Abstract
Objective To determine the methodological quality of clinical trials that examined possible interactions of St John's wort with conventional drugs, and to examine the results of these trials.
Design Systematic review.
Data sources Electronic databases from inception to April 2004, reference lists from published reports, and experts in the field.
Study selection Eligible studies were prospective clinical trials evaluating the pharmacokinetic effect of St John's wort on the metabolism of conventional drugs.
Data extraction Two reviewers selected studies for inclusion and independently extracted data.
Data synthesis 22 pharmacokinetic trials studied an average of 12 (SD 5) participants; 17 trials studied healthy volunteers and five studied patients. Most (17) studies used a “before and after” design; four studies used control groups other than the active group. Three studies randomised the sequence of administration or the participants to study arms or periods; three studies blinded participants or investigators. In 15 trials, investigators independently assayed the herb. Of 19 trials with available plasma data, three found no important interaction (change in area under the curve < 20%) and 17 found a decrease in systemic bioavailability of the conventional drug; in seven studies the 95% confidence interval excluded a decrease of < 20%.
Conclusion Clinicians and patients should beware of possible decreases in the systemic bioavailability of conventional drugs when taken concomitantly with St John's wort.
Introduction
Use of natural health products, including herbal medicines, vitamins, and supplements, is widespread.1 With expanding use, clinicians and researchers are focusing their attention on the possibility that these products interact with prescribed drugs and alter their metabolism. Case reports and clinical trials have found that St John's wort (Hypericum perforatum), in particular, may cause important interactions, possibly leading to adverse events or drug resistance. The purposes of this systematic review were to identify all clinical trials examining drug interactions of St John's wort, assess these trials' methodological quality, and summarise their findings.
Methods
Data sources
We searched the following databases (from inception to April 2004): AMED, CINAHL, E-Psyche, CISCOM (to December 2002), Cochrane CENTRAL, Medline, the National Research Register (United Kingdom), ClinicalTrials.gov (United States), and bibliographies of retrieved articles; we also contacted experts in the field.
Study selection and quality assessment
Two reviewers (EM, PW), working independently and in duplicate, identified relevant abstracts and assessed eligibility after reading the full reports, resolving disagreements by consensus or arbitration. Eligible studies were prospective clinical trials examining the pharmacokinetic effect of St John's wort on the metabolism of a conventional drug.
We assessed the quality of each study by examining the study design, the mechanism to allocate patients, the inclusion of control patients, the use of placebo, and justification of sample size. In addition, we determined whether the trial used single dose pharmacokinetics or studied St John's wort at its steady state (continuous use of the herb increases the likelihood of an interaction). We also determined if the investigators assayed St John's wort in each preparation.
Statistical analysis
We measured inter-rater agreement (adjusted for chance) for eligibility using the κ statistic. We describe the magnitude of the interaction between St John's wort and the conventional drug by differences in the area under the curve (AUC) of the drug before and after challenge with St John's wort. We used the difference in AUC reported as a percentage of the AUC at baseline and 95% confidence interval around this change; if the difference was not reported, we estimated the confidence interval from reported measures of variance or P values for the change in AUC.2 We considered an interaction to be important when the confidence interval of the AUC difference was completely outside of the standard deviation (SD) 20% equivalence limits; we considered that no important interaction existed when the confidence interval of the AUC difference was entirely within the SD 20% equivalence limits.3 Owing to statistical and methodological heterogeneity we did not pool results of the separate trials.
Results
Search results
The chance adjusted inter-rater agreement for inclusion of studies was κ = 0.8 (95% confidence interval 0.6 to 1.0); the 22 included trials (published between 1999 and April 2004) studied an average of 12 (SD 5) participants.4-25 (For more details of selection and of the included studies, see the flow chart and table on bmj.com) Five studies enrolled patients who needed the drugs for health reasons4-8; all others enrolled healthy volunteers.
Table 1.
Characteristics of methods used in 22 reviewed studies
| Name and year | Study design | Herb assayed | Control group | Blinding | Randomisation | Sample size discussed |
|---|---|---|---|---|---|---|
| Mathijssen, 20025 | Before and after | No | No | No | No | No |
| Markowitz, 200016 | Before and after | Yes | No | No | No | No |
| Burstein, 200014 | Before and after | Yes | No | No | No | No |
| Durr, 200020 | Before and after | No | No | No | No | No |
| Piscitelli, 200021 | Before and after | Yes | No | No | No | No |
| Mai, 20037 | Before and after | Yes | No | No | No | Yes |
| Hebert, 200423 | Before and after | Yes | No | No | No | No |
| Bauer, 20036 | Before and after | Yes | No | No | No | Yes |
| Hall, 20038 | Before and after | Yes | No | No | No | Yes |
| Jiang, 200422 | Before and after | Yes | No | No | Yes | Yes |
| Johne, 20024 | Before and after | Yes | No | No | No | Yes |
| Wang, 200110 | Before and after | Yes | No | No | No | Yes |
| Wang, 20029 | Before and after | Yes | No | No | No | No |
| Markowitz, 200324 | Before and after | No | No | No | No | Yes |
| Dresser, 200325 | Before and after | No | No | No | No | No |
| Roby, 200111 | Before and after | Yes | No | No | No | No |
| Wenk, 200412 | Before and after | No | No | No | No | No |
| Sugimoto, 200115 | Crossover | No | Yes (placebo) | Yes | Yes | No |
| Pfrunder, 200317 | Crossover | Yes | Yes | No | Yes | No |
| Wang, 200418 | Crossover | No | Yes (placebo) | Yes | Yes | No |
| Morimoto, 200419 | Crossover | Yes | No | No | No | No |
| Johne, 199913 | Parallel | Yes | Yes (placebo) | Yes | No | Yes |
Pharmacokinetic details
Two studies investigated both single dose and steady state pharmacokinetics,9,10 and 18 studies examined steady state pharmacokinetics. Two studies examined exclusively urine metabolites.11,12 The mean duration of St John's wort dosing for steady state studies was 17 (SD 11.5, range 3-56) days. In seven studies, participants received St John's wort concurrently with the conventional drug for the post-exposure AUC assay.4-6,13-16 Fifteen trials reported independent assays of the St John's wort preparation to determine whether the dosage of interest was truly contained in the product. Investigators assayed the serum concentration of the conventional drug for a mean of 37.5 (SD 47, range 7-216) hours to generate a “concentration over time” curve and the AUC.
