Abstract
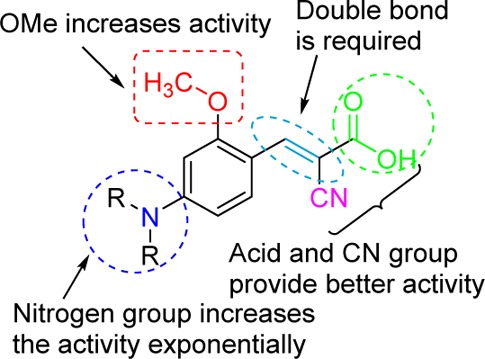
Potent monocarboxylate transporter 1 inhibitors (MCT1) have been developed based on α-cyano-4-hydroxycinnamic acid template. Structure–activity relationship studies demonstrate that the introduction of p-N, N-dialkyl/diaryl, and o-methoxy groups into cyanocinnamic acid has maximal MCT1 inhibitory activity. Systemic toxicity studies in healthy ICR mice with few potent MCT1 inhibitors indicate normal body weight gains in treated animals. In vivo tumor growth inhibition studies in colorectal adenocarcinoma (WiDr cell line) in nude mice xenograft models establish that compound 27 exhibits single agent activity in inhibiting the tumor growth.
Keywords: Warburg effect, reverse Warburg effect, monocarboxylate transporter 1, α-cyano-4-hydroxycinnamic acid, colorectal adenocarcinoma
Vigorous glycolysis is the hallmark of many advanced stage tumors.1−5 Malignant tumors are heterogeneous in nature, and tumor microenvironment contains aerobic and hypoxic regions.1 Tumor hypoxia leads to cancer advancement, metastasis, treatment failure, and patient mortality as these cells become resistant to standard chemo- and radiation therapy. Under hypoxic conditions, cancer cells consume glucose that is metabolized to lactate, ketone, and other energy rich molecules.1 The transport of these energy rich metabolites occurs via monocarboxylate transporters (MCTs).1,4,6,7 They are members of the solute carrier 16 gene family consisting of 14 known MCT isoforms (SLC16A1–14).6,7 Of these, MCTs 1–4 have been shown to transport monocarboxylates such as lactate, pyruvate, and some ketone bodies.1,6,7 MCT1 expression is elevated in an array of human tumors, and therefore, these transporters can be major selective targets for antitumor therapy.1,8−14 Recently, Lisanti et al. have carried out extensive studies to explain the glycolytic process in epithelial cancer cells.15−18 These studies strongly indicate that cancer cells initiate oxidative stress in their neighboring fibroblasts resulting in mitochondrial dysfunction and aerobic glycolysis (Warburg effect). These fibroblasts then produce lactate and pyruvate, which are taken up by adjacent cancer cells via MCT1 for mitochondrial oxidative phosphorylation and further proliferation. In essence, tumor associated stromal fibroblasts and epithelial cancer cells develop a metabolic symbiotic relationship (reverse Warburg effect).15−18
Owing to the importance of MCT1 in the above-mentioned tumor related biological events, development of MCT1 inhibitors should be of immense importance in broad-spectrum cancer treatment. In this regard, we initiated a project on the discovery of novel MCT1 inhibitors as potential anticancer agents based on α-cyano-4-hydroxycinnamic acid (CHC).19 Although CHC has been in biochemical usage as a low affinity MCT1 inhibitor (IC50 >100 μM), the derivatives of CHC have not been studied to improve its potency. We envisioned the systematic design, synthesis, and evaluation of several CHC analogues to understand the structure–activity relationship (SAR) and obtain lead molecules that inhibit MCT 1 at low concentrations for potential development as anticancer agents.
