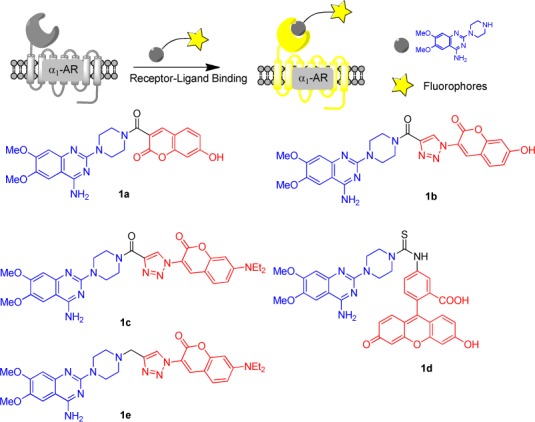
Abstract
α1-Adrenergic receptors (α1-ARs), as the essential members of G protein-coupled receptors (GPCRs), can mediate numerous physiological responses in the sympathetic nervous system. In the current research, a series of quinazoline-based small-molecule fluorescent probes to α1-ARs (1a–1e), including two parts, a pharmacophore for α1-AR recognition and a fluorophore for visualization, were well designed and synthesized. The biological evaluation results displayed that these probes held reasonable fluorescent properties, high affinity, accepted cell toxicity, and excellent subcellular localization imaging potential for α1-ARs.
Keywords: α1-Adrenergic receptors, fluorescent probes, quinazoline, cell imaging, subcellular localization
α1-Adrenergic receptors (α1-ARs), as the important members of G protein coupled receptors (GPCRs), distribute in a variety of organs, tissues, and cells, which mediate many crucial physiological effects in the human body. These receptors are classified into at least three subtypes (α1A, α1B, and α1D) based on their differences on the biological structure, tissue distributions, pharmacological properties, and signaling pathways.1 Various studies confirmed that α1-ARs are closely involved in benign prostatic hyperplasia (BPH), hypertension, prostate cancer, and other diseases.2−4 Functional experiments manifested that α1B-AR is mainly in charge of the vasomotion of small resistance vessels, while α1A- and α1D-ARs take responsibility for the contraction of the main arteries in animal species.5
So far, there are many challenges to fully understand the biological and pharmacological characteristics of each α1-AR subtype. This dilemma is mainly caused by the difficulties either to determine their distribution in various organs and tissues or to define the functional response mediated by each one in the different species by using classical approaches without their three-dimensional crystal structure and tissue-selective α1-AR antagonists.6 Fortunately, with the rapid development of fluorescence analysis technology in various areas, small-molecule fluorescent probes with many advantages, including high sensitivity, selectivity, and visualization, have been widely applied to track the biological macromolecules, especially GPCRs.7−10 Small-molecule fluorescent probes for GPCRs generally consist of two essential components: the pharmacophore moiety that is used to bind with the target through the receptor–ligand interaction and the fluorophore group that can trace the target by the fluorescent properties.11 Moreover, good optical characteristics and high affinity of probe can ensure its successful labeling of the target. It needs to be noted that in the case of α1-ARs, only one small-molecule fluorescent probe, BODIPY-FL-prazosin, is available. However, the application of this probe is restricted to its complicated preparation. Recently, some QD-based fluorescent probes have been reported as well.12,13 Although rational results about α1B-AR study were given by these QD probes, the studies of probes on purity, structure, cytotoxicity, and receptor affinity were inadequate.10 Therefore, more small-molecule fluorescent probes with diversity of structure and fluorescence property are demanded for the current molecular pharmacology study and drug discovery of α1-AR.
