Abstract
Molecules offering simultaneous detection and killing of cancer cells are advantageous. Hybrid of cancer cell-selective, ROS generator betulinic acid and bis-arylidene oxindole with amino propyl-linker is developed. With intrinsic fluorescence, the molecule exhibited cancer cell-specific residence. Further, it generated ROS, triggered apoptosis, and exhibited potent cytotoxicity in cancer cells selectively. We demonstrate the first example and use of isatins as betulinic acid conjugate for selective detection of cancer and subsequent killing of cancer cells via apoptosis.
Keywords: Fluorescent oxindole, betulinic acid, cancer cell detection, ROS, apoptosis
Cancer treatment needs multimodal strategies to restrain its aggressive malignancy. Strategies that include simultaneous detection/sensing and treatment should be specific for cancer to avoid unwanted side effects. Effective detection includes specific labeling of tumor cells. Desirably, detection, drug-sensitization, and effective cytotoxicity among tumor cell population and various infiltrating cells in tumor-microenvironment would be the most sought-after strategy for controlling tumor-aggressiveness. Among tumor-detecting molecular probes, intrinsically fluorescent small molecules are preferably and widely employed as they can enter live cells easily and offer screening through visual detection.1−5
Isatin-based molecules are biologically important, but there are limited reports toward establishing isatin-based fluorescent compounds for biological screening.6−8 To our knowledge, use of fluorescent isatins in the context of cancer detection is not documented before. This is probably because isatins so far developed did not show any specificity toward cancer cells. Recently we reported a new class of antiestrogenic, bis-arylidene oxindoles (certain derivatives of isatin) as antibreast cancer agent.9 In our pursuit for designing a cancer cell selective molecular probe, we have focused our attention toward these intrinsically fluorescent-isatin molecules.
Natural triterpenoid betulinic acid (BetA) exhibits a range of biological effects including potent antitumor activity.10−16 This anticancer property is linked to its ability to induce caspase-independent apoptotic cell death in cancer cells by depolarizing mitochondrial membrane potential.17 Since normal cells exhibit resistance against BetA-mediated toxicity, favorable therapeutic window is naturally expected. Evasion of apoptosis is one of the hallmarks of cancer.18 Therefore, BetA with natural tendency to trigger mitochondrial pathway of apoptosis may find use in treating drug resistant cancers.16 BetA is hence used in various combination strategies including chemotherapeutic drugs,19,20 death receptor ligand TRAIL,21 etc. BetA is a known reactive oxygen species (ROS) scavenger for normal cells22,23 but generates excessive ROS to promote a caspase-independent programmed cell death in cancer cells.17,24 Cancer cells generate moderate level of ROS, which accounts for DNA damage promoting enhanced genetic instability and drug resistance.25 However, the excessive increase in ROS-mediated oxidative stress misbalances cancer cells and overwhelms antioxidant-defense of endogenous reduced glutathion (GSH) and super oxide dismutase (SOD). Hence, selective and excessive ROS generation is a strategically useful anticancer tool.26,27 However, the entry of BetA in cancer cells is not selective as there is evidence for its entering in nonproliferating normal cells, wherein it acts as ROS-scavenger. However, ROS-scavenging or ROS-generating role of BetA in proliferating normal cells (such as in blood, skin, or hair cells) is not clear. Implicitly, in order to avoid any unwanted toxicity BetA should be properly targeted.
