Abstract
Background:
Since 2003, the incidence of community associated Clostridium difficile infection (CA-CDI) has increased; different types of food have been supposed to be the vectors of C. difficile strains. The purpose of this study is to investigate the occurrence of C. difficile strains in ready-to-eat salads distributed in food services.
Materials and Methods:
A total of 106 ready-made salad specimens were sampled from different restaurants and food services located in Isfahan, in the center of Iran. Positive isolates of C. difficile were identified and confirmed for the existence of three genes including tpi, tcdA and tcdB by multiplex PCR.
Results:
A total of six (5.66%) samples were positive for C. difficile strains. Of which, one strain (16.6%) was positive for A and B toxins.
Conclusion:
The existence of toxigenic C. difficile in ready-made salads could be a caution for public health. Further investigation is required to assess the relationship between the isolated strains in our study and those from diarrheic patients through molecular typing.
Keywords: Clostridium difficile, multiplex PCR, prevalence, salad, vegetable
INTRODUCTION
Clostridium difficile (C. difficile) is a Gram-positive, rod, sporogenic, anaerobic and toxin-producing bacteria.[1,2] This organism is recognized as one of the causative agents of hospital infectious incidence.[3] About 15% to 25% of the episodes of antibiotic-associated diarrhea (AAD) is linked with the pathogenic strains; also, 86.1% of C. difficile isolates from the suspected cases of C. difficile-associated diarrhea (CDAD) were characterized as toxigenic.[4,5] Pathogenic strains of C. difficile are capable of producing two enterogenic potential toxins including A (tcdA) and B (tcdB).[6] Toxin production leads to the spectrum of complications such as colitis, diarrhea, with or without the formation of a false membrane, toxic megacolon, perforation and even death.[7]
Recently another toxin known as C. difficile binary toxin (cdt) has been isolated from some certain strains. Of the total identified strains, 5% is known to have the capability of binary toxin production. Association between severe infectious form of disease in human with the latter toxin represents its particular importance.[8,9]
A variety of niches including hospital environments,[10] soil, drinking water, untreated water, river, lake, marine environments and domestic food animals are introduced as the main source of C. difficle spores.[11,12,13] Accordingly, animal originated foods have been considered as one of the probable means of transmission. The prevalence of C. difficile have been reported in a variety of food types including: Different kinds of meat and meat products,[14,15,16,17,18] fish, shellfish, edible bivalve molluscs and other seafoods,[12,19,20] egg,[21] vegetables[22] and ready-to-eat salads.[23] Considering the genetic overlap of the food isolates and the clinical ones, the hypothesis that C. difficle could be a “food-borne pathogen” has been highly confirmed by the findings of the above-mentioned studies.[24]
Ready-to-eat salads are recognized as risky meals, prone to different kinds of contamination by food-borne bacteria like Escherichia Coli, Staphylococcus aureus, Bacillus cereus, Salmonella listeria spp (L. monocytogenes), Yersinia spp (Y. intermedia and Y innocua).[25,26,27,28,29] Such contaminations sometimes lead to outbreaks.[30] Rarely investigated, the prevalence of C. difficle was reported to be 2/4% to 7/5%, respectively in green vegetable and ready-to-eat salads in some countries.[21,23] But there is no information on the incidence of C. difficile in these types of food items in Iran. In another point of view, as the main source of phytochemicals and fibers, fresh vegetables and salads are highly recommended in a daily diet by nutritionist. Present study deals with C. difficle contamination rate in ready-to-eat salads offered in the food services located in different districts of Isfahan.
MATERIALS AND METHODS
Ready-made salad specimens (n = 106) were sampled from 106 restaurants and food services in Isfahan using random sampling from April 9th to July 1st, 2013. Salads were composed of cabbage, lettuce, carrot, tomato, corn and pea. Samples were transferred under cool condition (portable insulated cold boxes) to food microbiology laboratory of Food Security Research Center in Isfahan University of Medical Sciences.