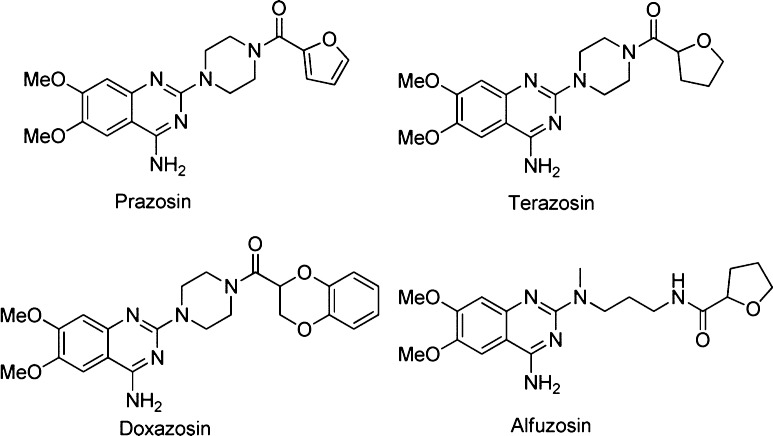
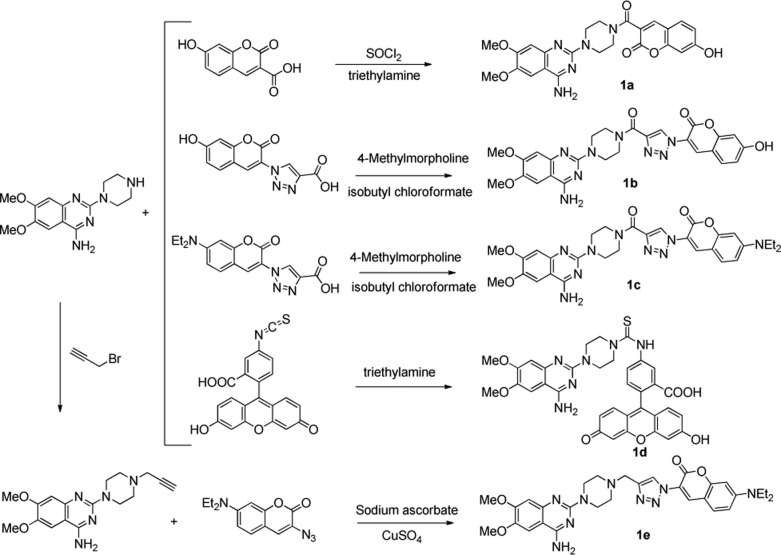
α1-Adrenergic receptor antagonists can be structurally categorized into several classes, including quinazolines, 1,4-benzodioxans, dihydropyridines and dihydropyrimidines, fused pyrimidindiones, pyridazinones, imidazolines, N-arylindoles, N-aryl, and N-heteroarylpiperazines, and miscellaneous compounds.14 Among all chemotypes, quinazoline compounds, including prazosin, terazosin, doxazosin, and alfuzosin, are the most clinically effective α1-AR antagonists (Scheme 1).15 In our previous study, a pharmacophore model based on quinazoline derivatives that contribute many effective α1-AR antagonists was well built, and the proposed interaction model of quinazoline-based antagonists with α1-ARs was well developed.16−19 It was found that there is a bulky space around the piperazine group to accommodate the fluorophore moiety, which does not influence the affinity of antagonists to receptors. Therefore, in the current research, we chose the quinazoline moiety as the pharmacophore to generate fluorescent probes for selectively binding with α1-ARs. In the meanwhile, coumarin and fluorescein groups were selected as fluorophores because of their preferred characteristics, such as high sensitivity, light stability, small molecular weight, water solubility, and reasonable cell permeability. The understanding on how α1-ARs bind with their ligands20 and the development of fluorescent labeling analysis method can efficiently promote the emergence of fluorescent probes for α1-ARs. In view of these evidence, a series of quinazoline derivatives (1a–1e) were well designed as small-molecule fluorescent probes for α1-ARs by conjugating pharmacophore (quinazoline) with fluorophores (coumarin and fluorescein) (Scheme 2).
Scheme 1. Structures of Quinazoline Derivatives as α1-AR Antagonists.
Scheme 2. Designed Quinazoline-Based Fluorescent Probes for α1-ARs.
To establish a preliminary understanding on if these quinazoline-based fluorescent probes can be recognized by α1-ARs, we first developed docking models of molecules 1a–e with α1A-AR homology model that we developed in 2008.19 As a result, the docking conformations and orientations of 1a–e around the active site of α1A-AR were highly consistent with of prazosin as depicted in Figures 1 and S1–S6.These computational results clearly propose that compounds 1a–e may be recognized by α1A-AR. More computational details can be found in Supporting Information.
