Abstract
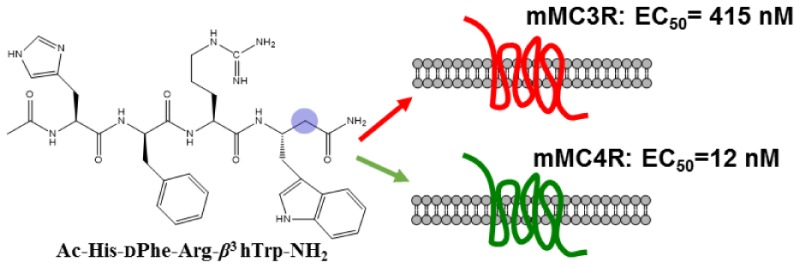
The melanocortin-3 and -4 receptors are expressed in the brain and play key roles in regulating feeding behavior, metabolism, and energy homeostasis. In the present study, incorporation of β3-amino acids into a melanocortin tetrapeptide template was investigated. Four linear α/β3-hybrid tetrapeptides were designed with the modifications at the Phe, Arg, and Trp residues in the agonist sequence Ac-His-dPhe-Arg-Trp-NH2. The most potent mouse melanocortin-4 receptor (mMC4R) agonist, Ac-His-dPhe-Arg-β3hTrp-NH2 (8) showed 35-fold selectivity versus the mMC3R. The study presented here has identified a new template with heterogeneous backbone for designing potent and selective melanocortin receptor ligands.
Keywords: β-amino acids, α/β3-peptides, peptidomimetics
The endogenous melanocortin agonists include α-, β-, and γ-melanocyte-stimulating hormones (MSH) are derived by posttranslational processing of the pro-opiomelanocortin (POMC) gene.1 All POMC derived melanocortin agonists possess a “His-Phe-Arg-Trp” domain in their primary amino acid sequence. The commonly used synthetic analogues NDP-MSH and MT-II possess a core His-dPhe-Arg-Trp sequence.2−4 The conserved tetrapeptide sequence has been determined to be important for the ligand binding/molecular recognition and stimulation of melanocortin receptors (MCRs).5,6 The MCRs are G protein-coupled receptors (GPCRs) that activate the cyclic adenosine monophosphate (cAMP) signal transduction pathway and generate a cascade of intracellular events, which result in physiological responses. Five melanocortin receptors (MC1–5R) have been cloned and characterized.7−13 All of the melanocortin receptors respond to the endogenous melanocortin hormones except for the MC2R, which only responds to ACTH. Therefore, the MC2R has been excluded from this study.8
The melanocortin-1 receptor (MC1R) is involved in skin and hair pigmentation.7,8 The melanocortin-5 receptor (MC5R) is found in a variety of tissues and identified to mediate exocrine gland function in mice.14 The melanocortin-3 and -4 receptors (MC3R and MC4R) are expressed in the brain and have been identified to regulate feeding behavior, metabolism, energy, and weight homeostasis.15−19 Central administration of α-MSH, or synthetic melanocortin agonists such as MT-II, results in decreased food intake. These effects produced by agonists can be blocked by preadministration of the synthetic MC3/MC4 receptor antagonist SHU9119.15 The MC4R has been extensively studied as a potential drug target for feeding related disorders due to its regulation of weight and energy homeostasis. The involvement of the MC3R in metabolism and energy homeostasis has been suggested by several studies, but the underlying mechanisms of actions are unclear.17−19 Selective and potent ligands are necessary to characterize these receptors in vivo. The endogenous peptide agonists are potent but nonselective at the melanocortin receptors.