Isolation method was adapted from Rodriguez-Palacios et al.;[14] in brief, C. difficile broth; Clostridium difficile moxalactam norfloxacin (CDMN) containing: 40 g/l proteose peptone, 5 g/l disodium hydrogen phosphate, 0.1 g/l magnesium sulfate, 2 g/l sodium chloride, 6 g/l fructose, 1 g/l sodium taurocholate was supplemented with 500 mg/l cysteine hydrochloride, 32 mg/l moxalactam, and 12 mg/l norfloxacin.
The sample was homogenized under aseptic conditions. Twenty grams of each sample was cultured into 30 ml of CDMN broth and incubated under anaerobic condition at 37°C for 10–15 days.
Spore selection by alcohol shock was performed by adding 2 ml of the cultured broth to 2 ml of 96% ethanol (1:1 [v/v]), then thoroughly homogenized by a vortex mixer (VELP Scientific, Italy) and incubated for 50 min at room temperature. The mixture was then centrifuged (BH 1200, IRAN) at 3800× g for 10 min and the sediment was streaked onto CDMN Agar (RM026-500G, Himedia, India) added with 5% horse blood and incubated in an anaerobic chamber (5% H2, l0% CO2, 85% N2) at 37°C (MART microbiology B.V, Drachten, the Netherlands). Up to two suspected colonies with the morphology characteristics such as grayish, swarming, rough, non-hemolytic and horse-like smell were considered presumably positive and sub-cultured on anaerobic blood agar (Merck, Germany). C. difficile was presumptively identified with Gram-stain appearance, Sub-terminal, green-stained endospores, ovoid-shape, vegetative, pink-stained cells and and being positive for l-proline aminopeptidase reaction (Pro Disc, Remel, Lenexa, KS, USA).[31]
DNA extraction was performed using a commercial kit (DNP™ kit, CinnaGen Inc, Iran). In brief, bacterial culture was centrifuged (BH 1200, IRAN) for 10 minutes at 7500g. Bacterial culture (10–20 mg) was collected in a 1.5 ml microcentrifuge tube and suspended in 100 μl of protease buffer. Five μl of protease was added to suspension, mixed and kept at 55°C for 30 minutes. One hundred of the prepared mixture was blended with 400 μl of lysis solution and vortexed (VELP Scientific, Italy) for 15–20 seconds. Precipitation solution (300 μl) was added to the prepared mixture, mixed for 5 s and centrifuged 12,000g for 10 minutes. The tube containing the obtained solution was decanted by gentle inverting on a tissue paper for 2–3 seconds Wash buffer (1 ml) was added to the tube, mixed by 3–5 s. and centrifuged at 12,000g for 5 min, then it was decanted. The precipitate was dried at 65°C for 5 min and suspended in 50 μl of solvent buffer by gentle shaking and kept at 65°C for 5 minutes. Insoluble particles were precipitated by centrifugation at 12,000g for 30 s and purified DNA was obtained from supernatant. The concentration of DNA was measured visually through electrophoresis in fresh 2% agarose gel. One μl of DNA solution was used for each 50 μl of PCR mixture.
Multiplex PCR was applied as described by Lemee et al.[32] Three pairs of primer were used containing tpi - specific primers (tpi - F [5’ AAAGAAGCTACTAAGGGTACAAA - 3’] and tpi - R [5’ - CATAATATTGGGTCTATTCCTAC - 3’]), tcdA - specific primers (tcdA - F [5’ - AGATTCCTATATTTACATGACAATAT - 3’] and tcdA - R [5’ GTATCAGGCATAAAGTAATATACTTT - 3’]) and tcdB - specific primers (tcdB - F [5’ GGAAAAGAGAATGGTTTTATTAA - 3’] and tcdB - R [5’ ATCTTTAGTTATAACTTTGACATCTTT - 3’]). First primers were inferred from alignments of internal fragments of the tpi gene that (as housekeeping gene) was used to distinguish Clostridium species and it produced a 230 bp amplified fragment specific for C. difficile. The tcdB - specific primers were deduced from the conserved 5’ region of tcdB and produced a 160 bp fragment. The tcdA - specific primers were designed to flank the smallest of the three deletions in the 3’ region of tcdA and produced a 369 - bp fragment for A + B + strains and a 110 - bp fragment for A-B+ strains.