Figure 1.
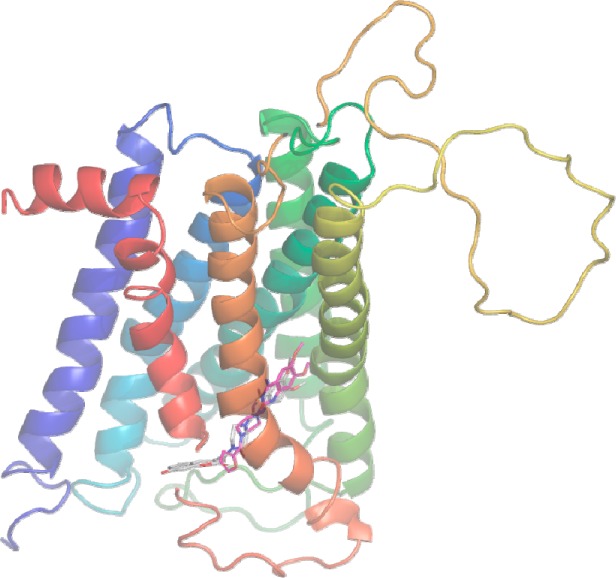
Proposed docking conformation of prazosin (red sticks) and 1a (white sticks) in the human α1A-AR binding site.
The convenient syntheses of these fluorescent probes were mainly based on CuAAC reaction and amide condensation with mild conditions, high yield, and easy purification (Scheme 3). The fluorescein derivative was obtained through the one-step condensation reaction of fluorescein isocyanate and the quinazoline parent. Synthesis of coumarin derivatives was started from the acetylation or azidation of coumarin. And then, the quinazoline was attached to coumarin by amide or triazole moiety. More synthetic details can be found in the Supporting Information.
Scheme 3. Synthetic Routes of Designed Fluorescent Probes.
These probes were first evaluated for in vitro affinities with three human cloned α1-adrenoceptors subtypes by the radioligand binding assay using [3H]prazosin in membrane from transfected CHO cells.21,22 All compounds demonstrated up to nanomolar affinities with three α1-AR subtypes, which are close to the positive control, phentolamine (Table 1). Compound 1e is approximately 100-fold less potent than compound 1c because of the lack of carbonyl group. In the absence of the triazole linker, compound 1a shows the similar affinity with 1b and 1c containing the triazole rings. This affinity result also proves that a bulky space exists around the piperazine group to accommodate the fluorophore moiety without influencing the affinity to receptors. Certainly, this space is not infinite. When the fluorophore is changed from coumarin (1a) to more bulky fluorescein (1d), the affinity might decrease slightly.
Table 1. Comparison of the Probes’ Affinity to α1-ARs.
|
Kia(nM) |
IC50 (nM) |
|||||
|---|---|---|---|---|---|---|
| compd | α1A | α1B | α1D | α1A | α1B | α1D |
| phentolamine | 0.6 | 4.8 | 7.6 | 1.1 | 10.8 | 12.5 |
| 1a | 2.1 | NAc | NAc | 3.9 | <5b | <5b |
| 1b | 1.3 | NAc | NAc | 2.5 | <5b | <5b |
| 1c | 0.3 | 0.1 | 0.4 | 0.6 | 1.2 | 2.2 |
| 1d | 4.7 | 7.3 | 20.7 | 8.8 | 16.3 | 34.0 |
| 1e | 29.9 | 29.9 | 27.5 | 52.4 | 52.4 | 45.2 |
Ki was calculated from IC50 using the Cheng–Prusoff equation.
Data was estimated from the unclassical binding-competitive curve.
Not available.
