Abstract
Background:
Autism spectrum disorder (ASD) is a complex, heterogeneous neurodevelopmental disorder with onset during early childhood. Most studies have reported an elevation in platelet serotonin in persons with autism. The serotonin (5-hydroxytryptamine; 5-HT) transporter in the brain uptakes 5-HT from extracellular spaces. It is also present in platelets, where it takes up 5-HT from plasma. Polymorphisms in serotonin transporter gene (SLC6A4) were frequently studied in many neuropsychiatric disorders.
Materials and Methods:
We have measured the plasma 5-HT levels in 20 autistic male children and 20 control male children by the enzyme-linked immunosorbent assay (ELISA) method. In addition, the SLC6A4 promoter region (5-HTTLPR) insertion/deletion (I/D) polymorphism was studied, using whole genomic DNA.
Results:
Plasma serotonin was significantly low in autistic children compared to control (P = 0.001), although correlation to severity of autism was not significant. The frequency of short (S) allele in autism cases was 10% and in the control group it was absent.
Conclusion:
Our study demonstrated an increased prevalence of 5-HTTLPR S allele in autism subjects. Significantly decreased plasma serotonin was detected in autism subjects, with no significant relationship between 5-HTTLPR genotype and plasma 5-HT being evident.
Keywords: Gene promoter, platelet, reuptake, serotonin, synapses, transport
INTRODUCTION
The incidence of autism is increasing worldwide, while the underlying pathophysiological mechanisms remain virtually uncharacterized. The rate of prevalence is estimated to be 1 in 110 in the US, and there is a 1.1% prevalence rate in the UK with similar incidences throughout the world (Brugha, et al., 2012).[1]
There are classes of different pharmacological agents that are found to be effective in improving behavioral symptoms of autism spectrum disorder (ASD), serotonin reuptake inhibitors being one of them (Hubbard, et al., 2012).[2] Therefore, serotonin (5-hydroxytryptamine; 5-HT), aneurotransmitter found throughout the brain, has been a potent interest in studies on autism.
There are many reports of elevated platelet 5-HT in autism, and of its dysfunctional signaling as a causal mechanism for the disorder (Mulder, et al., 2004; Yubero-Lahoz, et al., 2013).[3,4] A recent review study showed the similarity of platelet 5-HT to neuronal serotonin and thus the use of platelets’ 5-HT activity as a peripheral marker for its central activity (Yubero-Lahoz et al., 2013).[4] A disturbance of serotonin in autistic children is thought to be linked to carbohydrate-rich diets (Vered, et al., 2003)[5], as a significant elevation in 5-HT levels in autistic children was noted shortly after the meal with a significant decreased level in a steeper form thereafter that would be attributed to increased serotonin reuptake from the blood by either platelets or neurons in the brain.
Platelet serotonin, also known as platelet-poor plasma (PPP) serotonin, showed variable results in autism and altered handling of serotonin was suggested. The level of PPP 5-HT in autism was inversely related to the severity of autism (Spivak, et al., 2004; Anderson, et al., 2012).[6,7] The same trend was also found in mothers of autistic children during pregnancy, and an in-utero role for serotonin in fetal susceptibility for developing autism was highlighted (McBride, et al., 1989).[8] A recent study suggested the dependency of the raphe-prefrontal network (mainly the medial prefrontal cortex) on the 5-HT transporter during early development of the brain, and this area is responsible for many neurodevelopmental disorders (Witteveen et al., 2013).[9]
The serotonin transporter gene (SLC6A4) has been studied in the context of ASDM (Makkonen, et al., 2008; Buznikov, et al. 2001).[10,11] SLC6A4 is a transporter protein that transports serotonin from the synaptic cleft to the presynaptic neuron and thus terminates the action of serotonin. SLC6A4 gene transcription is influenced by polymorphisms, which were extensively studied in many neuropsychiatric disorders, with two commonly studied polymorphisms in its promoter region (Bonnin and Levitt, 2011; Lesurtel et al., 2006; Anderson et al., 2009).[12,14] Serotonin-transporter-linked polymorphic region (5-HTTLPR) was studied for association with ASD in many populations, with controversial results depending on ethnic diversity, methods of genetic analysis, and symptoms of ASD (Gordon, et al., 1993; McDougle, et al., 1996; Heils, et al., 1996; Lesch, et al., 1994).[15,16,17,18,19] 5-HTTLPR polymorphism due to a 44-base pair (bp) insertion/deletion (I/D) was linked to different protein expression and thus activity of corresponding protein. The long (L) allele of 5-HTTLPR is thought to be linked to higher 5-HT transporter expression (Yirmiya, et al., 2001; Kim, et al., 2002)[20,21] and thus lower blood levels of 5-HT.