The PCR was set for 25 μl reaction volume containing 10% (v/v) glycerol, 1 μM each primer, 200 μM each deoxynucleoside triphosphate, 0.5 U of Taq DNA polymerase in a 1X amplification buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2) aztd and 1–5 μl of each template DNA. The reaction was performed in the GeneAmp System 2400 thermal cycler.
The PCR mixtures were denatured at 95°C for 3 min in the beginning, then a touchdown procedure was executed at 95°C for 30 s, annealing for 30 s at temperatures decreasing from 65 to 55°C during the first 11 cycles (with 1°C steps in cycles 1 to 11). At the end, a final extension step at 72°C for 30 s was performed. Totally, 40 cycles were carried out. Kindly provided by the University of Guelph, C. difficile ribotype 027 was used as a positive control.
PCR products were analyzed on 2% agarose (progen, Australia) gel by electrophoresis and after staining the gel with DNA green viewer was visualized under UV light.
RESULT
The result of the present study revealed that the incidence rate of C. difficle in the tested ready-to-made salads was about 6% and toxic-producing strains were found among the isolates. Of which, one strain (16.6%) was positive for A and B toxins. None of the other identified strains showed toxin-producing property.
The results of electrophoresis of the PCR products were depicted in Figure 1. Generating a 230-bp fragment, all of the isolates were shown to carry the tpi gene, but only one of the six isolated strains were positive for tcdA and tcdB genes by producing a 369-bp fragment and a 160-bp fragment as their PCR products, respectively.
Figure 1.
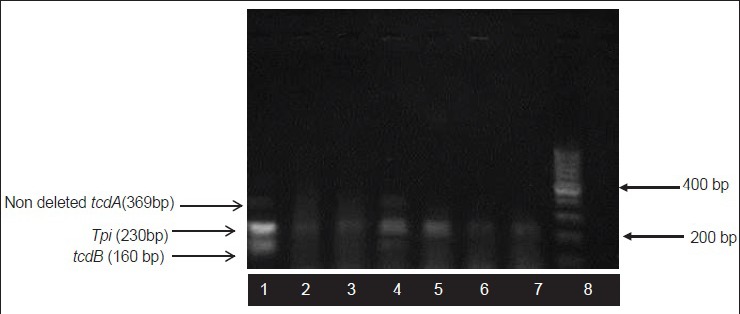
Agarose gel electrophoresis pattern shows multiplex PCR amplification products. Lane 1: Control strain that is positive for tpi, tcdA and tcdB genes, Lane 2,3,5,6,7: Isolates of C. difficile, lane 4: toxigenic (A+B+) C. difficile strain, lane 8: 100 bp DNA ladder
DISCUSSION
These results are in accordance with the results of the study carried out by Bakri et al.[23] in Scotland where C. difficile spores were isolated from 7.5% (3/40) of tested salad samples composed of baby leaf spinach, organic mixed leaf salad and organic lettuce. Two of the identified isolates were found to be toxigenic and characterized as A-B + and A + B+[23]. In a recent study performed by Eckert et al. in examining 60 ready-to-made salads and 44 vegetable samples, the prevalence rate of C. difficile was revealed to be in 3.3% and 2.27%, respectively.[33] Likewise, Al Saif and Brazier reported on the contamination rate of 2.4% for the raw vegetable marketed in Cardiff area of south Wales. In their study, C. difficile strains were detected from potato, onion, mushroom, carrot, radish and cucumber samples. About two third of the identified isolates in the latter study were capable of A toxin production.[34] However, in another study, C. difficile was not detected in any of the examined ready-to-made salads and sprouts samples in Slovenia. The absence of C. difficle strains in the latter study might be affected by its little sample size (n = 8)[21].