As far as the spectroscopic properties are concerned (Table 2), all target compounds showed excitation and emission wavelengths comparable to that reported in literature for the parent fluorophores. In addition, the fluorescence quantum yield was checked in a mixed solvent of methanol and PBS buffer. All compounds possessed reasonable fluorescence quantum yields. In particular, compound 1b showed high quantum yields, which is up to 42%. The solvent effect on fluorescence property was examined as well (Figure S6). The hydroxyl coumarin derivatives, 1a and 1b, emitted strong fluorescence in water; however, their fluorescence emissions were quenched in methanol and ethanol. Amino coumarin and fluorescein derivatives behaved in the opposite way.
Table 2. Characterization and Properties of Compounds 1a–1e.
| compd | λmax (nm) | λex (nm) | λem (nm) | Φ (%) |
|---|---|---|---|---|
| 1a | 391 | 391 | 456 | 8.5 ± 1 |
| 1b | 392 | 392 | 454 | 42 ± 1 |
| 1c | 420 | 420 | 500 | 7.8 ± 0.2 |
| 1d | 494 | 485 | 517 | 2.6 ± 0.3 |
| 1e | 430 | 430 | 505 | 4.7 ± 0.1 |
It should be pointed out that a reasonable fluorescent probe should be harmless while labeling the target. In view of this reason, the cytotoxicities of these probes were checked by a standard sulforhodamine B (SRB) colorimetric assay.23 As demonstrated in Table 3, the cytotoxicity of compounds 1c and 1e in HEK293A (human embryonic kidney) cells transfected with α1A-AR (HEK293A-α1A-AR) or HEK293A cells transfected with α1D-AR (HEK293A-α1D-AR) was similar to the positive control, doxazosin, while compounds 1a, 1b, and 1d had slight cytotoxicity. Obviously, as presented in Table 3, only compound 1c and 1e had moderate cytotoxicities with IC50 values at the micromolar level. The results showed that cell viability was not significantly changed upon treatment, indicating the low cytotoxicity and good biocompatibility of these probes in living cell study at the nanomolar concentration without any cell damage.
Table 3. Cytotoxicity Results of the Synthesized Probes.
| IC50 (μM) |
||
|---|---|---|
| compd | HEK293A-α1A-AR | HEK293A-α1D-AR |
| doxazosin | 19 ± 2 | 22 ± 2 |
| 1a | >100 | >100 |
| 1b | >100 | 85 ± 2 |
| 1c | 12 ± 0.2 | 32 ± 2 |
| 1d | >100 | >100 |
| 1e | 26 ± 3 | 17 ± 0.2 |
The above-mentioned results demonstrated that most of the probes displayed high affinities to α1-ARs and accepted cytotoxicities that laid a solid foundation for fluorescence imaging in living cells. Although their fluorescence quantum yield left something to be desired, cell imaging potential of high expression of α1-ARs was extensively evaluated. We incubated HEK293A cells transfected with α1A-AR and α1D-AR with the probes and DiD (a cell membrane dye, red, colocalization) at 37 °C for 5 min, in which normal HEK293A cells (without α1-AR expression) as a negative control, and HEK293A-α1A-AR and HEK293A-α1D-AR cells incubated with tamsulosin (a potent α1-AR antagonist) as another negative control. Indeed, the fluorescence of cells was not strong due to the poor quantum yield of compounds.24 However, as shown in Figure 1, the incubation of cells with compound 1a allowed visualization of the α1A- and α1D-ARs in stably transfected HEK293 cells using conventional fluorescence microscopy (Figure 2A,B), while a significant reduction in fluorescence labeling was observed in transfected HEK293 cells upon competition with 20-fold excess of tamsulosin (Figure 2D,E). Moreover, very weak fluorescence labeling in negative HEK293 cells showed a spot of nonspecific binding of compound 1a (Figure 2C). We also proved the application of our probe 1c in detecting cellular α1-ARs by flow cytometry. As shown in Figure S20, compound 1c could identify the HEK293 cells transfected with α1-ARs successfully. Although compound 1c has weaker affinity to α1d-ARs than α1a-ARs, stronger staining of α1d-ARs cells than that of α1a-ARs cells was found. This may be caused by the inconsistent expression levels of α1a-AR and α1d-AR in cells, and in this case, the expression level of α1d-AR is higher than α1a-AR.
Figure 2.