Many peptidic and nonpeptidic ligands for the MCRs are reported in the literature, but the structural requirements for designing receptor selective ligands are not straightforward. Various approaches have been explored to overcome this problem including the use of constrained and bulkier unnatural amino acids, backbone modifications, and cyclization.20−23 The use of β-amino acids have been successfully utilized in the past for the development of potent, selective, and stable ligands.24−26 In a study, Nunn et al. reported that β-tetrapeptide analogues of the hormone somatostatin behave as potent agonists at the somatostatin 4 receptor (SSTR4).26 More recently, Mollica et al. have shown that the opioid peptide biphalin with the β3-amino acid substitution resulted in increased enzymatic stability.25 Kulkarni et al. reported receptor subtype selectivity when the His residue was replaced by a β-amino acid in the melanocortin antagonist SHU9119 template.27 Peptides containing β-amino acids have an extra methylene group in the peptide backbone, either between the carbonyl and the α-carbons (β3) or between the α-carbon and nitrogen atoms (β2) (Figure 1).28 Because of their extended backbone, β-amino acid containing peptides are able to obtain multiple conformations. β-Amino acids are also well-known to induce secondary structures, which often enable them to mimic the structural and functional properties of native peptides.29,30 Additionally, β-amino acids in peptides are not readily recognized by peptidases and reported to be more stable against proteolytic degradation in vitro and in vivo.25,31,32 Therefore, replacement of one or more α-amino acid residues to β-amino acids can be a viable option to improve functional properties of a peptide.24,25
Figure 1.
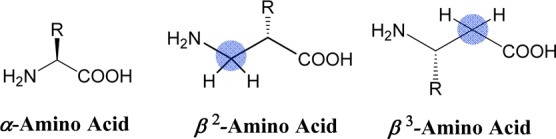
Structures of α- and β-amino acids. The blue circle indicates the position of an extra methylene group in the peptide backbone.
Previous structure–activity relationship (SAR) studies have identified the “His-Phe-Arg-Trp” and “Phe-Arg-Trp” as the minimal sequences required for generating the response in the frog and lizard skin bioassays.2−4 Subsequent truncation studies of the potent peptide NDP-MSH (Ac-Ser-Tyr-Ser-Nle-Glu-His-dPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2) established that Ac-His-dPhe-Arg-Trp-NH2 activated all four melanocortin receptors (MC1 and MC3–5R) at nanomolar concentrations. In comparison, the tripeptide, Ac-dPhe-Arg-Trp-NH2, possessed micromolar agonist activities at the MC1R, MC4R, and MC5R and only slightly activated the MC3R when tested up to 100 μM concentrations.6
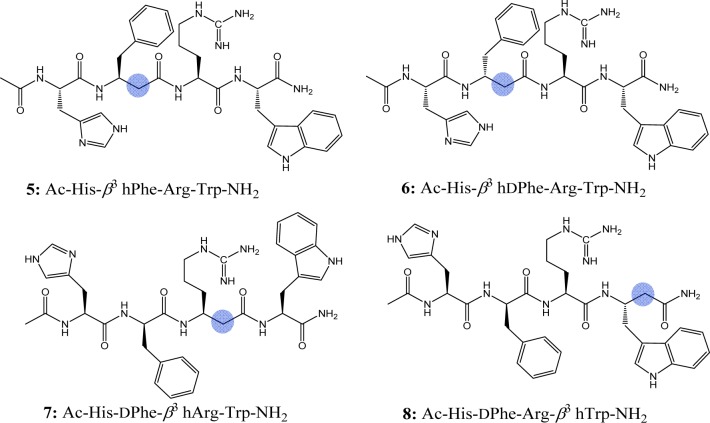
It is hypothesized that insertion of a β-amino acid in the melanocortin agonist “His-Phe-Arg-Trp” sequence could alter selectivity and/or potency at the melanocortin receptors. Based on the above rationale, this study was designed to introduce β3-amino acid analogues (β3 hPhe, β3 hdPhe, β3 hArg, and β3 hTrp) at the Phe, Arg, and Trp positions of the tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 (1). Four analogues containing β3 amino acids (β3 hXaa) were synthesized and pharmacologically characterized at the mouse mMCRs (Figure 2 and Table 1).
Figure 2.
Structures of the β3-amino acid containing tetrapeptides. The blue circle indicates position of an extra methylene group in the peptide backbone.
