Abstract
Background:
This study was aimed to evaluate the predictive value of fecal calprotectin in patients with ulcerative colitis from patients with irritable bowel syndrome (IBS).
Materials and Methods:
Between May and October 2013, 88 adult patients, between the age 18 and 65 years with a history of chronic diarrhea of unknown origin were assessed. Standard colonoscopies were performed in all patients to assess ulcerative colitis. Before colonoscopies, they were asked to supply a stool specimen. Fecal calprotectin value was measured using a commercial enzyme-linked immunosorbent assay kit.
Results:
The mean of age, gender combination, and body mass index were not significantly different between patients with ulcerative colitis or IBS. The duration of disease in ulcerative colitis patients was significantly higher than IBS patients (P < 0.0001). The level of calprotectin in ulcerative colitis patients was significantly higher than IBS patients (265.9 vs 115.8, respectively, P = 0.001). Also, cutoff value >164 μg/g with sensitivity and specify of 57 (CI: 41%–71.6%), and 75 (CI: 59.7%–56.8%), respectively, was the best for discrimination between patients with ulcerative colitis and those with IBS.
Conclusion:
Our results show that fecal calprotectin as a noninvasive method, which can be used to identify patients with ulcerative colitis from IBS patients has low sensitivity and specificity.
Keywords: Fecal calprotectin, inflammatory bowel diseases, irritable bowel syndrome, ulcerative colitis
INTRODUCTION
In adult population, chronic diarrhea is a relatively common condition and often poses a diagnostic challenge despite its frequency.[1] In fact, diagnosis based on initial history and physical examination occurred only in about one-third of all patients with chronic diarrhea.[2] One of the most frequent causes of chronic diarrhea in adults is probably irritable bowel syndrome (IBS), which in industrialized countries affecting 6%–22% of the general population with incidence ranging from 6% to 9%.[3] Also, inflammatory bowel diseases (IBD), such as ulcerative colitis, and Crohn's disease are chronic disorders characterized by alternating periods of remission and relapse.[4]
IBD are generally viewed as unpredictable diseases, and the putative risk of an unanticipated relapse is considered as one of the main fear by patients.[5] Gastroenterologists often are hampered by the diagnostic difficulty of differentiating between patients with IBS and those with IBD, the lack of a reliable and noninvasive index of bowel pathology.[6] Diarrhea, abdominal pain, bloating, and excessive flatus are common symptoms in both IBS and IBD conditions.[7]
The ensuing clinical evaluation in the majority of patients with chronic diarrhea can be invasive, prolonged, and resource-intensive.[8] Stool tests can be useful simple screening tool for discriminating the presence or absence of gastrointestinal pathology.[1] Calprotectin is a calcium-binding heterodimer of the S100 protein family, and compared with other candidates, may offer performance advantages based on its biological characteristics.[9] Previous studies have shown that fecal calprotectin levels in elevated patients with IBD seem to correlate with disease activity, and suggested that a high fecal calprotectin concentration might distinguish patients with IBD from patients with IBS.[10]
The diagnosis and management of IBD is still a challenge for physicians, and early studies focus on IBD in total; and data about the predictive value of fecal calprotectin in ulcerative colitis from IBS are limited, so, the present prospective study was designed to assess the predictive value of fecal calprotectin in patients with ulcerative colitis from patients with IBS.
MATERIALS AND METHODS
The present study was conducted from May, 2013 to October, 2013, on 88 adult patients with a history of chronic diarrhea of unknown origin, who had referred to “Al-Zahra” Hospital in Isfahan, Iran. This study was approved by the ethics committee of Isfahan University of Medical Sciences. Patients age range between 18 and 65 years in both the genders were eligible if they had a history of chronic diarrhea of unknown origin, lasting for more than 4 weeks, with or without abdominal pain. Also, patients with known colorectal neoplasia, family cancer syndromes (such as familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer), gastrointestinal endoscopy within the preceding 2 weeks, concurrent menstruation, or major epistaxis during the previous 48 h, history of infectious diarrhea during the previous 6 months, infection with intestinal parasites, colostomy or ileostomy up to 1 month before study enrollment, prior diagnosis with intestinal cancer, pregnancy, and long-term use of nonsteroidal anti-inflammatory drugs, aspirin, and anticoagulant medications were excluded from the study. Written informed consent was obtained from all participants after they were explained about and informed of the purposes of the study.