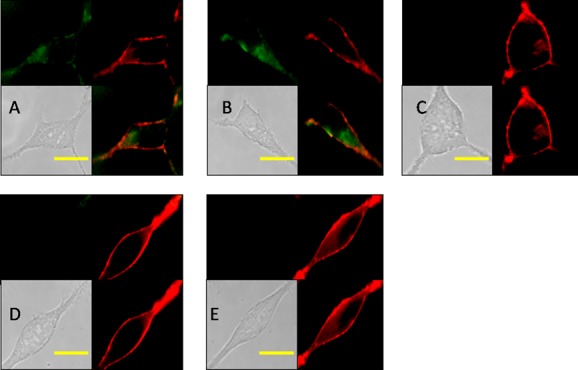
Confocal fluorescence image of HEK293 cell using compound 1a. All cells are incubated with 1a at 37 °C for 5 min and washed immediately, and the exposure time is the same. The background was adjusted by ImageJ software. (A) Image of HEK293A-α1A-AR cells incubated with 1a (15 nM) and DiD; (B) image of HEK293A-α1D-AR cells incubated with 1a (15 nM) and DiD; (C) image of HEK293A cells incubated with 1a (15 nM) and DID; (D) image of HEK293A-α1A-AR cells incubated with 1a (15 nM), DID, and tamsulosin (300 nM); (E) image of HEK293A-α1D-AR cells incubated with 1a (15 nM), DID, and tamsulosin (300 nM). Scale bar (yellow) = 20 μm.
In subcellular localization, the fluorescence of α1A-AR was found on the cell surface, and their location was almost overlapped with the red area stained by DiD. In the case of α1D-AR, its fluorescence mainly distributed in cell plasma, as well as a little bit on the cell surface. These subcellular localization results are highly in accordance with Piascik’s conclusion, where α1A-AR fluorescence was detected not only on the cell surface but also intracellularly, and α1D-AR fluorescence was detected mainly intracellularly.25 Fluorescence imaging of the other four compounds 1b–1e presents similar results (Figures S16–S19). Although the largest emission peak of 1a or 1b was near to blue, the blue filter did not give a clear imaging result; therefore, these images were recorded through the green filter.
Additionally, it is a known fact that α1-ARs are overexpressed in prostate cancer.26 After successful staining of α1A- and α1D-AR overexpressing HEK293A cells, we tested 1d on PC-3 prostate cancer cells because of its most reasonable fluorescence property. As shown in Figure 3, PC-3 cells could be “lighted up” after being incubated with 1d (1 μM), while HepG2 cells (low α1-AR expression) showed little fluorescence.27 Also, 1d shows high displaceable properties in PC-3 prostate cancer cells with doxazosin. These findings confirm probe 1d as a labeling tool in α1-AR overexpressing cells.
Figure 3.
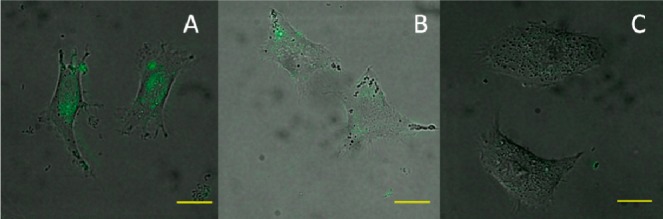
Fluorescence image of cancer cells using compound 1d. The background was adjusted by ImageJ software. (A) PC-3 cells were with incubated with 1d (1 μM) at 37 °C for 30 min, then washed immediately; (B) PC-3 cells were with incubated with 1d (1 μM) and doxazosin (10 μM) at 37 °C for 30 min, then washed immediately; (C) HepG2 cells were incubated with 1d (1 μM) at 37 °C for 30 min, then washed immediately. Scale bar (yellow) = 20 μm.