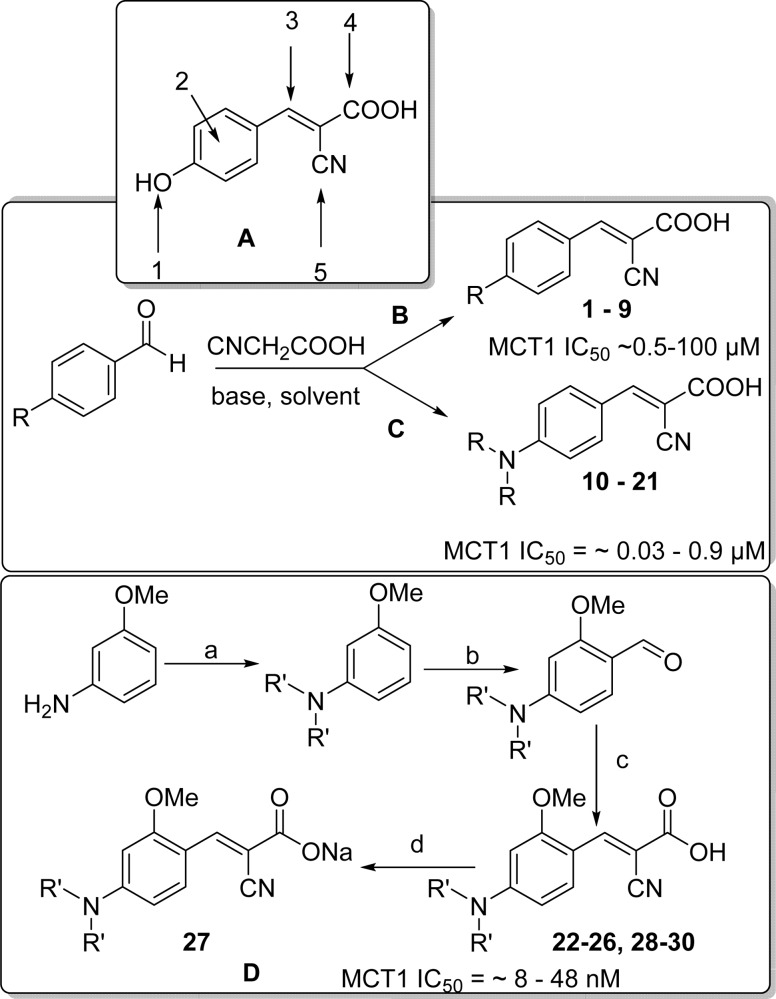
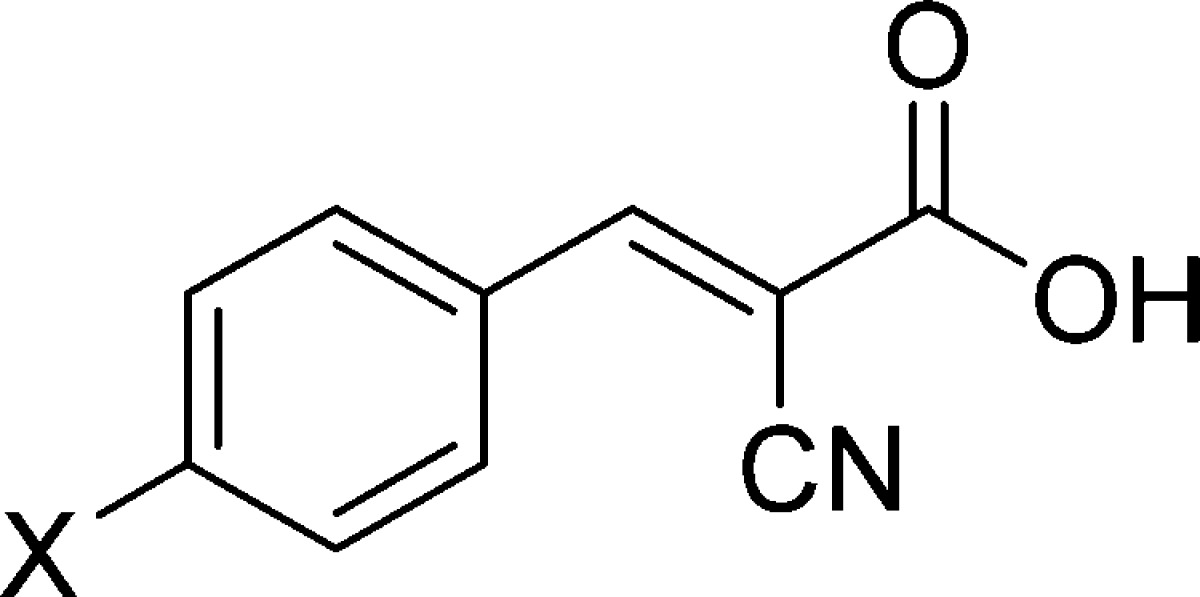
There are five positions of substitution on the CHC template (Scheme 1A), and we chose p-hydroxyl replacement as the starting point. For initial studies, hydroxyl group was substituted with alkyl (methyl, ethyl, 1–2), electron withdrawing (F, Cl, Br, CN, and NO2, 3–7), and electron donating groups (methoxy, 3,4-methylenedioxy, 8–9). These CHC derivatives of α-cyanocinnamic acids were synthesized from corresponding aldehydes via Knoevenagel type condensation (1–9, Scheme 1B).20
Scheme 1.
Reagents and conditions: (a) Alkyl bromide, K2CO3, tetrabutylammonium bromide, H2O/ethanol or isopropanol, 100 °C, 8–24 h; (b) (i) POCl3/DMF 0–80 °C, 2–4 h; (ii) Na2CO3; (c) CNCH2COOH, piperidine, CH3CN, 80 °C, 8–20 h; (d) NaHCO3, MeOH–H2O, rt, overnight.
The compounds 1–9 were evaluated for their MCT1 inhibition activity via [14C]-lactate uptake assay on rat brain endothelial 4 cells (RBE4). We utilized this cell line for initial lactic acid transport studies as they highly express MCT1 as confirmed by Western blotting.21,22 Gratifyingly, compounds 1–9 have exhibited several times higher potency (∼0.5–100 μM) than the parent CHC molecule (Scheme 1B).
We then explored the role of cyano (−CN), carboxylic acid (−COOH), and olefinic functionalities in CHC by replacing −CN group with H atom, replacing −COOH and introducing two −CN groups, removal of the −CN group, and also reduction of the olefin. In all these cases, we did not observe any appreciable MCT1 inhibition activity when compared to CHC (data not shown).