To assess ulcerative colitis, colonoscopies were performed in all patients by experienced gastroenterologists who were unaware of the fecal assay results. Mucosal abnormalities were recorded by anatomic location and biopsies were obtained routinely from each segment of the colon. Inflammation was defined and graded by standard histological criteria and subtyped by endoscopic and histological features as Crohn's colitis, ulcerative colitis, microscopic colitis, collagenous colitis, or others. Among all colonoscopies, patients with ulcerative colitis and normal colonoscopy were eligible and were evaluated for fecal assay. Also, patients with normal colonoscopy with a clinical history indicative of IBS according to Rome II criteria were then considered to have a diagnosis of IBS.
Before colonoscopic procedure, the patients were asked to supply a stool specimen, and then fecal assay on patients’ samples, which was diagnosed by colonoscopy and were eligible, was done by experienced laboratory technicians, who were unaware of the clinical diagnoses or details of the patients’ clinical histories. The stool specimens were stored at –20°C and fecal calprotectin was measured using a commercial enzyme-linked immunosorbent assay kit, based on a two-site sandwich technique with two selected monoclonal antibodies, which bind to human calprotectin, and an average of the two calprotectin measurements was recorded.
Age, gender, body mass index (BMI), duration of disease, and level of calprotectin were analyzed using SPSS-20 for windows (SPSS IBM, New York, USA). Based on estimates from the literature, the sample size of this study was calculated in order to have 80% power with two-sided log-rank test, α =0.05, to observe a significant differences of calprotectin levels. Results were reported using number (%) for categorical variables and mean ± SD for continuous variables. Categorical variables were compared using Chi-square test and continuous variables were compared using independent sample t-test between groups. For calprotectin level, a receiver operating characteristic (ROC) curve analysis was used to establish the cutoff values that optimized the ulcerative colitis from IBS in patients with gastrointestinal dysfunction. Sensitivity, specificity, positive predictive value (PPV), negative predictive values (NPV), and likelihood ratio (LR) were then calculated. P values less than 0.05 were considered to indicate statistical significance.
RESULTS
Of 125 patients who were assessed for eligibility, 16 patients did not enter the study (11 patients did not meet the inclusion criteria and five patients refused informed consent). Then, 109 patients underwent colonoscopy procedure, 88 patients with ulcerative colitis and normal colonoscopy based on colonoscopies findings were followed for fecal assay and 21 patients with other colonoscopy findings (Crohn's colitis, microscopic colitis, collagenous colitis, or other) were excluded. Finally, 88 patients (44 patients with ulcerative colitis and 44 patients with IBS) were evaluated and analyzed [Figure 1].
Figure 1.
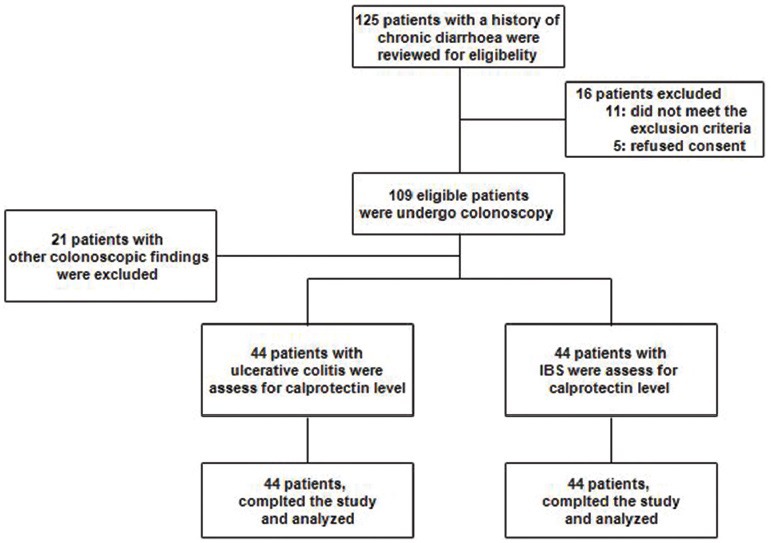
Study flowchart
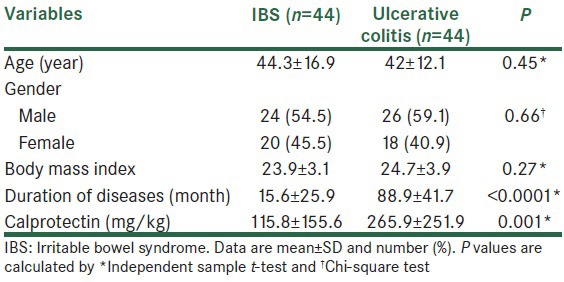
The mean age for the studied patients was 43.2 ± 15.2 years, 50 patients (57%) were male and 38 patients (43%) were female. Table 1 shows the differences between patients with regard to the results of colonoscopy for age, gender combination, BMI, duration of disease, and calprotectin level. There were no significant differences for age, gender combination, and BMI between groups (P > 0.05), but in ulcerative colitis patients mean of duration of disease was significantly more than IBS patients (P < 0.0001). Also, the mean of calprotectin in ulcerative colitis patients was higher than IBS patients (265.9 vs 115.8, respectively, P = 0.001).
