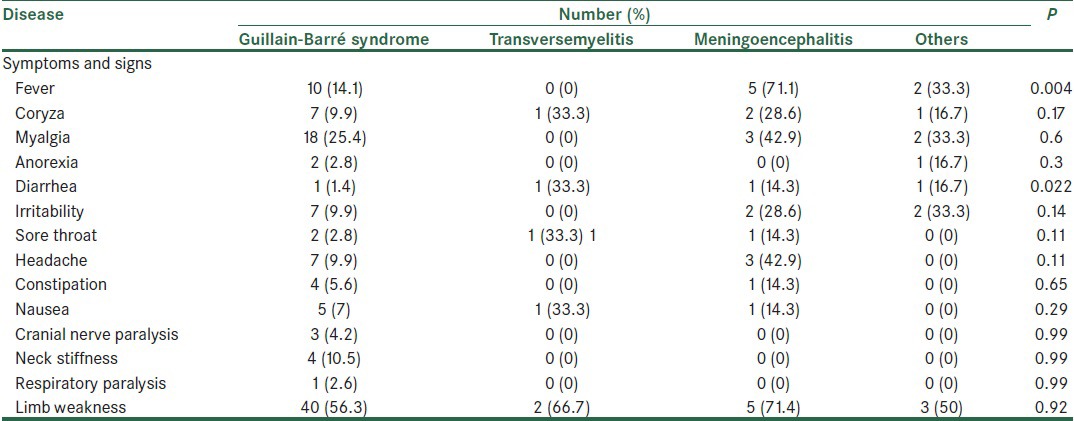
From 85 cases of AFP 54 (63.5%) were male and 31 (36.5%) were female. t-test showed no significant differences between the sex of patients (P = 0.95). Mean age of patients was 5.7 ± 3.9 years with an age range of 1-14 years old. Mean age of male and female patients was 5.8 ± 3.8 and 5.6 ± 4.1 years, respectively., t-test showed no significant differences between the mean age of patients and their sex (P = 0.2). Patients with TM had a higher mean age than other patients and one-way variance analysis showed no significant differences between age and cause of paralysis. 28 (33%) cases resided in rural areas and 57 (67%) were from urban. There was no significant difference between residing location and paralysis. The most common signs and symptoms were fever, muscle pain, anorexia, diarrhea, irritability, sore throat, headache, constipation, nausea, cough, neck stiffness, limb weakness and respiratory paralysis, which are shown in Table 1. As shown in Table 1, there is a relation between fever and diarrhea with diagnosis of AFP (P < 0.05). GBS was the most common cause of AFP with 71 cases (83.5%). Other registered diagnosis were encephalitis and acute disseminated encephalomyelitis in 5 cases, transversemyelitis in 3 cases, cerebellar ataxia in 2 cases, Trauma-related anterior horn cell disease in 2 cases, Syringomyelia in 1 case, and vaccine associated polio poliomyelitis (VAPP) in 1 case. Analysis by exact fisher test showed that there was no significant difference between number of limb paralysis and underlying diseases (P = 0.003). There was no significant difference between cranial nerve paralysis, respiratory muscle paralysis and acute flaccid diseases (P > 0.05).
Table 1.
Distribution of symptoms and signs
DISCUSSION
In this study, the most common cause of AFP was GBS. Majority of patients had symptoms of upper respiratory tract infection before paralysis. Quadriplegia was the most common type of limb involvement (49%). There was no significant difference between age, sex and residing area and the disease (P = 0.17). Detection of definite etiology for GBS needs an advanced laboratory facility, which is not available in developing countries. Nobuiro Yuki reported that two thirds of cases are preceded by symptoms of URT infection or diarrhea.[10] Recent reports suggested that infection with Campylobacter jejuni may cause GBS by triggering demyelination of peripheral nerves.[11] Many different infectious agents have been discriminated as the cause of GBS.[12,13,14,15] Encephalitis and acute disseminate encephalomyelitis (ADEM) are usually follow many infections or immunization. The brain MRI often is more extensive in ADEM. Brain computerized tomography may be normal in many patients.[16] TM associated with infection or associated immune-mediated processes are usually viral in origin, although other microorganisms such as Mycoplasma pneumoniae might be involved.[16,17,18] Lionnet et al. reported two patients with acquired immunodeficiency syndrome with myelitis due to varicella-zoster.[19] Acute cerebellar ataxia has a wide spectrum of causes including direct infectious or post infectious processes. Viral infections that might cause this include Chickenpox, Coxsackie virus, infectious mononucleosis, and echovirus infections. Post vaccination sequele and abscess of the cerebellum are other causes.[20] Syringomyelia is a slowly progressive cavity formation within the spinal cord, medulla oblongata, or both that is associated with gliosis.[21] In this study, we found no case of myelitis caused by wild polio virus. Since 1992, Iran has consistently reported high rate of routine vaccination coverage in infants. Annual national immunization day since 1994 has achieved high coverage among children aged <5 years old. In our study, we reported one case of VAPP. Since 1995, the rate of reported cases of non-polio AFP in Iran has exceeded 1.0 case per 100,000 children under 15 years.[22] Shahmahmoodi et al. reported 6 cases of VAPP in Iran from 1995 to 2008.[23] We believe that this case might also be among them because samples from all over the country are transferred to the same referral center. In this report, all of these infants had a kind of primary immunodeficiency. Infants with immunodeficiency are 3000 times more at risk for VAPP.[24] Changing to an IPV schedule and introducing or screening of newborns for immune deficiencies could reduce the risk for VAPP infection.