We then introduced N,N-dialkyl group in place of hydroxyl moiety owing to the biological significance of nitrogen atom in many pharmaceutical compounds. Condensation of commercially available p-(N,N-dimethyl) benzaldehyde with cyanoacetic acid in the presence of piperidine provided the corresponding cyanocinnamic acid 10 in ∼72% yield. To our delight, 10 exhibited potent MCT1 inhibition activity with a mean IC50 = 0.14 μM. Taking 10 as a lead derivative, we further explored the SAR (Table 1) by structural modification on the nitrogen atom. In this regard, we synthesized several p-N,N-dialkyl and aryl substituted cyanocinnamic acids starting from aniline (Scheme 1C). Dialkylation of aniline followed by Vilsmeier–Haack formylation23 and subsequent base-mediated condensation with cyanoacetic acid provided cinnamic acids 11–21 in good yields (Scheme 1C and Table 1). Compounds 11–21 upon evaluation of their MCT1 inhibition activities on RBE4 cell line exhibited a clear pattern of SAR. From these studies, it was found that increasing the alkyl chain increased the potency up to four carbon chain length (11–13, 13–66 nM, Table 1). Further increase in the chain length to pentyl 15 and hexyl 16 decreased the activity. Aryl-substituted cyanocinnamic acid 19 also showed excellent activity (26 nM). Cyclic amines such as pyrrolidine 20 and piperidine 21 decreased the activity to a moderate range of IC50 values (70 and 142 nM, Table 1).