Table 1.
Baseline characteristics of studied patients
The results of ROC analyses, describing the values of the mean level of calprotectin in predicting ulcerative colitis from IBS in patients with gastrointestinal dysfunction are shown in Figure 2. There was a significant relationship between calprotectin level and gastrointestinal dysfunction in cutoff >164 μg/g (area under the curve, 0.67; standard error, 0.057; P = 0.003). Also, the sensitivity, specificity, PPV, NPV, and LR for calprotectin level to predict ulcerative colitis from IBS in patients with gastrointestinal dysfunction are shown in table 2. As shown for this cutoff point sensitivity and specificity were 56.8% and 75%, respectively.
Figure 2.
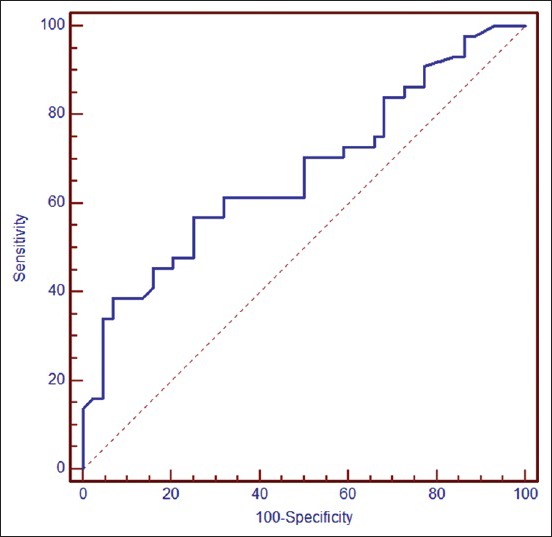
Receiver–operating characteristic curves for calprotectin level (area under the curve, 0.67; standard error, 0.057; confidence interval 95%, 0.562–0.767; P = 0.003) for predicting ulcerative colitis from irritable bowel syndrome in patients with chronic diarrhea
Table 2.
The prognostic value of evaluation of calprotectin level in patients with gastrointestinal dysfunction for prediction of ulcerative colitis

DISCUSSION
Colonoscopy with biopsy, which is an invasive and expensive procedure is the gold standard for IBD, and a normal colonoscopy as an expensive procedure can exclude the possibility of ulcerative colitis.[11] Therefore, a reliable, simple, cheap, and noninvasive test is needed to be used to discriminate patients with functional bowel disorders from those with organic bowel disorders.