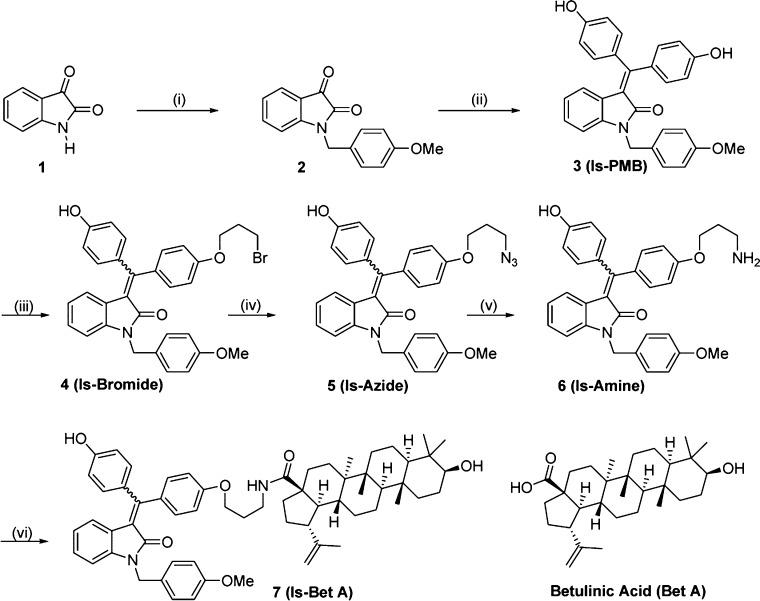
Toward developing a new hybrid molecule strategically exhibiting cancer cell selective detection and killing, we synthesized bis-arylidene oxindole-conjugated BetA. Since these isatins exhibit fluorescence properties, we reasoned that upon conjugation we could visualize the fate of BetA conjugate in cells. The synthetic procedure for the hybrid molecule is illustrated in Scheme 1. The precursor bis-phenol derivative 3 (Is-PMB) was synthesized using two-step reaction sequences starting from isatin following our published protocol.9 Mono O-alkylation of compound 3 using 1,3-dibromo propane and potassium carbonate as a base in acetone gave compound 4 in 60% yield. As our expectation, compound 4 was obtained as an inseparable mixture of Z and E isomers. Nucleophilic substitution of this bromo-compound (4) using sodium azide in DMSO yielded compound 5 in 98% yield. Staudinger reduction of compound 5 in the presence of triphenylphosphine in THF provided compound 6 (Is-amine) in 75% yield. Finally compound 7 (Is-BetA) was synthesized by reacting compound 6 with BetA using N,N′-dicyclohexylcarbodimide (DCC), 4-dimethylaminopyridine (DMAP), and hydroxybenzotriazole (HOBt) in DMF with 85% yield.
Scheme 1. Synthesis of bis-Arylidene Oxindole–Betulinic Acid Conjugate (Is-BetA, 7).
Reagents and conditions: (i) (a) NaH, THF, r.t., 1 h; (b) PMB-Br, DMF, r.t., 5 h, 78%; (ii) 4,4′-dihydroxy benzophenone, Zn, TiCl4, THF, reflux, 3 h, 65%; (iii) (a) K2CO3, acetone, reflux, 1 h; (b) 1,3-dibromo propane, reflux, 3 h, 60%; (iv) NaN3, DMSO, r.t., overnight, 98%; (v) (a) PPh3, THF, rt, 2 h; (b) H2O, r.t., overnight 75%; (vi) betulinic acid, DCC, DMAP, HOBT, DMF, r.t., overnight, 85%.
All the three synthesized compounds and intermediates, Is-BetA (compound 7), Is-PMB (compound 3), and Is-amine (compound 6) incidentally showed red fluorescent properties, with their excitation λmax ranged within 369–383 nm, and emission λmax, 552–565 nm, respectively (Figure S1). Betulinic acid (or BetA) does not possess any fluorescent property. In order to visualize the extent of cellular entry of BetA, which is originally nonfluorescent, we conjugated BetA with green fluorescent fluorescein molecule to form BetA-FITC. The fluorescent modification of BetA was done similar to that of the synthesis of Is-BetA, wherein carboxylic acid group of BetA was involved. We hypothesized that BetA-FITC and Is-BetA would not largely differ in their extent of cellular entry as the conjugation pattern (involving BetA) did not differ. Synthesis of BetA-FITC was accomplished as per the described synthetic procedure (Figure S2).