Table 1. MCT1 IC50 Values of N,N-Dialkyl/Aryl Cyanocinnamic Acids.
| Sl. No. | X | % yielda | MCT1 IC50 (μM)b |
|---|---|---|---|
| 10 | N,N-dimethyl | 72 | 0.14 ± 0.05 |
| 11 | N,N-diethyl | 75 | 0.07 ± 0.02 |
| 12 | N,N-dipropyl | 67 | 0.01 ± 0.01 |
| 13 | N,N-dibutyl | 65 | 0.03 ± 0.00 |
| 14 | N,N-diisobutyl | 61 | 0.10 ± 0.05 |
| 15 | N,N-dipentyl | 70 | 0.14 ± 0.02 |
| 16 | N,N-dihexyl | 68 | 0.88 ± 0.18 |
| 17 | N,N-diallyl | 60 | 0.11 ± 0.03 |
| 18 | N,N-dipropargyl | 62 | 0.40 ± 0.02 |
| 19 | N,N-diphenyl | 76 | 0.03 ± 0.00 |
| 20 | pyrrolidinyl | 62 | 0.07 ± 0.01 |
| 21 | piperidinyl | 67 | 0.14 ± 0.03 |
Starting from aldehydes.
Average IC50 values of minimum three separate experiments. Values reported as avg ± SEM.
On the basis of the significantly improved activity of N,N-dialkyl series, we further carried out SAR studies by introducing substitutions at the 2-position of the aromatic ring (Scheme 1A). For initial studies, we started with cyanocinnamic acid 22 derived from o-methoxy-N,N-dipropyl benzaldehyde. Compound 22 exhibited higher MCT1 inhibitory activity (IC50 = 12 nM) compared to its nonmethoxy derivative 12. To understand further SAR of this lead candidate, we introduced several alkyl and aryl substitutions on the nitrogen atom. The required aldehydes were synthesized from m-anisidine (Scheme 1D). Alkylation of m-anisidine with alkyl halides in the presence of K2CO3 and catalytic tetrabutylammonium bromide followed by Vilsmeier–Haack formylation23 provided the corresponding aldehydes. These aldehydes were then condensed with cyanoacetic acid in the presence of piperidine to synthesize cyanocinnamic acids 22–30 (Scheme 1D and Table 2). MCT1-based [14C]-lactate uptake studies on RBE4 cells revealed that these derivatives 22–30 have superior inhibitory activity (IC50 = 8–48 nM) when compared to their nonmethoxy homologues 10–21 (Table 1). In fact, cyanocinnamic acids 22–24 and 26 have exhibited inhibitory activity in low nanomolar range (8–12 nM, Table 2).
Table 2. MCT1 IC50 Values of o-Methoxy p-N,N-Dialkyl/Aryl Cyanocinnamic Acids.