Table 1. Agonist Potency (nM) Pharmacology of the Tetrapeptides at the Mouse Melanocortin Receptors Using the AlphaScreen cAMP (Perkin Elmer) Assaya.
| peptide | sequence | mMC1R potency (nM) | mMC3R potency (nM) | mMC4R potency (nM) | mMC5R potency (nM) |
|---|---|---|---|---|---|
| α-MSH | 0.30 ± 0.11 | 0.40 ± 0.03 | 1.7 ± 0.16 | 0.22 ± 0.05 | |
| 1 | Ac-His-dPhe-Arg-Trp-NH2 | 18 ± 9.0 | 38 ± 5.0 | 5.6 ± 2.6 | 4.0 ± 1.3 |
| 2 | Ac-His-Phe-Arg-Trp-NH2 | 2360 ± 530 | 960 ± 225 | 1210 ± 460 | 70 ± 36 |
| 3 | Ac-DPhe-Arg-Trp-NH2 | 10,000 ± 4200 | 40% @ 100 μM | 70% @ 100 μM | 70% @ 100 μM |
| 4 | Ac-Phe-Arg-Trp-NH2 | 60% @ 100 μM | >100,000 | >100,000 | >100,000 |
| 5 | Ac-His-β3 hPhe-Arg-Trp-NH2 | 6577 ± 3619 | 70% @ 100 μM | 7678 ± 2618 | 4810 ± 674 |
| 6 | Ac-His-β3 hdPhe-Arg-Trp-NH2 | 7034 ± 3076 | 50% @ 100 μM | 60% @ 100 μM | 60% @ 100 μM |
| 7 | Ac-His-dPhe-β3 hArg-Trp-NH2 | 37 ± 14 | 854 ± 165 | 70 ± 26 | 73 ± 28 |
| 8 | Ac-His-dPhe-Arg-β3 hTrp-NH2 | 33 ± 13 | 415 ± 38 | 12 ± 4.0 | 5.6 ± 0.72 |
The indicated errors represent the standard error of the mean determined from at least three independent experiments. A value >100,000 means the compound was examined but lacked agonist activity at up to 100 μM concentrations. A % indicates that at the 100 μM highest concentration tested, some stimulatory response was observed relative to control.
These peptides were synthesized by microwave-assisted Fmoc SPPS using Rink Amide MBHA resin in a manual microwave synthesizer (Discover SPS CEM Corp.). All synthesized peptides were acetylated at the N-terminus and amidated at the C-terminus. Purification was achieved by semipreparative RP-HPLC, and characterization was done using analytical HPLC and mass spectrometry (see Supporting Information). Table 1 summarizes the functional agonist pharmacology of the synthesized peptides at the mouse MCRs using the cAMP-based AlphaScreen assay (PerkinElmer).
The tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 (1) was used as a control in this study and possessed nanomolar agonist activity at all the MCRs with ∼6 nM potency at the mMC4R, consistent with earlier studies.6 The melanocortin agonist core peptide Ac-His-Phe-Arg-Trp-NH2 (2), possessed 130-, 25-, 200-, and 18-fold decreased potency compared to 1 at the mMC1R and mMC3–5Rs, respectively. The tripeptide Ac-dPhe-Arg-Trp-NH2 (3) possessed 550-fold decreased activity at the mMC1R compared to 1 and was only able to stimulate the mMC3R, mMC4R, and mMC5R at 40%, 70%, and 70% of the maximum cAMP response, respectively, at 100 μM concentrations. Tripeptide Ac-Phe-Arg-Trp-NH2 (4) resulted in a loss of stimulatory activity at the mMC3–5Rs and showed slight agonist activity at the mMC1R at 100 μM concentrations. The data reported herein for the endogenous peptide α-MSH tetrapeptide 1 and 2 are in agreement with the values reported in the literature, using a cAMP β-galactosidase reporter gene assay.6
The β3 hPhe (5) and β3 hdPhe (6) peptides were the least tolerated substitutions at the MCRs. Compared to 1, the tetrapeptide Ac-His-β3 hPhe-Arg-Trp-NH2 (5) resulted in 365-, 1280-, and 1200-fold decreased activity at the mMC1R, mMC4R, and mMC5R, respectively, and showed only 70% maximal cAMP response at the mMC3R at 100 μM concentrations (Figure 3). Peptide 6 resulted in a 7 μM full agonist at the mMC1R but was only able to stimulate the mMC3R, mMC4R, and mMC5R to 50–60% maximal cAMP response at 100 μM concentrations (Figure 3). Substitution of the Arg residue with β3 hArg and the Trp replacement by β3 hTrp in the reference tetrapeptide resulted in peptides 7 and 8, respectively. Peptides 7 and 8 showed full agonist