The aim of this study is to report the difference in PPP 5-HT levels and determine 5-HTTLPR I/D polymorphism in a sample of autistic children and age-matched controls.
MATERIALS AND METHODS
Patients and participants
Our study included 20 autistic male children aged 7.4 ± 2.6 years and 20 control children aged 9 ± 1.6 years. Their age ranged 5-15 years (mean age 6.6 ± 4.4 years).
The diagnosis of autism was confirmed by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) and the Childhood Autism Rating Scale (CARS) (Schopler et al., 1999).[22] The cases were invited to participate in the study and the Research Ethics Committee of the National Research Centre approved all procedures for blood donation and research. The caregivers’ consent was obtained for all the studied cases.
Molecular analysis
Whole genomic DNA was isolated from the blood of both the autistic children and the control group. The DNA purity and concentration were measured by NanoDrop (ND)-1000 (Wilmington, DE 19810 USA). All used samples showed a concentration of 200 ng/μl and their purity was determined by the 260/280 ratio. The 5-HTTLPR I/D genotype was determined via polymerase chain reaction (PCR), following a previously described protocol (McCauley, et al., 2004).[23] The forward primer 50 - GGCGTTGCCGCTCTGAATGC - 30 and the reverse primer 50- GAGGGACTGAGCTGGACAACCAC - 30 were used. Cycling conditions included an initial denaturation at 95°C for 10 min, followed by 50 cycles of 94°C for 15 s, optimal annealing temperature (Ta opt°C) for 30 s, 72°C for 15 s, and a final extension at 72°C for 10 min. The PCR products were run on 3% agarose gel stained with ethidium bromide. The L and S alleles corresponded to 523- and 484-bp fragments, respectively, [Figure 1].
Figure 1.
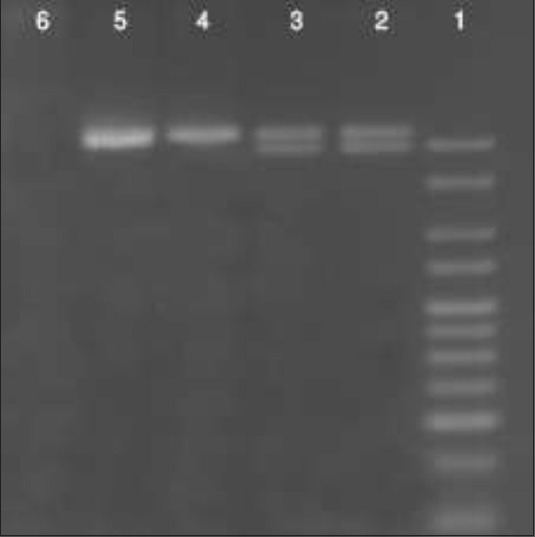
Agarose gel 3% showing the PCR products for 5-HTTLPR I/D genotype of 4 autism cases (lane 2&3 are L/S heterozygotes, lane 4&5 are L/L homozygotes, while lane 6 is a negative control)
Heterozygous samples showed both the alleles. Two investigators scored the allele sizes independently. Any inconsistency was reviewed and procedures were repeated if necessary. Genotyping was conducted blind to both the cases and the control samples without access to the clinical information.
Determination of PPP 5-HT
The serotonin assay was done using Serotonin ELISA kit (DIASource ImmunoAssays S.A. 8, Rue de l’Industrie, B-1400 Nivelles, Belgium), using the competitive ELISA kit (the microtiter plate format).
All statistical analysis was done using the SPSS version 14 software package (IBM SPSS Statistics, USA) and graph prism program. The Chi-square test was used to evaluate the difference in gene alleles between the studied groups, and P values of 0.05 were considered statistically significant. Multinomial logistic regression was used to study interacting factors.
RESULTS
The autistic patients and the control group showed matched ages with no significant difference (P = 0.3). They are all male children. The PCR results for autistic patients showed the allele frequencies to be 10% for the S allele and 90% for the L allele [Table 1]. Four autistic cases had the L/S 5-HTTLPR genotype, and the distribution of L/L, L/S, and S/S genotypes among patients were 80%, 20%, and 0% (with 10% and 90% allele frequency for S and L alleles, respectively). In the control group, the S allele was not detected.
Table 1.