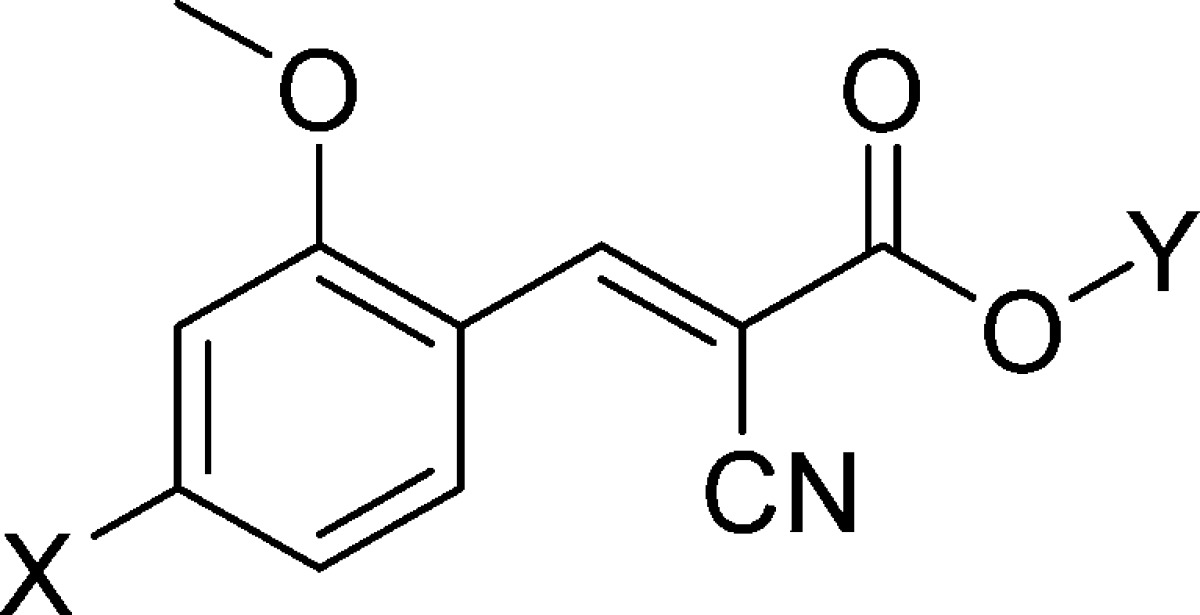
| Sl. No. | X | Y | % yielda | MCT1 IC50 (nM)c |
|---|---|---|---|---|
| 22 | N,N-dipropyl | H | 62 | 12 ± 1 |
| 23 | N,N-dibutyl | H | 60 | 9 ± 1 |
| 24 | N,N-diisobutyl | H | 58 | 11 ± 4 |
| 25 | N,N-dipentyl | H | 74 | 34 ± 7 |
| 26 | N,N-diphenyl | H | 66 | 8 ± 1 |
| 27 | N,N-diphenyl | Na | 78b | 11 ± 3 |
| 28 | N,N-diallyl | H | 70 | 29 ± 2 |
| 29 | pyrrolidinyl | H | 68 | 48 ± 20 |
| 30 | piperidinyl | H | 85 | 25 ± 5 |
Starting from aldehydes.
Starting from acid.
Average IC50 values of minimum three separate experiments. Values reported as avg ± SEM.
We chose 26 as a representative example for further evaluation based on its potent MCT1 inhibitory profile. To improve the water solubility, we prepared its sodium salt 27 by treating the corresponding acid 26 with NaHCO3 in methanol–water. The sodium salt 27 has water solubility of ∼1 mg/mL. Compound 27 retained its potent MCT1 inhibitory activity (11 nM, Table 2). Similarly, we have also synthesized sodium salts of 29 and 30, and they exhibited water solubility of >7 mg/mL. Having achieved the initial goal of discovering potent MCT1 inhibitors, we then carried out systemic toxicity analysis of some of these derivatives in healthy ICR mice.
Compound 27 was chosen for initial studies, and the mice were treated with 27 in 0.1% DMSO in saline for 3 weeks (6.67 mg/kg, ip, bid). All mice survived, and weight gains were similar when compared to the control group (Supporting Information). We then carried out another study with increased dosages of 50 mg/kg, oral gavage, bid, and 50 mg/kg, ip, qd. In these cases also, all mice survived, and weight gains were similar to the control group (Supporting Information). Because of the higher water solubility of sodium salts of 29 and 30, toxicity studies were carried out at the higher dosage of 70 mg/kg/ip/bid. In these cases also, no animal mortality was observed, and the body weight gains were similar to the control group (Supporting Information).
We also carried out a preliminary pharmacokinetic analysis of 27 in ICR mice. Sixty ICR mice were divided into two groups and group 1 was administered with 27 intraperitoneally and group 2 intragastrically at 100 mg/kg. Blood samples were collected at different time points (n = 3 for each time point). The peak plasma concentrations were observed at 0.25 h for group 1 and 0.30 h for group 2. In both the cases, compound 27 was completely eliminated from the system within 24 h (Supporting Information).