To determine if any slight structural change would affect the cellular uptake, we opted to test Is-PMB and Is-amine compounds along with Is-BetA for their uptake properties in various cells. As absorptivity of tested molecules emitting at red region were not the same, therefore, for comparison of quantitative cellular uptake of each molecule, we represented data as % uptake following normalization with fluorescent absorption values of respective molecules. As a positive control we used anticancer drug, doxorubicin (Dox), which also possesses red fluorescence. One noncancer cell, NIH 3T3, and two cancer cells, B16F10 (mouse melanoma) and PANC-1 (human pancreatic cancer), were used for this uptake study. The microscopic visualization of cells (Figure 1A) treated with various molecules revealed the following: (a) noncancer (NIH3T3) cells did not take up Is-BetA, Is-amine, and BetA-FITC, but took up Is-PMB and Dox quite efficiently. (b) Contrastingly, cancer cells took up all treated molecules including Is-BetA, Is-amine, and BetA-FITC. Quantitative analysis of this uptake study maintains this microscopically visualized trend (Figure 1B). In consonant with microscopic observation, Is-PMB and Dox showed significantly higher percentage of uptake in both cancer and noncancer cells nonspecifically. Interestingly, Is-amine and Is-PMB, which bear minimum structural difference, exhibited contrasting uptake behaviors. It is the presence of O-propyl amine side chain spacer in Is-amine that possibly bolstered its (and also Is-BetA’s) selective uptake in cancer cells. Moreover, BetA-FITC individually exhibited selective uptake in cancer cells. We believe this might have synergized the cellular uptake of Is-BetA conjugate in cancer cells. Detailed microscopic images are provided in Figures S3–S5.
Figure 1.
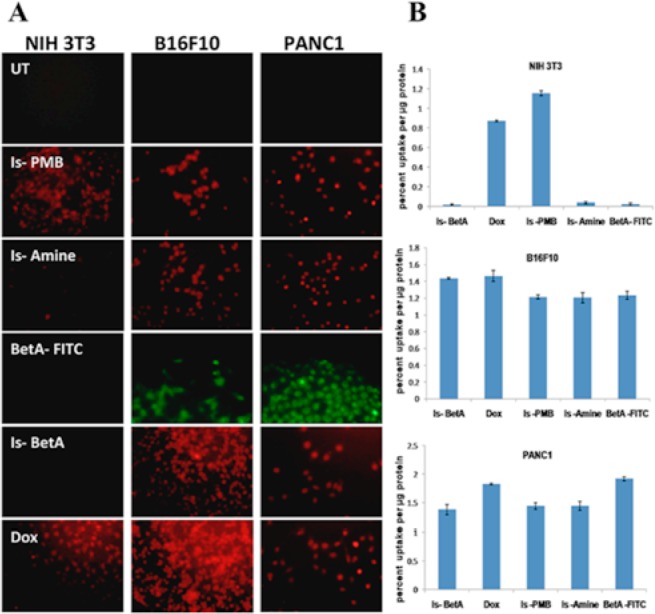
(A) Fluorescence images of normal cells (NIH 3T3) or cancer cells (B16F10, PANC1) either kept untreated (UT) or treated with Is-PMB, Is-amine, BetA-FITC, Is-BetA, and Dox, respectively. (B) Quantitative uptake of Is-BetA, Dox, Is-PMB, Is-amine, and Bet-FITC in cells as depicted. The data is represented as percentage of fluorescent molecules’ uptake per microgram of protein.