5- HTTLPR genotype and allele frequencies among autistic and control children
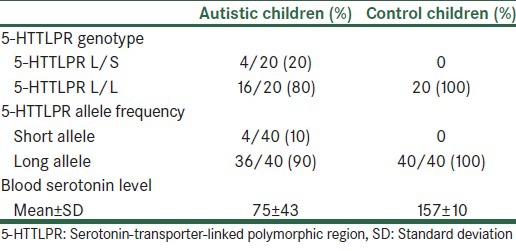
The Chi-square test of allele distribution among autistic patients and controls shows a significant association of the S allele with autism (P = 0.035). No effect of age was noted. As all cases are males, the effect of sex on polymorphism could not be studied.
Blood serotonin
Measured serotonin levels in autism cases ranged 43-159 ng/ml (mean 75 ± SD 43), and in control 139-171 ng/ml (mean 157 ± SD 10) [Figure 2]. There is a significant decrease in the serotonin level in autistic children compared to control, as seen by using the Mann—Whitney test (P = 0.001) [Table 1].
Figure 2.
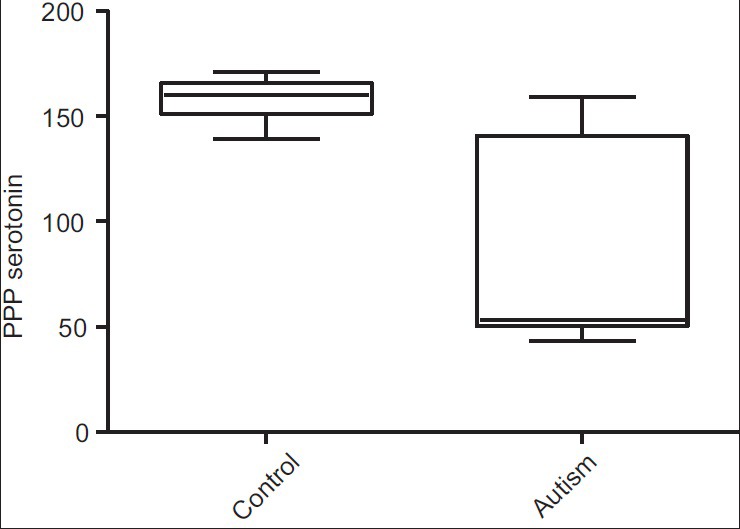
Plasma serotonin between autism and control groups (P = 0.001)
The present study included 11 patients with severe autism (CARS 39-60) and 9 patients with mild to moderate autism (CARS 30-38). No significant difference in blood serotonin levels between the moderate group (mean 73.11 ± SD 40.87) and the severe autistic features group (mean 85.54 ± SD 50.18) was found.
We could not detect a significant difference in blood serotonin with relation to different 5-HTTLPR genotyping among autism cases (P = 0.14).
DISCUSSION
Our study shows a significant decreased PPP 5-HT level in autistic children compared to control.
Measurement of the PPP levels of 5-HT appears to provide the best available index of in vivo exposure of the platelet to 5-HT (Anderson, et al., 2012).[7] An interesting study measuring 5-HT axons that were immunoreactive to a serotonin transporter (5-HTT) antibody in a number of postmortem brains from autistic patients, showed that the number of serotonin axons was increased in them (Azmitia, et al., 2011).[24] Another study found similarities in brain and plasma 5-HT levels in response to dietary factors (Kot, et al., 2012).[25]
Our results of decreased PPP 5-HT represented an indicator of decreased central 5-HT due to increased neuronal uptake. A study done on pigs found an interrelated value between the brain (hippocampus) and the platelet serotonin. Both values were related to some exploratory tests in pigs (Ursinus, et al., 2013).[26] It is suspected that the relationship would be more complicated in autism, with serotonin being one of the implicated central neurotransmitters and its metabolism being a predisposing mechanism.
Serotonin neuronal cellular effects were studied recently (Mercado et al., 2013)[27] using microarray technology, the main biological pathways affected being cytoskeletal remodeling, protein signaling, and apoptosis. In the present work, autism cases showed significantly increased 5-HTTLPRS allele compared to controls (P = 0.035). The findings of the present study are consistent with those of a previous study by Anderson et al. 2012.[7] However, they included first-degree relatives, e.g., mothers, fathers, and sibling as control groups, which is considered one of the limitations of that study, while the present study enrolled unrelated, normal, healthy children.