activities ranging from nanomolar to micromolar potencies at the mouse MC1R and MC3–5Rs. Peptide Ac-His-dPhe-β3 hArg-Trp-NH2 (7) possessed 22-, 12-, and 18-fold decreased potency at the mMC3R, mMC4R, and mMC5R, respectively, and showed equipotency at the mMC1R compared to 1. Peptide (7) was 12-fold more selective at the mMC4R over the mMC3R. The tetrapeptide Ac-His-dPhe-Arg-β3 hTrp-NH2 (8) was the most potent compound in the series showing equipotent activities compared with reference peptide 1 at the mMC1R, mMC4R, and mMC5R, and resulted in 11-fold decreased mMC3R potency. Peptide (8) is 35-fold selective toward the mMC4R versus the mMC3R, but showed no selectivity relative to the mMC1R and the mMC5R (Table 1).
Figure 3.
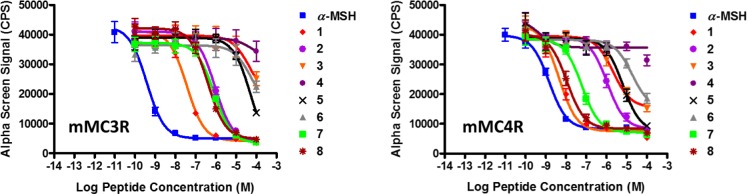
Illustration of the pharmacological agonist dose–response curves for the tetrapeptides reported.
The MC3R and MC4R are involved in the regulation of food and energy homeostasis, but the underlying mechanism of their overlapping/synergistic roles are not clearly understood. Therefore, discovery of potent and selective ligands for these receptors are highly desirable. The strategic replacement of key melanocotin residues with corresponding β3-amino acids in the pharmacophore region of the tetrapeptide resulted in potent and selective compounds in this study. Peptide 5 (β3 hPhe in place of dPhe) maintained full agonist activity at the mMC1R, mMC4R, and mMC5R, albeit with decreased potencies compared to 1, and had slight agonist activity at the mMC3R. Peptide 6 resulted in 220-fold decreased potency at the mMC1R and showed only slight agonist activity at the mMC3R, mMC4R, and mMC5Rs, relative to (1). These results support the hypothesis regarding the Phe amino acid side chain as a key residue in the core melanocortin tetrapeptide sequence and suggest that the introduction of an extra methylene group between the Phe and Arg residues greatly disrupt putative key interactions of aromatic phenyl group and/or neighboring arginine residue with the MCRs.
Peptides 7 and 8 showed nanomolar potencies and selectivity of 12- and 35-fold for the mMC4R over the mMC3R, respectively. It is worth mentioning that peptide 8 showed increased selectivity for the mMC4R over the mMC3R, while retaining the nanomolar equipotent functional activity as compared to 1. The improved selectivity of the tetrapeptide 8 for mMC4R may be postulated to the receptor subtype-preferred bioactive conformation of the peptide due to the placement of hβ3-amino acid in the tetrapeptide sequence. The additional methylene group near the Trp residue in peptide 8 may alter backbone dihedral angles (phi and psi) and the orientation of indole side chain in comparison to peptide 1. The side chain of Trp is unique, with its amphipathic nature enabling it to participate in additional structure stabilizing interactions.33 In a recent study, participation of the Trp residue in stabilizing bioactive conformation at melanocortin receptors has been reported.22 Biophysical studies suggested that the Trp residue was interacting with the His residue to stabilize the reverse turn in the pharmacophore region, which resulted in a 50-fold selective mMC4R ligand over the mMC3R. The lack of selectivity of compound 8 for the mMC4R relative to the mMC1R and the mMC5R may be suggestive of similar ligand conformational preferences by these receptors unlike the mMC3R. Results from the current study indicate that the Trp position in the putative core sequence of melanocortin agonists can be exploited to differentiate pharmacology at the mMC3R and mMC4R. Further SAR studies are required to test this hypothesis.