The conjugate molecule Is-BetA, its precursors such as Is-Amine and Is-PMB, and its conjugate partner BetA were tested in several cancer and noncancer cells for their cytotoxic effect. Dox was also included in this study. This study was done with following cancer cells: MCF-7 (human breast cancer), A549 (human lung cancer), PANC-1 (human pancreatic cancer), B16F10 (mouse melanoma), OVCAR-3 (human ovarian cancer), and MDA-MB-231 (human breast cancer). The noncancer cells as included for this study were CHO (chinese hamster ovary) and NIH3T3 (human fibroblast). The result is presented in Table 1. Even though Is-PMB showed nonspecific cellular uptake in both cancer and noncancer cells, it did not show any toxicity in these cells. Dox was as usual toxic in both kinds of cells. BetA and Is-amine, which were selectively taken up by cancer cells, showed mixed trend of cytotoxicity in cancer cells. Both BetA and BetA-FITC exhibited similar levels of toxicity in selected cancer cells and no toxicities in noncancer cells. This indicates that final fates (i.e., killing) of cells treated with BetA or BetA-FITC are similar even though the cellular uptake of these molecules could only be observed in cells treated with BetA-FITC. Clearly, even though BetA-FITC and Is-BetA exhibited more or less similar levels of entry in cancer cells, Is-BetA could induce selectively more toxicity in cancer cells than BetA-FITC (or BetA).
Table 1. Cytotoxicity of Is-BetA and Other Derivatives in Cancer (MCF-7, B16-F10, OVCAR 3, MDA-MB-231, PANC-1, and A549) and Noncancer Cells (CHO, NIH3T3) Following 72 h of Continuous Treatment (ND, Not Determined).
| MCF7 (IC50, μM) | B16-F10 (IC50, μM) | OVCAR 3 (IC50, μM) | MDA-MB-231 (IC50, μM) | PANC1 (IC50, μM) | A549 (IC50, μM) | CHO (IC50, μM) | NIH 3T3 (IC50, μM) | |
|---|---|---|---|---|---|---|---|---|
| Is-BetA | 7.396 ± 0.034 | 2.296 ± 0.037 | 6.048 ± 0.018 | 1.256 ± 0.004 | 2.630 ± 0.032 | 2.560 ± 0.022 | >20 | >20 |
| BetA | 10.527 ± 0.024 | >20 | 8.366 ± 0.008 | >20 | 10.223 ± 0.022 | >20 | >20 | >20 |
| BetA-FITC | ND | 20 | ND | ND | 9.778 ± 0.033 | ND | ND | >20 |
| Is-amine | ND | >20 | ND | >20 | >20 | 1.359 ± 0.004 | ND | ND |
| Is-PMB | >20 | >20 | >20 | >20 | >20 | ND | >20 | >20 |
| Dox | ND | 0.663 ± 0.024 | ND | ND | 1.026 ± 0.036 | ND | ND | 7.211 ± 0.029 |
However, Is-amine induced no cytotoxicity in either cancer or noncancer cells, except with a glaring example of cytotoxicity in A549 cancer cells. However, as expected from the selective uptake of Is-BetA in cancer cells, Is-BetA clearly showed selective and consistent cytotoxicity in only cancer cells. There were no visible Is-BetA-mediated cytotoxicities in noncancer cells. Clearly, the conjugate (Is-BetA) is significantly more efficient in selectively killing cancer cells when compared to the individual partners, i.e., Is-amine and BetA. The data in Figure 1 and Table 1 clearly indicates that the fluorescent uptake of Is-BetA was directly proportional to the killing of cells. Since noncancer cells did not take up Is-BetA, we did not witness any killing effect. Conversely, since cancer cells efficiently took up Is-BetA, it induced effective killing of cancer cells. Dox is known to have nonspecific toxicity in proliferating noncancer cells. We obtained nonspecific uptake of Dox in all tested cells. This might have a role in maintaining Dox-mediated indiscriminate toxicity in both cancer and noncancer cells.