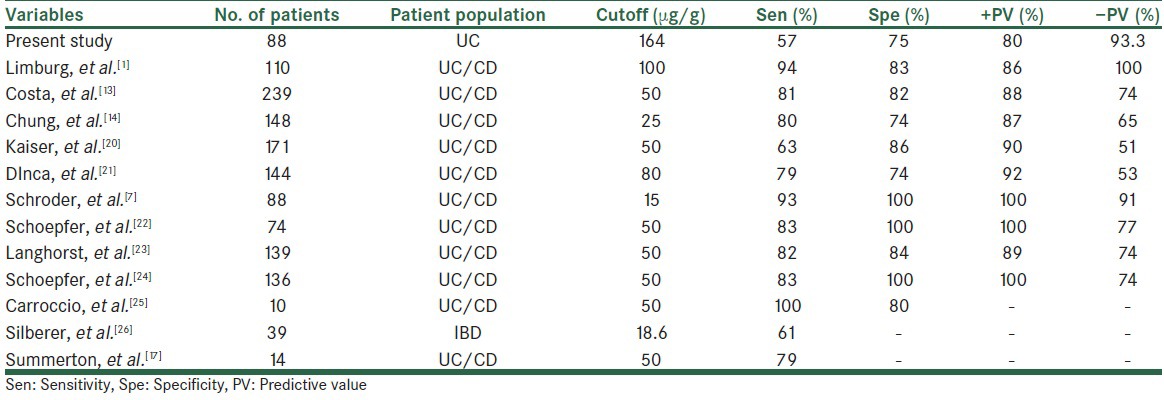
The present study was aimed to assess whether noninvasive measurement of fecal calprotectin improves the diagnostic accuracy of ulcerative colitis from IBS in patients with chronic diarrhea. The results show that measurement of fecal calprotectin was significantly different between IBS in patients with chronic diarrhea. Median estimate for fecal calprotectin in our study was 230 μg/g in the patient sample corresponding well with 118, 167, 151, 285, 137, and 267 μg/g reported in earlier studies using the same assay.[7,12,13,14,15,16,17] Also, we found that cutoff values of >164 μg/g with a sensitivity and specificity of 57% and 75%, respectively, was the best value in the prognosis of ulcerative colitis from IBS in patients with chronic diarrhea.
In a cohort study by Schroder et al.,[7] fecal calprotectin was screened in 88 adult patients with a history of chronic diarrhea of unknown origin, and 45 patients were diagnosed with IBD and 31 patients were diagnosed with IBS. Also, authors reported that the sensitivity and specificity of calprotectin for IBD were 93% and 100%, respectively. In a meta-analysis by von Roon et al.,[18] nine studies in adults were included to assess the predictive value of fecal calprotectin in patients with ulcerative colitis from patients with IBS. Reported sensitivities and specificities of fecal calprotectin in differentiating IBD from, in particular, IBS were 86% (CI: 83%–89%) and 81% (CI: 78%–84%), respectively. In another meta-analyses by van Rheenen et al.,[19] adult patients in six studies were included and the pooled sensitivity and specificity of fecal calprotectin testing were reported in 93 (CI: 85%–97%) and in 96 (CI: 79%–99%), respectively. So authors in these two studies suggested that fecal calprotectin has a good diagnostic precision for separating IBD from IBS overall. Similarly, our results show sensitivity and specificity of 57 (CI: 41%–71.6%), and 75 (CI: 59.7%–56.8%), respectively, for fecal calprotectin in differentiating ulcerative colitis from, in particular, IBS. As shown, these results with different rang show the usefulness of fecal calprotectin assessment in predicting of IBD from IBS, but the distinct lower diagnostic values found in our study here may be due to dissimilarities in the study design and studied population, whereas in Schroder et al.,[17] van Rheenen et al.,[18] and von Roon et al.[19] 's studies, patients were included only if they had IBD but in the present study patients with ulcerative colitis were assessed.
The cutoff of 100 μg/g of the calprotectin assay was reported in a review article. Also, the cutoff values, sensitivity, and specificity in the present study in comparison to previous studies are shown in table 3. Previous studies reported the cutoff values ranging from 15 to 100 μg/g of the calprotectin assay with the sensitivity between 61% and 100% and specificity between 74% and 100% for discrimination between patients with IBD and those without IBD.[1,7,13,14,17,20,21,22,23,24,25,26] In the present study, a cutoff 164 μg/g of calprotectin with the sensitivity and specificity of 57% and 75%, respectively, was obtained as the best for discrimination between patients with ulcerative colitis and those with IBS, which was higher than in the previous reports. It seems that differences in sample size and studied population are the main cause of difference in cutoff values. Although results are different, all the studies suggested that fecal calprotectin may be helpful to identify patients with IBD from those without IBD, despite the large variations of sensitivity and specificity been reported and different cutoffs proposed.
Table 3.