In summary, we herein well developed a series of quinazoline-based small-molecule fluorescent probes with high sensitivity, high affinity, and low toxicity for convenient detection of α1-ARs. The probes have up to nanomolar affinities with three α1-AR subtypes. These fluorescent probes at the nanomolar level have been successfully used in visualization and subcellular localization of α1-AR in cell imaging, including both α1-AR transfected HEK293 cells and prostate cancer cells, thus supplanting the application of radioligand for drug screening and providing extra dimensions of probing receptors that simple competitive radioligands do not present. The study is a preliminary work to establish that the strategy can be useful for cell staining. In addition, these probes have the advantage of easy synthesis from readily available inexpensive starting materials. It is expected that these probes now can be added to the armamentarium of fluorescent ligands that may be utilized as versatile and extremely useful tools for nowadays molecular pharmacology and drug discovery in the area of α1-ARs.
Glossary
ABBREVIATIONS
- α1-ARs
α1-adrenergic receptors
- α1A/B/D-ARs
α1A/B/D-adrenergic receptors
- CHO
Chinese hamster ovary
- CuAAC
Cu-catalyzed azide–alkyne cycloaddition
- GPCRs
G protein coupled receptors
- HEK
Human embryonic kidney
- QD
quantum dot
- SRB
sulforhodamine B
Supporting Information Available
Full experimental procedures; analytical and spectral characterization data of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
† These authors contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The present work was supported by grants from the Fok Ying Tong Education Foundation (No. 122036), the Program of New Century Excellent Talents in University (No. NCET-11-0306), the Shandong Natural Science Foundation (No. JQ201019), and the Independent Innovation Foundation of Shandong University, IIFSDU (No. 2010JQ005). Our cell imaging work was performed at the Microscopy Characterization Facility, Shandong University. We also thank Professor Youyi Zhang from Peking University for her generous gift, the α1A-AR- and α1D-AR-transfected HEK293A cells.
The authors declare no competing financial interest.
Supplementary Material
References
- Li W.; Du L.; Li M. Alkaloids and flavonoids as α1-adrenergic receptor antagonists. Curr. Med. Chem. 2011, 18, 4923–4932. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R. Jr; Hieble J. P. Adrenoceptor pharmacology: urogenital applications. Eur. Urol. 1999, 36, 17–22. [DOI] [PubMed] [Google Scholar]
- Forray C.; Noble S. A. Subtype selective α1-adrenoceptor antagonists for the treatment of benign prostatic hyperplasia. Expert Opin. Invest. Drug. 1999, 8, 2073–2094. [DOI] [PubMed] [Google Scholar]
- Nagarathnam D.; Wetzel J.; Miao S.; Marzabadi M.; Chiu G.; Wong W.; Hong X.; Fang J.; Forray C.; Branchek T. Design and synthesis of novel α1a adrenoceptor-selective dihydropyridine antagonists for the treatment of benign prostatic hyperplasia. J. Med. Chem. 1998, 41, 5320–5333. [DOI] [PubMed] [Google Scholar]
- Jarajapu Y. P. R.; Coats P.; McGrath J. C.; Hillier C.; MacDonald A. Functional characterization of α1-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br. J. Pharmacol. 2001, 133, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D. M. Structure-function of α1-adrenergic receptors. Biochem. Pharmacol. 2007, 73, 1051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M.; Lacivita E.; Berardi F.; Perrone R. Developments in fluorescent probes for receptor research. Drug Discovery Today 2009, 14, 706–712. [DOI] [PubMed] [Google Scholar]
- Kuder K.; Kiec-Kononowicz K. Fluorescent GPCR ligands as new tools in pharmacology. Curr. Med. Chem. 2008, 15, 2132–2143. [DOI] [PubMed] [Google Scholar]