Betulinic acid has dual role in different cells. It is a known scavenger of ROS in normal cells but induces limited ROS generation in cancer cells. As Is-BetA exhibited efficient cancer cell killing, it is of interest to know the status of ROS in Is-BetA-treated cells. In fact, only Is-BetA, among its precursors and conjugate partners, triggered excessive ROS production in B16F10 cells (Figures 2 and S6). The ROS-generating effect of Is-BetA in cancer cells was hence significantly and strikingly more than that of even the ROS generating parent molecule, BetA. Notably, neither Is-BetA nor its precursors Is-amine and Is-PMB, and its conjugate partner, BetA, could generate ROS in noncancer cells. However, ROS production was nil in Is-BetA-treated noncancer cells (Figure S7). This could be simply because of its least uptake in these cells. Evidently and as expected, Dox induced the production of ROS in both cancer and noncancer cells.
Figure 2.
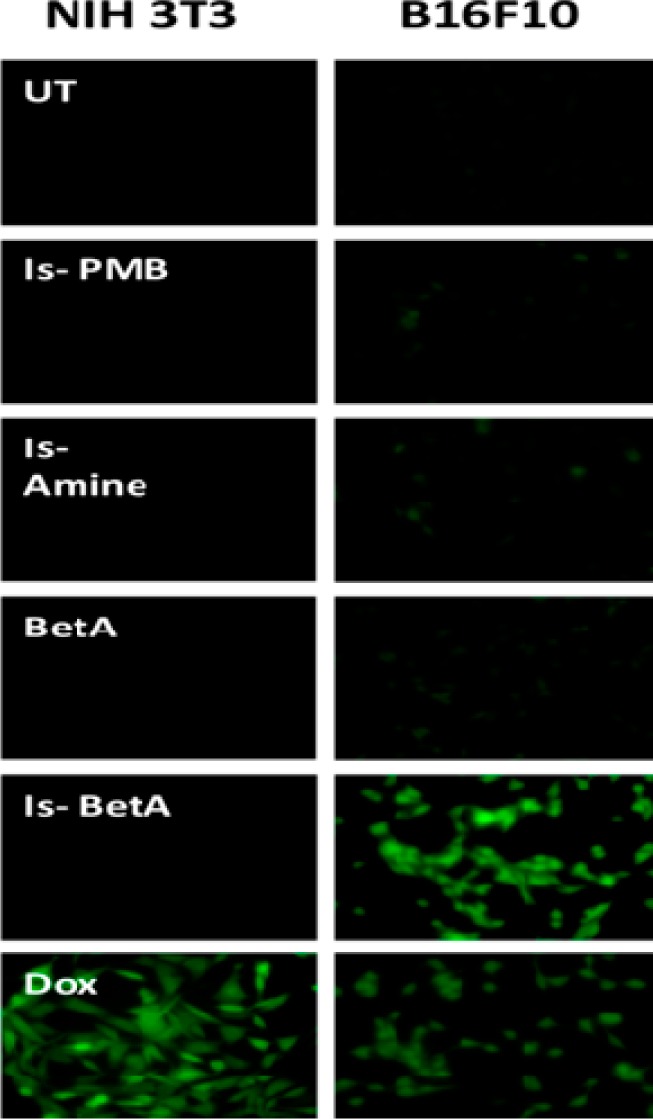
Is-BetA selectively generates reactive oxygen species (ROS) in cancer cells. Noncancer (NIH 3T3) and cancer cells (B16F10) were treated with Is-PMB, Is-amine, BetA, Is-BetA, and Dox, respectively, or kept untreated (UT). ROS as generated in respective cells were microscopically visualized in 20× magnification.
Further, using flow cytometry technique we determined the apoptosis-inducing capabilities of tested molecules in cancer cells. Following the treatments of respective molecules, B16F10 melanoma cells were assayed for apoptosis by doubly staining with annexin V/propidium iodide (PI). Annexin V and PI doubly stained cells show the characteristic of postapoptotic cells, which contain cellular population with apoptotic and necrotic properties simultaneously. As depicted in individual subfigures of Figure 3, this postapoptotic population appears in the right upper corner (Q2), whereas cells undergoing only necrosis appear in the left upper corner (Q1), cells with apoptotic-only feature appear in right lower corner (Q4), and healthy cells with no apoptotic or necrotic feature appear in left lower corner (Q3). It is clear that, among Is-BetA-treated cells, maximum cancer cells exhibited features of postapoptotic conditions. This population is significantly more than the corresponding population from cells treated with other individual components, Is-amine and BetA. This data is in consonant with the significant ROS generating ability and selectively high cytotoxicity of Is-BetA in cancer cells.