Encouraged by the generally nontoxic nature of potent MCT1 inhibitors in healthy animals, we next carried out the in vivo tumor growth inhibition study with 27 using flank-based tumor xenografts in nude mice. For initial studies, we utilized a MCT1 expressing and highly tumorigenic colorectal adenocarcinoma cell line WiDr.1 These cancer cells (7 × 106) in 1:1 PBS–matrigel were inoculated into the flank of female BALB/c nude mice and allowed to grow until the tumor volume reached ∼100 mm3 (∼3 weeks). Mice were administered with 27 by intraperitoneal injection (10 mg/kg, bid) and by oral gavage (50 mg/kg, bid). The treatment was continued for 3 weeks, and tumor volumes were determined with calipers every 3 days (Figure 1). At the end of the study, mice were euthanized, and tumors were isolated and weighed to validate the caliper measurements. On the basis of these studies, ∼29% and 45% tumor growth inhibition was observed in ip and oral gavage groups, respectively.
Figure 1.
In vivo efficacy with 27 as a single agent in WiDr tumor xenograft model: WiDr cells were implanted subcutaneously in the right flanks of female BALB/c nude mice. After tumors reached ∼100 mm3, mice were randomly assigned to three groups (n = 8 mice per group). Group 1 was administered with 50 mg/kg, oral gavage, bid; group 2 was treated with 10 mg/kg, ip, bid. Tumor volumes were recorded every 3–4 days. The study was carried out for 22 days. Mann–Whitney test was used to compute significance. *P ≤ 0.05.
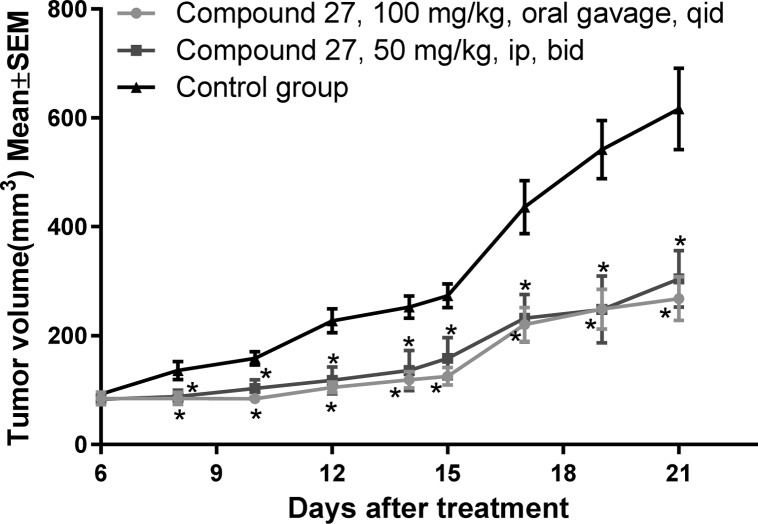
Similarly, another in vivo tumor growth inhibition study was conducted by starting the treatment from day 6 following WiDr tumor inoculation. This time, 27 was administered at a higher dosage of 100 mg/kg, oral gavage, qid and 50 mg/kg, ip, bid in groups 1 and 2, respectively. By the end of the study (21 days, tumor volumes in Figure 2), 56% and 45% tumor growth inhibition was observed in groups 1 and 2, respectively, based on the weights of the isolated tumors.
Figure 2.
In vivo efficacy with 27 in WiDr tumor xenograft model: WiDr cells were implanted subcutaneously in the right flank of female BALB/c nude mice. Treatments were started on day 6 of implant. Mice were randomly assigned to three groups (n = 8 mice per group). Group 1 was administered with 100 mg/kg, oral gavage, qid; group 2 received 50 mg/kg, ip, bid. Tumor volumes were recorded every 2 days. The study was carried out for 21 days. Mann–Whitney test was used to compare the treated and untreated groups. *P ≤ 0.05.