In this study like other previous studies performed in Iran, no poliomyelitis cases with wild polio virus was reported and this would indicate that polio virus immunization schedule in our country is highly efficient; surveillance activity should be continued and accurate surveillance for polio is essential for eradication.
ACKNOWLEDGMENT
The authors would like to express their special thanks to M. Rostami, M.D., E. Akhtar, M.S of health and E. Bahrani for their valuable helps.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Alberta Health and Wellness. Public Health Notifiable Diseases Management Guidelines; August. 2011 [Google Scholar]
- 2.Kliegman RM, Stanton BF, Schor NF, St Geme JW, Behrman RE. 20th ed. Vol. 241. Philadelphia: Elsevier Saunders; 2011. Nelson Textbook of Pediatrics; pp. 1084–6. [Google Scholar]
- 3.Zahraei SM, Sadrizadeh B, Gouya MM. Eradication of poliomyelitis in Iran, a historical perspective. Iran J Public Health. 2010;38:124. [Google Scholar]
- 4.Global Polio Eradication Initiative. [Last cited on 2010 Apr 13]. Available from: http://www.polioeradication.Org .
- 5.Progress towards interrupting wild poliovirus transmission worldwide: January 2010-March 2011. Wkly Epidemiol Rec. 2011;86:199–204. [PubMed] [Google Scholar]
- 6.Salk JE. Studies in human subjects on active immunization against poliomyelitis. I A preliminary report of experiments in progress. J Am Med Assoc. 1953;151:1081–98. [PubMed] [Google Scholar]
- 7.Mandell GL, Bennet JE, Dolin R. 8th ed. Vol. 171. Philadelphia: Elsevier Saunders; 2015. Principles and Practice of Infectious Diseases; pp. 2345–50. [Google Scholar]
- 8.Cochi SL, Kew O. Polio today: Are we on the verge of global eradication? J Am Med Assoc. 2008;300:839. doi: 10.1001/jama.300.7.839. [DOI] [PubMed] [Google Scholar]
- 9.Acute Flaccid Paralysis Surveillance. HSE Health Protection Surveillance Centre. Available from: http://www.hpsc.ie .
- 10.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294–304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 11.Poropatich KO, Walker CL, Black RE. Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: A systematic review. J Health Popul Nutr. 2010;28:545–52. doi: 10.3329/jhpn.v28i6.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam CC, O’Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS One. 2007;2:e344. doi: 10.1371/journal.pone.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JH, Sheu JJ, Lin HC. Increased risk of Guillain-Barré Syndrome following recent herpes zoster: A population-based study across Taiwan. Clin Infect Dis. 2010;51:525–30. doi: 10.1086/655136. [DOI] [PubMed] [Google Scholar]
- 14.Orlikowski D, Porcher R, Sivadon-Tardy V, Quincampoix JC, Raphaël JC, Durand MC, et al. Guillain-Barré syndrome following primary cytomegalovirus infection: A prospective cohort study. Clin Infect Dis. 2011;52:837–44. doi: 10.1093/cid/cir074. [DOI] [PubMed] [Google Scholar]
- 15.Goldschmidt B, Menonna J, Fortunato J, Dowling P, Cook S. Mycoplasma antibody in Guillain-Barré syndrome and other neurological disorders. Ann Neurol. 1980;7:108–12. doi: 10.1002/ana.410070203. [DOI] [PubMed] [Google Scholar]
- 16.Kaye EM, Van der Knaap MS. Disorders primarily of white matter. In: Swaiman KF, Ashwal S, Ferriero DM, editors. Principle and Practice of Pediatric Neurology. Philadelphia: Elsevier Saunders; 2012. pp. 1351–2. [Google Scholar]
- 17.Candler PM, Dale RC. Three cases of central nervous system complications associated with Mycoplasma pneumonia. Pediatr Neurol. 2004;31:133–8. doi: 10.1016/j.pediatrneurol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Wolf VL, Lupo PJ, Lotze TE. Pediatric acute transverse myelitis overview and differential diagnosis. J Child Neurol. 2012;27:1426–36. doi: 10.1177/0883073812452916. [DOI] [PubMed] [Google Scholar]
- 19.Lionnet F, Pulik M, Genet P, Petitdidier C, Davous P, Lebon P, et al. Myelitis due to varicella-zoster virus in two patients with AIDS: Successful treatment with acyclovir. Clin Infect Dis. 1996;22:138–40. doi: 10.1093/clinids/22.1.138. [DOI] [PubMed] [Google Scholar]
- 20.U.S. National Library of Medicine, National Institute of Health. URL of this page. Available from: http//www.nlm.nih.gov .
- 21.Newman PK, Terenty TR, Foster JB. Some observations on the pathogenesis of syringomyelia. J Neurol Neurosurg Psychiatry. 1981;44:964–9. doi: 10.1136/jnnp.44.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). Wild poliovirus transmission in bordering areas of Iran, Iraq, Syria, and Turkey, 1997-June 1998. MMWR Morb Mortal Wkly Rep. 1998;47:588–92. [PubMed] [Google Scholar]
- 23.Shahmahmoodi S, Mamishi S, Aghamohammadi A, Aghazadeh N, Tabatabaie H, Gooya MM, et al. Vaccine-associated paralytic poliomyelitis in immunodeficient children, Iran, 1995-2008. Emerg Infect Dis. 2010;16:1133–6. doi: 10.3201/eid1607.091606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín J. Vaccine-derived poliovirus from long term excretors and the end game of polio eradication. Biologicals. 2006;34:117–22. doi: 10.1016/j.biologicals.2006.02.005. [DOI] [PubMed] [Google Scholar]