Figure 3.
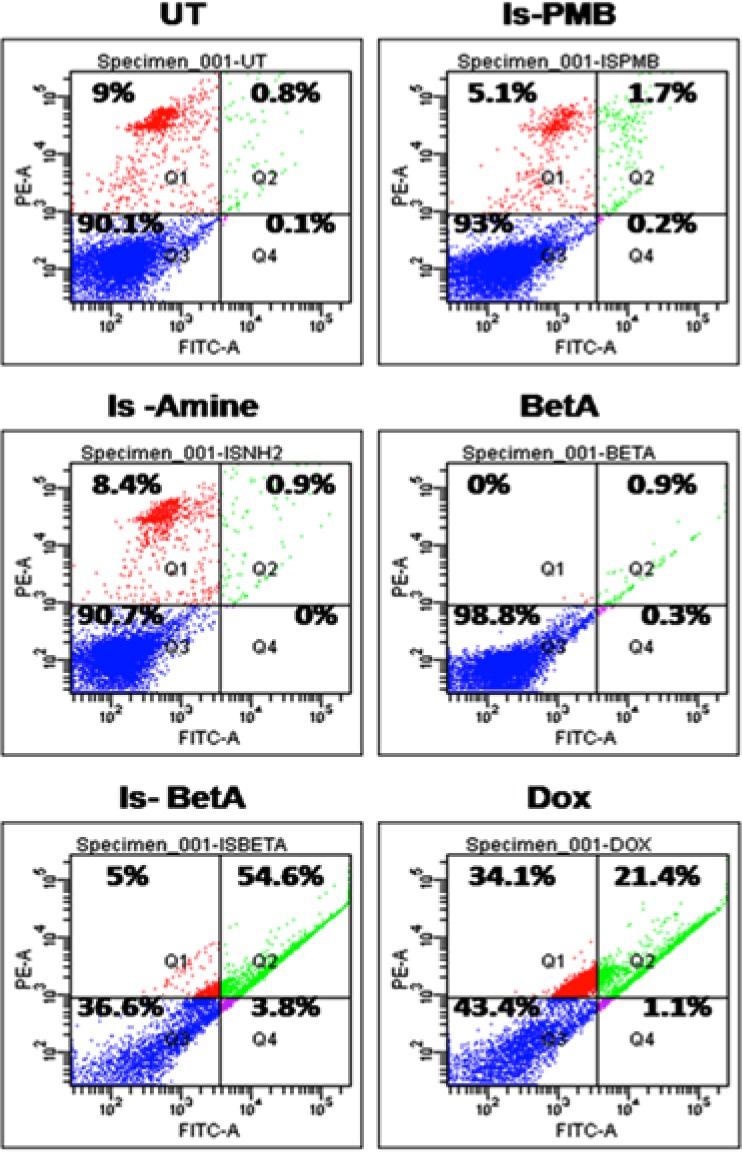
Is-BetA induces apoptosis in cancer cells. Flow cytometric analyses of B16F10 cancer cells either kept untreated (UT) or treated with Is-PMB, Is-amine, BetA, Is-BetA, and Dox. Among all treatments, Is-BetA-treated cells represent maximum postapoptotic features (Q2: 54.6%).
Evidently, Is-BetA-mediated excellent ROS generation in cancer cells induced significant apoptosis in them. This could be the primary reason for selective and efficient killing of various cancer cells. Clearly, those cells (i.e., cancer cells), which were fluoresced due to efficient uptake of Is-BetA, generated huge excess of ROS and eventually were killed via apoptosis. Hence, we accomplished the development of a single molecule, which can effectively serve to first detect and finally kill cancer cells, both selectively and efficiently. The drug sensitizing property and in vivo antitumor potential of this 2-in-1 therapeutic molecule is currently under progress.