These studies clearly demonstrate that MCT1 inhibitors have potential to develop them as anticancer agents. However, detailed mechanistic studies need to be conducted with low MCT1 expressing cell line or with MCT1 knock out cell lines to evaluate the in vivo anticancer efficacy of 27. Also, studies to determine whether efficacy is directly due to MCT1 inhibition and whether any potential off-target effects are present must be conducted.
In conclusion, we have carried out a systematic SAR study of CHC and discovered several potent MCT1 inhibitors that are active at low nanomolar concentration. Preliminary in vivo toxicology studies indicate that these potent inhibitors are well tolerated without significant serious side effects. In vivo anticancer efficacy studies in MCT1 expressing colorectal cancer (WiDr) indicate an efficient tumor growth reduction. In general, these MCT1 inhibitors are highly potent, easy to synthesize, water-soluble, generally nontoxic, orally bioavailable, and effective in arresting the tumor growth in vivo as single agents. We believe that MCT1 inhibitors can be developed as single agent cancer chemotherapeutics and that they should also be highly amenable for combination therapy with other drugs with different mechanisms of action. The potential outcome of this project will be a new class of broad spectrum anticancer agents with higher affinity and specificity than previously available.
Glossary
ABBREVIATIONS
- CHC
α-cyano-4-hydroxycinnamic acid
- MCT
monocarboxylate transporter
- IC50
the concentration of test compound causing 50% transport inhibition
- SAR
structure–activity relationship
- RBE4
rat brain endothelial 4 cells
- K2CO3
potassium carbonate
- NaHCO3
sodium bicarbonate
- SEM
standard error of the mean
- ICR mice
mice initiated at Institute for Cancer Research
- DMSO
dimethyl sulfoxide
- PBS
phosphate buffered saline
- ip
intraperitoneal
- qd
once daily
- bid
twice daily
- qid
four times daily
Supporting Information Available
Synthetic methods and procedures of all the compounds, spectroscopic data, and elemental analysis, and in vitro and in vivo biological protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by Engebretson Drug Discovery Program, College of Pharmacy, University of Minnesota; Whiteside Clinical Research Institute, Duluth, Minnesota; and Minnesota Partnership for Biotechnology and Medical Genomics.
The authors declare the following competing financial interest(s): A patent application has been filed, but it has not been licensed nor has generated any revenue to date.
Supplementary Material
References
- Sonveaux P.; Végran F.; Schroeder T.; Wergin M. C.; Verrax J.; Rabbani Z. N.; De Saedeleer C. J.; Kennedy K. M.; Diepart C.; Jordan C. F.; Kelley M. J.; Gallez B.; Wahl M. L.; Feron M.; Dewhirst M. W. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008, 11, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V.; Thangaraju M.; Prasad P. D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [DOI] [PubMed] [Google Scholar]
- Lunt S. Y.; Vander H. M. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [DOI] [PubMed] [Google Scholar]
- Ganapathy K. S.; Geschwind J. F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miran J.; Sung S. K.; Jinhwa L. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P.; Price N. T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The SLC16 gene family: Structure, role and regulation in health and disease. Mol. Aspects Med. 2013, 34, 337–349. [DOI] [PubMed] [Google Scholar]
- Sonveaux P.; Copetti T.; Saedeleer C. J. D.; Vegran F.; Verrax J.; Kennedy K. M.; Moon E. J.; Dhup S.; Danhier P.; Frerart F.; Gallez B.; Ribeiro A.; Michiels C.; Dewhirst M. W.; Feron O. Targeting the lactate transporter mct1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One 2012, 7, e33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J. R.; Yang C.; Scott K. E.; Cameron M. D.; Fallahi M.; Li W.; Hall M. A.; Amelio A. L.; Mishra J. K.; Li F.; Tortosa M.; Genau H. M.; Rounbehler R. J.; Lu Y.; Dang C. V.; Kumar K. G.; Butler A. A.; Bannister T. D.; Hooper A. T.; Unsal-Kacmaz K.; Roush W. R.; Cleveland J. L. Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014, 74, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Yang C.; Doherty J. R.; Roush W. R.; Cleveland J. L.; Bannister T. D. Synthesis and structure activity relationships of pteridine dione and trione monocarboxylate transporter 1 inhibitors. J. Med. Chem. 2014, 57, 7317–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow S. E.; Tate L. Use of a MCT1 inhibitor in the treatment of cancers expressing MCT1 over MCT4. PCT Int. Appl. WO 2010089580A120100812, 2010.