It was also shown in another study in Canada that the contamination rate of vegetables to C. difficle was 4.5% (5/111). Toxin-producing strains with the capability of Aand B toxin production were found among the detected isolates.[22]
Providing important vitamins and minerals as well as a wide range of health-promoting secondary metabolites, vegetables are important components of a healthy diet. Regular daily consumption of vegetables in sufficient amounts can prevent some diseases. In recent years, ready-to-made salads, prepared from raw vegetables have become one of the inseparable components of Iranian food tables in the restaurants. Salads and fresh vegetables are usually considered as the food items with high potentials for several microbial contaminations. The occurrence of C. difficle in ready-to-made salads has been investigated in few studies in different countries.[[21].[23]33] Although history of antibiotic treatments, age (over 65) and hospitalization were known to be the main risk factors for C. difficile infection (CDI).[3] Recent studies have demonstrated that the individuals without any of the given risk factors are still susceptible to infection by C. difficle strains.[35] Considering the high nutritional value of a vegetable meal as a ready-to-made salad, evaluation of its microbial quality is far important.
It seems that the prevalence of C. difficle among the salads and vegetable samples in all similar works is very close and ranged from 0% to 7.5%, noticeably lower than that reported about other food sources. A prevalence rate of 42% was previously shown for meat and seafoods.[15,16,17,18,19,20]
Iranian clinical studies demonstrated that C. difficile strains isolated from hospitalized diarrheic patients at the incidence rate of 6% to 22.2%[36,37,38,39,40] with 078 and 014 ribotypes as the most prevalent strains. These ribotypes were reported to be isolated from salad and fresh vegetable samples in other studies.[23,34]
To examine the relationship between the isolated strains in our study and those ones obtained from diarrheic patients, molecular typing studies are required.
ACKNOWLEDGMENTS
The authors appreciate the environmental health aministration of Isfahn for their collaboration. Furthermore, Dr. Parisa Shoaei's corporation is kindly appreciated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McFee RB, Abdelsayed GG. Clostridium difficile. Dis Mon. 2009;55:439–70. doi: 10.1016/j.disamonth.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Kuijper E, Coignard B, Tüll P. Emergence of Clostridium difficile associated disease in North America and Europe. Clin Microbiol Infec. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 3.Rupnik M. Is Clostridium difficile-associated infection a potentially zoonotic and foodborne disease? Clin Microbiol Infect. 2007;13:457–9. doi: 10.1111/j.1469-0691.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- 4.Hull MW, Beck PL. Clostridium difficile-associated colitis. Can Fam Physician. 2004;50:1536–40. 1543-5. [PMC free article] [PubMed] [Google Scholar]
- 5.Barbut F, Mastrantonio P, Delmee M, Braziar J, Kuijper E, Poxton I. Clin Microbiol Infec. 2007;13:1048–57. doi: 10.1111/j.1469-0691.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 6.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: New challenges from an established pathogen. Clev Clin J Med. 2006;73:187–97. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Geric B, Johnson S, Gerding DN, Grabnar M, Rupnik M. Frequency of binary toxin genes among Clostridium difficile strains that do not produce large clostridial toxins. J Clin Microbiol. 2003;41:5227–32. doi: 10.1128/JCM.41.11.5227-5232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Songer JG. Clostridia as agents of zoonotic disease. Vet Microbiol. 2010;140:399–404. doi: 10.1016/j.vetmic.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.McEllistrem MC, Carman RJ, Gerding DN, Genheimer CW, Zheng L. A hospital outbreak of Clostridium difficile disease associated with isolates carrying binary toxin genes. Clin Infect Dis. 2005;40:265–72. doi: 10.1086/427113. [DOI] [PubMed] [Google Scholar]