The present study demonstrates the first example among isatin derivatives conjugated with natural product betulinic acid, as a new anticancer therapeutic with dual role of selective detection and killing of cancer cells.
Acknowledgments
We acknowledge Prof. Amitabha Sarkar (IACS, Kolkata) for his generous suggestions and support. We also thank Biswajit Chakraborty (IICB, Kolkata) for his assistance in the isolation of betulinic acid (in bulk quantity) from methanol extract of the Dillenia Indica fruit commonly known as “Chalta” or Elephant Apple.
Glossary
ABBREVIATIONS
- BetA
betulinic acid
- ROS
reactive oxygen species
- Dox
doxorubicin
- FITC
fluorescein isothiocyanate
Supporting Information Available
Spectroscopic details of compounds 3, 4, 5, 6, and 7 and analytical profiles of novel compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ These authors contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
A.P., M.Y., and A.K.B. acknowledge fellowship supports from CSIR, New Delhi. A.G. is thankful to UGC for a fellowship. S.C. acknowledges DST, and A.G. and R.K. acknowledge CSIR Network projects for respective project fellowships. This project is supported by CSIR Network projects CSC0302, BSC0123, Govt. of India [to R.B.] and DST project (No. SR/S1/OC-101/2012] [to S.A.].
The authors declare no competing financial interest.
Supplementary Material
References
- Leong C.; Lee S. C.; Ock J.; Li X.; See P.; Park S. J.; Ginhoux F.; Yun S. W.; Chang Y. T. Microglia specific fluorescent probes for live cell imaging. Chem. Commun. 2014, 50, 1089–1091. [DOI] [PubMed] [Google Scholar]
- Fernández-Suárez M.; Ting A. Y. Fluorescent probes for super resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 929–943. [DOI] [PubMed] [Google Scholar]
- Hama Y.; Urano Y.; Koyama Y.; Bernardo M.; Choyke P. L.; Kobayashi H. A comparison of the emission efficiency of four common green fluorescence dyes after internalization into cancer cells. Bioconjugate Chem. 2006, 17, 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltke C.; Wallbrunn A. V.; Kopka K.; Schober O.; Heindel W.; Schäfers M.; Bremer C. A fluorescent photoprobe for the imaging of endothelin receptors. Bioconjugate Chem. 2007, 18, 685–694. [DOI] [PubMed] [Google Scholar]
- Sampedro A.; Villalonga-Planells R.; Vega M.; Ramis G.; Fernández de Mattos S.; Villalonga P.; Costa A.; Rotger C. Cell uptake and localization studies of squaramide based fluorescent probes. Bioconjugate Chem. 2014, 25, 1537–1546. [DOI] [PubMed] [Google Scholar]
- Kundu A.; Pathak S.; Pramanik A. Synthesis and fluorescence properties of isatin-based spiro compounds: switch off chemosensing of copper (II) ions. Asian J. Org. Chem. 2013, 2, 869–876. [Google Scholar]
- Dabiri M.; Tisseh Z. N.; Bazgir A. An efficient synthesis of fluorescent spiro [benzopyrazoloquinolineindoline] triones and spiro [acenaphthylenebenzopyrazoloquinoline] triones. Monatsh. Chem. 2012, 143, 139–143. [Google Scholar]
- Trigoso C. I.; Ibanez N.; Stockert J. C. A specific fluorogenic reaction for tryptophan residues using isatin in organic solvents. J. Histochem. Cytochem. 1993, 41, 1557–1561. [DOI] [PubMed] [Google Scholar]
- Pal A.; Ganguly A.; Ghosh A.; Yousuf M.; Rathore B.; Banerjee R.; Adhikari S. Bis-arylidene oxindoles as anti-breast-cancer agents acting via the estrogen receptor. ChemMedChem 2014, 9, 727–732. [DOI] [PubMed] [Google Scholar]