- Draoui N.; Schicke O.; Fernandes A.; Drozak X.; Nahra F.; Dumont A.; Douxfils J.; Hermans E.; Dogné J. M.; Corbau R.; Marchand A.; Chaltin P.; Sonveaux P.; Feron O.; Riant O. Synthesis and pharmacological evaluation of carboxycoumarins as a new antitumor treatment targeting lactate transport in cancer cells. Bioorg. Med. Chem. 2013, 21, 7107–7117. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Wu M. S.; Zou C.; Tang Q.; Lu J.; Liu D.; Wu Y.; Yin J.; Xie X.; Shen J.; Kang T.; Wang J. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett. 2014, 342, 150–158. [DOI] [PubMed] [Google Scholar]
- Draoui N.; Schicke O.; Seront E.; Bouzin C.; Sonveaux P.; Riant O.; Feron O. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol. Cancer Ther. 2014, 13, 1410–1418. [DOI] [PubMed] [Google Scholar]
- Pavlides S.; Whitaker-Menezes D.; Castello-Cros R.; Flomenberg N.; Witkiewicz A. K.; Frank P. G.; Casimiro M. C.; Wang C.; Fortina P.; Addya S.; Pestell R. G.; Martinez-Outschoorn U. E.; Sotgia F.; Lisanti M. P. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [DOI] [PubMed] [Google Scholar]
- Witkiewicz A. K.; Dasgupta A.; Sammons S.; Er O.; Potoczek M. B.; Guiles F.; Sotgia F.; Brody J. R.; Mitchell E. P.; Lisanti M. P. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol. Ther. 2010, 10, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P.; Martinez-Outschoorn U. E.; Chiavarina B.; Pavlides S.; Whitaker-Menezes D.; Tsirigos A.; Witkiewicz A.; Lin Z.; Balliet R.; Howell A.; Sotgia F. Understanding the lethal drivers of tumor-stroma co-evolution. Cancer Biol. Ther. 2010, 10, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnieszka K. W.; Whitaker-Menezes D.; Dasgupta A.; Philp N. J.; Lin Z.; Gandara R.; Sneddon S.; Martinez-Outschoorn U. E.; Sotgia1 F.; Lisanti M. P. Using the reverse Warburg effect to identify high-risk breast cancer patients. Cell Cycle. 2012, 11, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereddy V. R.; Drewes L. R.; Alam M. A.; Jonnalagadda S. K.; Gurrapu S. Therapeutic compounds. PCT Appl. WO 2013109972A2, 2013.
- Freeman F. Properties and reactions of ylidenemalononitriles. Chem. Rev. 1980, 80, 329–350. [Google Scholar]
- Drewes L. R.; Spanier J. A. Monocarboxylate transporters. Drug Transp. 2007, 147–170. [Google Scholar]
- Smith J. P.; Drewes L. R. Modulation of monocarboxylic acid transporter-1 kinetic function by the cAMP signaling pathway in rat brain endothelial cells. J. Biol. Chem. 2006, 281, 2053–2060. [DOI] [PubMed] [Google Scholar]
- Campaigne E.; Archer W. L. Formylation of dimethylaniline. Org. Synth. 1953, 33, 27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.