In summary, four hybrid α/β3 tetrapeptides were synthesized by incorporating β3-amino acids into the melanocortin agonist core sequence. Two compounds resulted, 7 and 8, with nanomolar agonist potencies and improved selectivity at the mMC4R versus the mMC3R. These results provide information on the consequences of incorporating β3-amino acid residues into the putative message sequence of melanocortin agonists. The most potent peptide Ac-His-dPhe-Arg-β3 hTrp-NH2 (8) in this study possessed 12 nM potency and 35-fold selectivity at the mMC4R over the mMC3R. The template presented herein may serve as a scaffold for the design of the potent and selective MCR ligands.
Glossary
ABBREVIATIONS
- SPPS
solid phase peptide synthesis
- MBHA
methylbenzhydryl
- NDP
[Nle4,dPhe7]-α-MSH
- MT-II
melanotan-II
- RP-HPLC
reversed phase high performance liquid chromatography
Supporting Information Available
Detailed procedure for the synthesis of peptides, analytical data, and AlphaScreen cAMP assay information. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by NIH R01 DK091906 (C.H.-L.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Eipper B. A.; Mains R. E. Structure and Biosynthesis of Pro-Adrenocorticotropin/Endorphin and Related Peptides. Endocr. Rev. 1980, 1, 1–26. [DOI] [PubMed] [Google Scholar]
- Hruby V. J.; Wilkes B. C.; Hadley M. E.; Al-Obeidi F.; Sawyer T. K.; Staples D. J.; de Vaux A. E.; Dym O.; Castrucci A. M.; Hintz M. F.; Riehm J. P.; Rao K. R. alpha-Melanotropin: the Minimal Active Sequence in the Frog Skin Bioassay. J. Med. Chem. 1987, 30, 2126–2130. [DOI] [PubMed] [Google Scholar]
- Castrucci A. M.; Hadley M. E.; Sawyer T. K.; Wilkes B. C.; al-Obeidi F.; Staples D. J.; de Vaux A. E.; Dym O.; Hintz M. F.; Riehm J. P.; Rao K. R.; Hruby V. J. Alpha-Melanotropin: the Minimal Active Sequence in the Lizard Skin Bioassay. Gen. Comp. Endrocrinol. 1989, 73, 157–163. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C.; Sawyer T. K.; Hendrata S.; North C.; Panahinia L.; Stum M.; Staples D. J.; Castrucci A. M.; Hadley M. F.; Hruby V. J. Truncation Studies of α-Melanotropin Peptides Identify Tripeptide Analogues Exhibiting Prolonged Agonist Bioactivity. Peptides 1996, 17, 995–1002. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C.; Hendrata S.; North C.; Sawyer T. K.; Hadley M. E.; Hruby V. J.; Dickinson C.; Gantz I. Discovery of Prototype Peptidomimetic Agonists at the Human Melanocortin Receptors MC1R and MC4R. J. Med. Chem. 1997, 40, 2133–2139. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C.; Holder J. R.; Monck E. K.; Bauzo R. M. Characterization of Melanocortin NDP-MSH Agonist Peptide Fragments at the Mouse Central and Peripheral Melanocortin Receptors. J. Med. Chem. 2001, 44, 2247–2252. [DOI] [PubMed] [Google Scholar]