- 10.Martirosian G. Recovery of Clostridium difficile from hospital environments. J Clin Microbiol. 2006;44:1202–3. doi: 10.1128/JCM.44.3.1202-1203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simango C. Prevalence of Clostridium difficile in the environment in a rural community in Zimbabwe. Trans R Soc Trop Med Hyg. 2006;100:1146–50. doi: 10.1016/j.trstmh.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Pasquale V, Romano VJ, Rupnik M, Dumontet S, Cižnár I, Aliberti F, et al. Isolation and characterization of Clostridium difficile from shellfish and marine environments. Folia Microbiol. 2011;56:431–7. doi: 10.1007/s12223-011-0068-3. [DOI] [PubMed] [Google Scholar]
- 13.Pirs T, Ocepek M, Rupnik M. Isolation of Clostridium difficile from food animals in Slovenia. J Med MicrobiL. 2008;57:790–2. doi: 10.1099/jmm.0.47669-0. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Palacios A, Staempfli HR, Duffield T, Weese JS. Clostridium difficile in retail ground meat, Canada. Emerg infect dis. 2007;13:485–7. doi: 10.3201/eid1303.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Songer JG, Trinh HT, Killgore GE, Thompson AD, McDonald LC, Limbago BM. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis. 2009;15:819–21. doi: 10.3201/eid1505.081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esfandiari Z, Jalali M, Ezzatpanah H, Weese JS, Chamani M. The Frequency of Clostridium difficile in processing steps of hamburger. J HSR. 2013:1460–8. [Google Scholar]
- 17.Rahimi E, Jalali M, Weese JS. Prevalence of Clostridium difficile in raw beef, cow, sheep, goat, camel and buffalo meat in Iran. BMC Public Health. 2014;14:119. doi: 10.1186/1471-2458-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esfandiari Z, Jalali M, Ezzatpanah H, Weese JS, Chamani M. Prevalence and characterization of Clostridium difficile in beef and mutton meats of Isfahan region, Iran. Jundishapur J Microbiol. 2014;7:e16771. doi: 10.5812/jjm.16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalf D, Avery BP, Janecko N, Matic N, Reid-Smith R, Weese JS. Clostridium difficile in seafood and fish. Anaerobe. 2011;17:85–6. doi: 10.1016/j.anaerobe.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Pasquale V, Romano V, Rupnik M, Capuano F, Bove D, Aliberti F, et al. Occurrence of toxigenic Clostridium difficile in edible bivalve molluscs. Food Microbiol. 2012;31:309–12. doi: 10.1016/j.fm.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zidaric V, Rupnik M. Bled, Slovenia: In 4th International Clostridium difficile Symposium, 20-22 September, 2012; Clostridium difficile in meat products, eggs and vegetables in Slovenia. abstract: Poster 118. [Google Scholar]
- 22.Metcalf DS, Costa MC, Dew WM, Weese JS. Clostridium difficile in vegetables, Canada. Lett Appl Microbiol. 2010;51:600–2. doi: 10.1111/j.1472-765x.2010.02933.x. [DOI] [PubMed] [Google Scholar]
- 23.Bakri MM, Brown DJ, Butcher JP, Sutherland AD. Clostridium difficile in ready-to-eat salads, Scotland. Emerg Infect Dis. 2009;15:817–8. doi: 10.3201/eid1505.081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould LH, Limbago B. Clostridium difficile in food and domestic animals: A new foodborne pathogen? Clin Infect Dis. 2010;51:577–82. doi: 10.1086/655692. [DOI] [PubMed] [Google Scholar]