Our study is the first work on Egyptian autistic children to study changes in blood serotonin and the 5-HTTLPR L/S polymorphism. A previous study carried out on South African autistic patients showed a significantly higher prevalence of 5-HTTLPR S allele among autistic patients, with a frequency of 33%, and the absence of the same in the control group (Arieff, et al., 2010),[28] which is similar to our results. The discrepancy between our genotyping results and other studies could be attributed to the various ancestral populations that were proved regarding 5-HTTLPR polymorphism (Esau, et al., 2008).[29]
The 5-HTTLPR L/S genotyping in the present study results is consistent with earlier reports on other populations such as the Indian population, as a significant preferential transmission of S allele from parents to the affected offspring was noted (P = 0.006), indicating an association of 5-HTTLPR with autism (Guhathakurta, et al., 2006).[30]
No difference is seen in serotonin levels in the autism cases with various HTTLPR genotyping. Serotonin levels could be influenced by other genetic markers than HTTLPR in SLC6A4, as this is supported by a recent study on autism in families that did not show any association of some social impairment tests with 5-HTTLPR polymorphism (Neves Mde, et al., 2011),[31] while another study found an association between SLC6A4 SNP rs16965628, located in intron one, and some behavioral problems (Lindholm Carlstrφm et al., 2012).[32] It was also found that vitamin D (calcitriol) activates the transcription of the serotonin-synthesizing gene tryptophan hydroxylase 2 (TPH 2) in the brain (Patrick and Ames, 2014).[33]
5-HTTLPR polymorphism was found to be associated with other psychiatric disturbances, e.g. childhood depression (Comasco, et al., 2013),[34] which would affect the symptoms of autism and the age of diagnosis. Fewer studies showed no main effect of 5-HTTLPR genotype and childhood depression (Tomoda, et al., 2013).[35]
The role of polymorphism in autism risk is thought to be influenced by environmental circumstances, e.g. social and parental rejection (Lindell, et al., 2012).[36]
To conclude, our study showed a significant correlation of the S allele of 5-HTTLPR with autism, and decreased serotonin in blood was detected among autistic children with no relationship between the studied polymorphism and serotonin levels.
This finding supports the role of 5-HTTLPR S allele as a risk factor for autism, although the correlation of genotyping with peripheral serotonin level would represent a complex biological process with many interacting genetic factors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Brugha TS, McManus S, Smith J, Scott FJ, Meltzer H, Purdon S, et al. Validating two survey methods for identifying cases of autism spectrum disorder among adults in the community. Psychol Med. 2012;42:647–56. doi: 10.1017/S0033291711001292. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard L, McNealy K, Scott-Van Zeeland AA, Callan E, Bookheimer SY, Dapretto M. Altered integration of speech and gesture in children with autism spectrum disorders. Brain Behav. 2012;2:606–19. doi: 10.1002/brb3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–9. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Yubero-Lahoz S, Robledo P, Farré M, de laTorre R. Platelet SERT as a peripheral biomarker of serotonergic neurotransmission in the central nervous system. Curr Med Chem. 2013;20:1382–96. doi: 10.2174/0929867311320110003. [DOI] [PubMed] [Google Scholar]
- 5.Vered Y, Golubchik P, Mozes T, Strous R, Nechmad A, Mester R, et al. The platelet-poor plasma 5-HT response to carbohydrate rich meal administration in adult autistic patients compared with normal controls. Hum Psychopharmacol. 2003;18:395–9. doi: 10.1002/hup.489. [DOI] [PubMed] [Google Scholar]
- 6.Spivak B, Golubchik P, Mozes T, Vered Y, Nechmad A, Weizman A, et al. Low platelet-poor plasma levels of serotonin in adult autistic patients. Neuropsychobiology. 2004;50:157–60. doi: 10.1159/000079108. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GM, Hertzig ME, McBride PA. Brief report: Platelet-poor plasma serotonin in autism. J Autism Dev Disord. 2012;42:1510–4. doi: 10.1007/s10803-011-1371-1. [DOI] [PubMed] [Google Scholar]
- 8.McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, et al. Serotonergic responsivity in male young adults with autistic disorder. Results of a pilot study. Arch Gen Psychiatry. 1989;46:213–21. doi: 10.1001/archpsyc.1989.01810030019003. [DOI] [PubMed] [Google Scholar]
- 9.Witteveen JS, Middelman A, van Hulten JA, Martens GJ, Homberg JR, Kolk SM. Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci. 2013;7:143. doi: 10.3389/fncel.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–7. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 11.Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–86. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 12.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BM, Schnetz-Boutaud NC, Bartlett J, Wotawa AM, Wright HH, Abramson RK, et al. Examination of association of genes in the serotonin system to autism. Neurogenetics. 2009;10:209–16. doi: 10.1007/s10048-009-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon CT, State RC, Nelson JE, Hamburger SD, Rapoport JL. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry. 1993;50:441–7. doi: 10.1001/archpsyc.1993.01820180039004. [DOI] [PubMed] [Google Scholar]