- Chhajlani V.; Wikberg J. E. Molecular Cloning and Expression of the Human Melanocyte Stimulating Hormone Receptor cDNA. FEBS Lett. 1992, 309, 417–420. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Cone R. D. The Cloning of a Family of Genes that Encode the Melanocortin Receptors. Science 1992, 257, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L.; Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Low M. J.; Tatro J. B.; Entwistle M. L.; Simerly R. B.; Cone R. D. Identification of a Receptor for Gamma Melanotropin and Other Proopiomelanocortin Peptides in the Hypothalamus and Limbic System. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy K. G.; Mortrud M. T.; Low M. J.; Simerly R. B.; Cone R. D. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endocrinol. 1994, 8, 1298–1308. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Konda Y.; Tashiro T.; Shimoto Y.; Miwa H.; Munzert G.; Watson S. J.; DelValle J.; Yamada T. Molecular Cloning of a Novel Melanocortin Receptor. J. Biol. Chem. 1993, 268, 8246–8250. [PubMed] [Google Scholar]
- Gantz I.; Miwa H.; Konda Y.; Shimoto Y. Molecular Cloning, Expression, and Gene Localization of a Fourth Melanocortin Receptor. J. Biol. Chem. 1993, 268, 15174–15179. [PubMed] [Google Scholar]
- Gantz I.; Shimoto Y.; Konda Y.; Miwa H.; Dickinson C.; Yamada T. Molecular Cloning, Expression, and Characterization of a Fifth Melanocortin Receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1214–1220. [DOI] [PubMed] [Google Scholar]
- Chen W.; Kelly M. A.; Opitz-Araya X.; Thomas R. E.; Low M. J.; Cone R. D. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91, 789–798. [DOI] [PubMed] [Google Scholar]
- Fan W.; Boston B. A.; Kesterson R. A.; Hruby V. J.; Cone R. D. Role of Melanocortinergic Neurons in Feeding and the Agouti Obesity Syndrome. Nature 1997, 385, 165–168. [DOI] [PubMed] [Google Scholar]
- Huszar D.; Lynch C. A.; Fairchild-Huntress V.; Dunmore J. H.; Fang Q.; Berkemeier L. R.; Gu W.; Kesterson R. A.; Boston B. A.; Cone R. D.; Smith F. J.; Campfield L. A.; Burn P.; Lee F. Targeted Disruption of the Melanocortin-4 receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [DOI] [PubMed] [Google Scholar]
- Chen A. S.; Marsh D. J.; Trumbauer M. E.; Frazier E. G.; Guan X. M.; Yu H.; Rosenblum C. I.; Vongs A.; Feng Y.; Cao L.; Metzger J. M.; Strack A. M.; Camacho R. E.; Mellin T. N.; Nunes C. N.; Min W.; Fisher J.; Gopal-Truter S.; MacIntyre D. E.; Chen H. Y.; Van der Ploeg L. H. Inactivation of the Mouse Melanocortin-3 Receptor Results in Increased Fat Mass and Reduced Lean Body Mass. Nat. Genet. 2000, 26, 97–102. [DOI] [PubMed] [Google Scholar]
- Butler A. A.; Kesterson R. A.; Khong K.; Cullen M. J.; Pelleymounter M. A.; Dekoning J.; Baetscher M.; Cone R. D. A Unique Metabolic Syndrome Causes Obesity in the Melanocortin-3 Receptor-Deficient Mouse. Endocrinology 2000, 141, 3518–3521. [DOI] [PubMed] [Google Scholar]
- Irani B. G.; Xiang Z.; Yarandi H. N.; Holder J. R.; Moore M. C.; Bauzo R. M.; Proneth B.; Shaw A. M.; Millard W. J.; Chambers J. B.; Benoit S. C.; Clegg D. J.; Haskell-Luevano C. Implication of the Melanocortin-3 Receptor in the Regulation of Food Intake. Eur. J. Pharmacol. 2011, 660, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. R.; Haskell-Luevano C. Melanocortin Ligands: 30 years of Structure-Activity Relationship (SAR) Studies. Med. Res. Rev. 2004, 24, 325–356. [DOI] [PubMed] [Google Scholar]