- Pisha E.; Chai H.; Lee I. S.; Chagwedera T. E.; Farnsworth N. R.; Cordell G. A.; Beecher C. W. W.; Fong H. H. S.; Kinghorn A. D.; Brown D. M.; Wani M. C.; Wall M. E.; Hieken T. J.; Das Gupta T. K.; Pezzuto J. M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Rieber M.; Rieber M. S. Induction of p53 without increase in p21WAFl in betulinic acid-mediated cell death is preferential for human metastatic melanoma. DNA Cell Biol. 1998, 17, 399–406. [DOI] [PubMed] [Google Scholar]
- Galluzzi L.; Larochette N.; Zamzami N.; Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 2006, 25, 4812–4830. [DOI] [PubMed] [Google Scholar]
- Fulda S.; Friesen C.; Los M.; Scaffidi C.; Mier W.; Benedict M.; Nuñez G.; Krammer P. H.; Peter M. E.; Debatin K. M. Betulinic acid triggers CD95 (APO-lIFas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997, 57, 4956–4964. [PubMed] [Google Scholar]
- Zuco V.; Supino R.; Righetti S. C.; Cleris L.; Marchesi E.; Gambacorti-Passerini C.; Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H.; Fulda S.; Führer M.; Debatin K. M.; Jeremias I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia 2004, 18, 1406–1412. [DOI] [PubMed] [Google Scholar]
- Thurnher D.; Turhani D.; Pelzmann M.; Wannemacher B.; Knerer B.; Formanek M.; Wacheck V.; Selzer E. Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head Neck 2003, 25, 732–740. [DOI] [PubMed] [Google Scholar]
- Tan Y. M.; Yu R.; Pezzuto J. M. Betulinic acid-induced programmed cell death in human melanoma cells involves mitogen-activated protein kinase activation. Clin. Cancer Res. 2003, 9, 2866–2875. [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. The hallmarks of cancer. Cell 2000, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Fulda S.; Debatin K. M. Sensitization for anticancer drug-induced apoptosis by betulinic acid. Neoplasia 2005, 7, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N.; Kataoka K.; Kondo K.; Arimochi H.; Fujino H.; Takahashi Y.; Miyoshi T.; Kuwahara T.; Monden Y.; Ohnishi Y. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br. J. Cancer 2004, 90, 1672–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S.; Jeremias I.; Debatin K. M. Cooperation of betulinic acid and TRAIL to induce apoptosis in tumor cells. Oncogene 2004, 23, 7611–7620. [DOI] [PubMed] [Google Scholar]
- Yoon J. J.; Lee Y. J.; Kim J. S.; Kang D. G.; Lee H. S. Protective role of betulinic acid on TNF-α-induced cell adhesion molecules in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 96–101. [DOI] [PubMed] [Google Scholar]
- Ciesielska A. S.; Plewka K.; Daniluk J.; Szerszeń M. K. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-α, TGF-β) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [DOI] [PubMed] [Google Scholar]
- Wick W.; Grimmel C.; Wagenknecht B.; Dichgans J.; Weller M. Betulinic acid-induced apoptosis in glioma cells: a sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing. J. Pharmacol. Exp. Ther. 1999, 289, 1306–1312. [PubMed] [Google Scholar]
- Pelicano H.; Carney D.; Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [DOI] [PubMed] [Google Scholar]
- Pérez-Galán P.; Roué G.; Villamor N.; Montserrat E.; Campo E.; Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood 2006, 107, 257–264. [DOI] [PubMed] [Google Scholar]
- Lecane P. S.; Karaman M. W.; Sirisawad M.; Naumovski L.; Miller R. A.; Hacia J. G.; Magda D. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-Cell lymphoma lines. Cancer Res. 2005, 65, 11676–11688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.