- 25.Delaquis P, Bach S, Dinu LD. Behavior of Escherichia coli O157: H7 in leafy vegetables. J Food Prot. 2007;70:1966–74. doi: 10.4315/0362-028x-70.8.1966. [DOI] [PubMed] [Google Scholar]
- 26.Meldrum R, Little C, Sagoo S, Mithani V, McLauchlin J, de Pinna E. Assessment of the microbiological safety of salad vegetables and sauces from kebab take-away restaurants in the United Kingdom. Food Microbiol. 2009;26:573–7. doi: 10.1016/j.fm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Sagoo SK, Little CL, Ward L, Gillespie IA, Mitchell RT. Microbiological study of ready-to-eat salad vegetables from retail establishments uncovers a national outbreak of salmonellosis. J Food Prot. 2003;66:403–9. doi: 10.4315/0362-028x-66.3.403. [DOI] [PubMed] [Google Scholar]
- 28.Pingulkar K, Kamat A, Bongirwar D. Microbiological quality of fresh leafy vegetables, salad components and ready-to-eat salads: An evidence of inhibition of Listeria monocytogenes in tomatoes. Int J food Sci and Nutr. 2001;52:15–23. doi: 10.1080/09637480020027219. [DOI] [PubMed] [Google Scholar]
- 29.Sagoo SK, Little CL, Mitchell RT. The microbiological examination of ready-to-eat organic vegetables from retail establishments in the United Kingdom. Lett Appl Microbiol. 2001;33:434–9. doi: 10.1046/j.1472-765x.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 30.MercanogluTaban B, Halkman AK. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe. 2011;17:286–7. doi: 10.1016/j.anaerobe.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 31.on Fedorko DP, Williams EC. Use of cycloserine-cefoxitin-fructose agar and L-proline-aminopeptidase (PRO Discs) in the rapid identification of Clostridium difficile. J Clin Microbiol. 1997;35:1258–9. doi: 10.1128/jcm.35.5.1258-1259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, et al. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (Toxin A), and tcdB (Toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol. 2004;42:5710–4. doi: 10.1128/JCM.42.12.5710-5714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckert C, Burghoffer B, Barbut F. Contamination of ready-to-eat raw vegetables with Clostridium difficile, France. J Med Microbiol. 2013;62:1435–8. doi: 10.1099/jmm.0.056358-0. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC). Severe Clostridium difficile-associated disease in populations previously at low risk - four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–5. [PubMed] [Google Scholar]
- 35.al Saif N, Brazier JS. The distribution of Clostridium difficile in the environment of South Wales. J Med Microbiol. 1996;45:133–7. doi: 10.1099/00222615-45-2-133. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghifard N, Salari MH, Ghassemi MR, Eshraghi S, Amin Harati F. The Incidence of nosocomial toxigenic Clostridium difficile associated diarrhea in Tehran tertiary medical centers. Acta Med Iran. 2010;48:320–5. [PubMed] [Google Scholar]
- 37.Salari MH, Badami N, Sadeghifard N, Amin Harati F. Investigation of various tissue culture monolayers sensitivity in detection of Clostridium difficile toxin. Iran J Public Health. 2008;37:99–102. [Google Scholar]
- 38.Sadeghifard N, Salari M, Ghassemi M, Shirazi M, Feizabadi M, Kazemi B, et al. Prevalence of Clostridium difficile-associated diarrhea in hospitalized patients with nosocomial diarrhea. Iran J Public Health. 2005;34:67–72. [Google Scholar]
- 39.Nasri M, Khorvash F, Zolfaghari M, Mobasherizadeh S. The Relative Frequency of Clostridium difficile in Fecal Samples of Hospitalized Patients with Diarrhea by ELISA Method. Journal of Isfahan Medical School. 2012;29:2376–82. [Google Scholar]
- 40.Jalali M, Khorvash F, Warriner K, Weese JS. Clostridium difficile infection in an Iranian hospital. BMC Res Notes. 2012;5:159. doi: 10.1186/1756-0500-5-159. [DOI] [PMC free article] [PubMed] [Google Scholar]