- Cairo C. W.; Key J. A.; Sadek C. M. Fluorescent small-molecule probes of biochemistry at the plasma membrane. Curr. Opin. Chem. Biol. 2010, 14, 57–63. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Du L.; Li M. Toward fluorescent probes for G-protein-coupled peceptors (GPCRs). J. Med. Chem. 2014, 57, 8187–8203. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A. Functionalized congener approach to the design of ligands for G protein-coupled receptors (GPCRs). Bioconjugate Chem. 2009, 20, 1816–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Hou Z.; Song Y.; Wang L.; Guo E. Visual and quantitative screening of α1-adrenoceptor antagonists in living cells using quantum dots. ACS Comb. Sci. 2014, 16, 155–159. [DOI] [PubMed] [Google Scholar]
- Zhou G.; Wang L.; Ma Y.; Wang L.; Zhang Y.; Jiang W. Synthesis of a quinazoline derivative: a new α1-adrenoceptor ligand for conjugation to quantum dots to study α1-adrenoceptors in living cells. Bioorg. Med. Chem. Lett. 2011, 21, 5905–9. [DOI] [PubMed] [Google Scholar]
- Rosini M.; Bolognesi M. L.; Giardina D.; Minarini A.; Tumiatti V.; Melchiorre C. Recent advances in α1-adrenoreceptor antagonists as pharmacological tools and therapeutic agents. Curr. Top. Med. Chem. 2007, 7, 147–62. [DOI] [PubMed] [Google Scholar]
- Desiniotis A.; Kyprianou N. Advances in the design and synthesis of prazosin derivatives over the last ten years. Expert Opin. Ther. Targets 2011, 15, 1405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. Y.; Tsaib K. C.; Xia L. Pharmacophore identification of a1A-adrenoceptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 657–664. [DOI] [PubMed] [Google Scholar]
- Du L.; Li M. Modeling the interactions between 1-adrenergic receptors and their antagonists. Curr. Comput.-Aided Drug Des. 2010, 6, 165–178. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Chen L.; Ma Z.; Du L.; Li M. Design, synthesis and biological evaluation of naphthalimide-based fluorescent probes for α1-adrenergic receptors. Drug Discovery Ther. 2014, 8, 11–17. [DOI] [PubMed] [Google Scholar]
- Li M.; Fang H.; Du L.; Xia L.; Wang B. Computational studies of the binding site of α1A-adrenoceptor antagonists. J. Mol. Model. 2008, 14, 957–66. [DOI] [PubMed] [Google Scholar]
- Jain K. S.; Bariwal J. B.; Kathiravan M. K.; Phoujdar M. S.; Sahne R. S.; Chauhan B. S.; Shah A. K.; Yadav M. R. Recent advances in selective α1-adrenoreceptor antagonists as antihypertensive agents. Bioorg. Med. Chem. 2008, 16, 4759–4800. [DOI] [PubMed] [Google Scholar]
- Greengrass P.; Bremner R. Binding characteristics of 3H-prazosin to rat brain α-adrenergic receptors. Eur. J. Pharmacol. 1979, 55, 323–326. [DOI] [PubMed] [Google Scholar]
- Nagatoma T.; Tsuchihashi H.; Sasaki S.; Nakagawa Y.; Nakahara H.; Imai S. Displacement by α-adrenergic agonists and antagonists of 3H-prazosin bound to the α-adrenoceptors of the dog aorta and the rat brain. Jpn. J. Pharmacol. 1985, 37, 181–187. [DOI] [PubMed] [Google Scholar]
- Vichai V.; Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [DOI] [PubMed] [Google Scholar]
- Baker J. G.; Adams L. A.; Salchow K.; Mistry S. N.; Middleton R. J.; Hill S. J.; Kellam B. Synthesis and characterization of high-affinity 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-labeled fluorescent ligands for human β-adrenoceptors. J. Med. Chem. 2011, 54, 6874–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D.; McCune D. F.; Edelmann S. E.; García-Cazarín M. L.; Gozoh T.; Piascik M. T. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol. Pharmacol. 2002, 61, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Shi T.; Gaivin R. J.; McCune D. F.; Gupta M.; Perez D. M. Dominance of the alpha1B-adrenergic receptor and its subcellular localization in human and TRAMP prostate cancer cell lines. J. Recept. Signal Transduction 2007, 27, 27–45. [DOI] [PubMed] [Google Scholar]
- Spector M.; Nguyen V.-A.; Sheng X.; He L.; Woodward J.; Fan S.; Baumgarten C. M.; Kunos G.; Dent P.; Gao B. Activation of mitogen-activated protein kinases is required for α 1-adrenergic agonist-induced cell scattering in transfected HepG2 cells. Exp. Cell Res. 2000, 258, 109–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.