- Singh A.; Wilczynski A.; Holder J. R.; Witek R. M.; Dirain M. L.; Xiang Z.; Edison A. S.; Haskell-Luevano C. Incorporation of a Bioactive Reverse-Turn Heterocycle into a Peptide Template Using Solid-Phase Synthesis to Probe Melanocortin Receptor Selectivity and Ligand Conformations by 2D 1H NMR. J. Med. Chem. 2011, 54, 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Dirain M. L.; Wilczynski A.; Chen C.; Gosnell B. A.; Levine A. S.; Edison A. S.; Haskell-Luevano C. Synthesis, Biophysical, and Pharmacological Evaluation of the Melanocortin Agonist AST3–88: Modifications of Peptide Backbone at Trp 7 Position Lead to a Potent, Selective, and Stable Ligand of the Melanocortin 4 Receptor (MC4R). ACS Chem. Neurosci. 2014, 5, 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa C.; Scrima M.; Grimaldi M.; D’Ursi A. M.; Dirain M. L.; Lubin-Germain N.; Singh A.; Haskell-Luevano C.; Chorev M.; Rovero P.; Papini A. M. 1,4-Disubstituted-[1,2,3]triazolyl-Containing Analogues of MT-II: Design, Synthesis, Conformational Analysis, and Biological Activity. J. Med. Chem. 2014, 57, 9424–9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesma G.; Salvadori S.; Airaghi F.; Murray T. F.; Recca T.; Sacchetti A.; Balboni G.; Silvani A. Structural and Biological Exploration of Phe(3)-Phe(4)-Modified Endomorphin-2 Peptidomimetics. ACS Med. Chem. Lett. 2013, 4, 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Pinnen F.; Costante R.; Locatelli M.; Stefanucci A.; Pieretti S.; Davis P.; Lai J.; Rankin D.; Porreca F.; Hruby V. J. Biological Active Analogues of the Opioid Peptide Biphalin: Mixed alpha/beta(3)-Peptides. J. Med. Chem. 2013, 56, 3419–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn C.; Rueping M.; Langenegger D.; Schuepbach E.; Kimmerlin T.; Micuch P.; Hurth K.; Seebach D.; Hoyer D. beta(2)/beta(3)-di- and alpha/beta(3)-Tetrapeptide Derivatives as Potent Agonists at Somatostatin sst(4) Receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 95–103. [DOI] [PubMed] [Google Scholar]
- Kulkarni V. V.; Cai M.; Muthu D.; Hruby V. J., Incorporation of β-Amino Acids in MT-II and SHU9119. In Proceedings of the 22nd American Peptide Symposium; Lebl M., Ed.; Prompt Scientific Publishing: San Diego, CA, 2011; pp 336–337. [Google Scholar]
- Seebach D.; Gardiner J. Beta-Peptidic Peptidomimetics. Acc. Chem. Res. 2008, 41, 1366–1375. [DOI] [PubMed] [Google Scholar]
- Seebach D.; Hook D. F.; Glattli A. Helices and Other Secondary Structures of beta- and gamma-Peptides. Biopolymers 2006, 84, 23–37. [DOI] [PubMed] [Google Scholar]
- Schievano E.; Mammi S.; Carretta E.; Fiori N.; Corich M.; Bisello A.; Rosenblatt M.; Chorev M.; Peggion E. Conformational and Biological Characterization of Human Parathyroid Hormone hPTH(1–34) Analogues Containing beta-Amino Acid Residues in Positions 17–19. Biopolymers 2003, 70, 534–547. [DOI] [PubMed] [Google Scholar]
- Frackenpohl J.; Arvidsson P. I.; Schreiber J. V.; Seebach D. The Outstanding Biological Stability of beta- and gamma-Peptides Toward Proteolytic Enzymes: An In Vitro Investigation with Fifteen Peptidases. ChemBioChem 2001, 2, 445–455. [DOI] [PubMed] [Google Scholar]
- Schreiber J. V.; Frackenpohl J.; Moser F.; Fleischmann T.; Kohler H. P.; Seebach D. On the Biodegradation of beta-Peptides. ChemBioChem 2002, 3, 424–432. [DOI] [PubMed] [Google Scholar]
- Samanta U.; Pal D.; Chakrabarti P. Packing of Aromatic Rings Against Tryptophan Residues in Proteins. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1999, 55, 1421–1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.