- 16.McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53:1001–8. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- 17.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, et al. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 19.Zhong N, Ye L, Ju W, Brown WT, Tsiouris J, Cohen I. 5-HTTLPR variants not associated with autistic spectrum disorders. Neurogenetics. 1999;2:129–31. doi: 10.1007/s100480050064. [DOI] [PubMed] [Google Scholar]
- 20.Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I, et al. Evidence for an association with the serotonin transporter promoter region polymorphism and autism. Am J Med Genet. 2001;105:381–6. doi: 10.1002/ajmg.1365. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Cox N, Courchesne R, Lord C, Corsello C, Akshoomoff N, et al. Transmission disequilibrium mapping at the serotonin transporter gene (SLC6A4) region in autistic disorder. Mol Psychiatry. 2002;7:278–88. doi: 10.1038/sj.mp.4001033. [DOI] [PubMed] [Google Scholar]
- 22.Schopler E, Reichler RJ, Renner BR. Los Angeles, CA: Western Psychological Services; 1999. Childhood Autism Rating Scale. [Google Scholar]
- 23.McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, et al. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:104–12. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- 24.Azmitia EC, Singh JS, Whitaker-Azmitia PM. Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology. 2011;60:1347–54. doi: 10.1016/j.neuropharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Kot M, Pilc A, Daniel WA. Simultaneous alterations of brain and plasma serotonin concentrations and liver cytochrome P450 in rats fed on a tryptophan-free diet. Pharmacol Res. 2012;66:292–9. doi: 10.1016/j.phrs.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Ursinus WW, Bolhuis JE, Zonderland JJ, Rodenburg TB, de Souza AS, Koopmanschap RE, et al. Relations between peripheral and brain serotonin measures and behavioural responses in a novelty test in pigs. Physiol Behav. 2013;118:88–96. doi: 10.1016/j.physbeh.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Mercado CP, Byrum S, Beggs ML, Ziu E, Singh P, Raj VR, et al. Impact of elevated plasma serotonin on global gene expression of murine megakaryocytes. PLoS One. 2013;8:e72580. doi: 10.1371/journal.pone.0072580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arieff Z, Kaur M, Gameeldien H, van der Merwe L, Bajic VB. 5-HTTLPR polymorphism: Analysis in South African autistic individuals. Hum Biol. 2010;82:291–300. doi: 10.3378/027.082.0303. [DOI] [PubMed] [Google Scholar]
- 29.Esau L, Kaur M, Adonis L, Arieff Z. The 5 HTTLPR polymorphism in South African healthy populations: A global comparison. J Neural Transm. 2008;115:755–60. doi: 10.1007/s00702-007-0012-5. [DOI] [PubMed] [Google Scholar]
- 30.Guhathakurta S, Ghosh S, Sinha S, Chatterjee A, Ahmed S, Chowdhury SR, et al. Serotonin transporter promoter variants: Analysis in Indian autistic and control population. Brain Res. 2006;1092:28–35. doi: 10.1016/j.brainres.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 31.Neves Mde C, Tremeau F, Nicolato R, Lauar H, Romano-Silva MA, Correa H. Facial emotion recognition deficits in relatives of children with autism are not associated with 5HTTLPR. Rev Bras Psiquiatr. 2011;33:261–7. doi: 10.1590/s1516-44462011000300009. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm Carlström E, Saetre P, Rosengren A, Thygesen JH, Djurovic S, Melle I, et al. Association between a genetic variant in the serotonin transporter gene (SLC6A4) and suicidal behavior in patients with schizophrenia. Behav Brain Funct. 2012;17:8–24. doi: 10.1186/1744-9081-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28:2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 34.Comasco E, Åslund C, Oreland L, Nilsson KW. Three-way interaction effect of 5-HTTLPR, BDNF Val66Met, and childhood adversity on depression: A replication study. Eur Neuropsychopharmacol. 2013;23:1300–6. doi: 10.1016/j.euroneuro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Tomoda A, Nishitani S, Matsuura N, Fujisawa TX, Kawatani J, Toyohisa D, et al. No interaction between serotonin transporter gene (5-HTTLPR) polymorphism and adversity on depression among Japanese children and adolescents. BMC Psychiatry. 2013;13:134. doi: 10.1186/1471-244X-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindell SG, Yuan Q, Zhou Z, Goldman D, Thompson RC, Lopez JF, et al. The serotonin transporter gene is a substrate for age and stress dependent epigenetic regulation in rhesus macaque brain: Potential roles in genetic selection and gene × environment interactions. Dev Psychopathol. 2012;24:1391–400. doi: 10.1017/S0954579412000788. [DOI] [PMC free article] [PubMed] [Google Scholar]