Different diagnostic precision of fecal calprotectin for inflammatory bowel disease
One of the limitations in the present study is that the fecal calprotectin is useful for discriminating IBD from IBS because it is a nonspecific disease biomarker, and it is not useful for discriminating ulcerative colitis from Crohn's disease or to discriminate active IBD from infectious gastrointestinal disease. Also, the lack of a control group of healthy adults is one of the limitations of our study. Comparing the level of fecal calprotectin in patients with IBD with level from healthy adults is reasonable. Finally, we believe that small sample size in our study is another limitation and larger prospective studies are suggested to be carried out to validate the cutoff values in clinical practice in patients with ulcerative colitis for monitoring intestinal inflammation.
In conclusion, although possibly limited, the results of the present study implicate an advantage of fecal calprotectin in the detection of ulcerative colitis when compared with the noninflammatory condition, IBS. And based on our findings, the sensitivity and specificity of fecal calprotectin are low and the large variations of sensitivity and specificity are reported in previous studies, and to assess the use of fecal calprotectin level in the clinical evaluation of patients with chronic diarrhea further studies are needed.
ACKNOWLEDGEMENTS
This study was supported by a grant from Isfahan university of medical sciences, Isfahan, IRAN, (No, 291308).
Footnotes
Source of Support: Nil
Conflict of Interest: The authors have no financial conflicts of interests in relation to this study.
REFERENCES
- 1.Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, et al. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–7. doi: 10.1111/j.1572-0241.2000.03194.x. [DOI] [PubMed] [Google Scholar]
- 2.Donowitz M, Kokke FT, Saidi R. Evaluation of patients with chronic diarrhea. N Engl J Med. 1995;332:725–9. doi: 10.1056/NEJM199503163321107. [DOI] [PubMed] [Google Scholar]
- 3.Delvaux M. Functional bowel disorders and irritable bowel syndrome in Europe. Aliment Pharmacol Ther. 2003;18(Suppl 3):75–9. doi: 10.1046/j.0953-0673.2003.01728.x. [DOI] [PubMed] [Google Scholar]
- 4.Irvine EJ. Review article: Patients’ fears and unmet needs in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):54–9. doi: 10.1111/j.1365-2036.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 5.Laharie D, Mesli S, El Hajbi F, Chabrun E, Chanteloup E, Capdepont M, et al. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: A prospective study. Aliment Pharmacol Ther. 2011;34:462–9. doi: 10.1111/j.1365-2036.2011.04743.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoff G, Grotmol T, Thiis-Evensen E, Bretthauer M, Gondal G, Vatn MH. Testing for faecal calprotectin (PhiCal) in the Norwegian Colorectal Cancer Prevention trial on flexible sigmoidoscopy screening: Comparison with an immunochemical test for occult blood (FlexSure OBT) Gut. 2004;53:1329–33. doi: 10.1136/gut.2004.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schröder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: Combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007;26:1035–42. doi: 10.1111/j.1365-2036.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 8.Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1464–86. doi: 10.1016/s0016-5085(99)70513-5. [DOI] [PubMed] [Google Scholar]
- 9.Berni Canani R, Rapacciuolo L, Romano MT, Tanturri de Horatio L, Terrin G, Manguso F, et al. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liver Dis. 2004;36:467–70. doi: 10.1016/j.dld.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2008;41:56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 11.El-Badry A, Sedrak H, Rashed L. Faecal calprotectin in differentiating between functional and organic bowel diseases. Arab J Gastroenterol. 2010;11:70–3. [Google Scholar]
- 12.Mayer EA. Clinical practice. Irritable bowel syndrome. New Engl J Med. 2008;358:1692–9. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642–7. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 14.Chung-Faye G, Hayee B, Maestranzi S, Donaldson N, Forgacs I, Sherwood R. Fecal M2-pyruvate kinase (M2-PK): A novel marker of intestinal inflammation. Inflamm Bowel Dis. 2007;13:1374–8. doi: 10.1002/ibd.20214. [DOI] [PubMed] [Google Scholar]
- 15.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364–8. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–91. doi: 10.1097/01.mib.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 17.Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: A marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841–5. doi: 10.1097/00042737-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 18.von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–13. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 19.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–13. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–37. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 22.Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum. 2007;50:1697–706. doi: 10.1007/s10350-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 23.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–9. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 25.Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: A prospective study in adults and children. Clin Chem. 2003;49:861–7. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 26.Silberer H, Küppers B, Mickisch O, Baniewicz W, Drescher M, Traber L, et al. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–26. [PubMed] [Google Scholar]