Study design
The table shows the study design and methodological quality of the 22 included trials. Eighteen did not have a control group—that is, a group treated equally but not receiving the active drug concomitantly. Of the four studies with control groups, three were randomised (procedure to generate the randomisation sequence not described)13,17,18; three control groups received placebos (placebo used not described).13,15,18
Effects of St John's wort
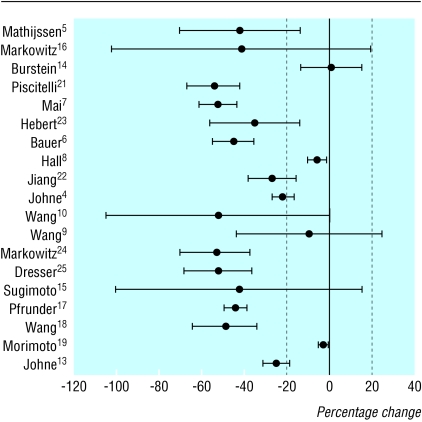
The figure shows the effect of St John's wort on the plasma AUC of the conventional drug; it uses a forest plot with studies grouped by study design. In three studies, the 95% confidence interval excluded a reduction in AUC of > 20%.8,14,19 Of the 16 other studies, point estimates of 15 indicated a decrease of > 20% in availability of the conventional drug. Of these 15, the confidence interval excluded a reduction of < 20% in AUC in seven. One study assessed primary mechanisms of interactions and did not report AUC data.20
Figure 1.
Mean percentage reported change (with 95% confidence intervals) in systemic bioavailability of drugs in plasma, after exposure to St John's wort. Trials are ordered according to study design (Mathijssen to Dresser are “before and after,” Sugimoto to Morimoto are crossover, and Johne is a parallel design)
Discussion
Our review shows that St John's wort has the potential to reduce systemic bioavailability of many conventional drugs. However, the studies had several shortcomings. Firstly, they were small: in 10 of the 22 trials the 95% confidence interval overlapped the 20% threshold for an important reduction in the area under the curve (AUC), so the results were inconclusive, and no study was large enough to examine subgroups meaningfully. Secondly, they contained few safeguards against bias (such as random and concealed allocation of participants, inclusion of controls, and blinding. Thirdly, their methods varied widely, with inadequate use of widely accepted standards of research practice.
The variable effect of St John's wort on different conventional drugs may be related to the variety of metabolic pathways that these drugs use. We could not, however, discern a clear relation between the nature of the conventional drug being tested or its posited metabolism and the magnitude of the interaction.
Our study is the first systematic review of St John's wort that examines the methodological quality of the clinical trials. Previous reviews have described the findings of clinical trials but have not assessed the trial validity or effect sizes.26-28
Our review uncovers several threats to the validity of pharmacokinetic studies that examine herb-drug interactions. A serious concern about conducting any research with herbs is the correspondence of the herb studied with the products that are available to and preferred by consumers. Assaying the herb is critical, as the product's content can differ from the dosage reported on the label and the composition varies from batch to batch.29 Only 15 of the 22 studies reported that they assayed the St John's wort content of the preparations they used. Furthermore, the duration of dosing used in these trials was highly variable, and few studies reported their rationale for the dosing regimen tested.
The methodological quality of the included studies was limited. Most studies used a “before and after” design that lacked contemporary controls or randomisation of order of administration, in particular to control for carryover effects, time dependent metabolic variability, and co-ingestions. When investigators used controls, they did not clearly describe how they allocated participants, how they generated the allocation sequence, and how they concealed this sequence from investigators enrolling participants. The US Food and Drug Administration's guidance on conducting pharmacokinetic trials,3 a commonly used guide, may consider these important safeguards against bias in future recommendations.
What is already known on this topic
Some research has suggested decreased systemic bioavailability of conventional drugs when St John's wort is taken concomitantly
What this study adds
Studies examining drug interactions of St John's wort are limited in scope and methodological quality, particularly in the areas of controls and randomisation
Clinicians and patients do not currently have high quality information to guide their decisions to use herbal products, particularly in conjunction with conventional drugs
We used a somewhat arbitrary threshold (20%) in AUC change to identify important changes in systemic bioavailability of the conventional drug after exposure to St John's wort; we could not identify clearly established drug specific thresholds beyond which reductions in AUC might lead to important reductions in drug effect. Lack of such thresholds may seriously hinder the clinical interpretation of pharmacokinetic trials.
Clinicians and patients should beware of possible reductions in systemic bioavailability of conventional drugs when taken concomitantly with St John's wort. Higher quality research is necessary to provide reliable information to guide clinical practice. In particular, trials that include adjustments in the dose of the conventional drug to achieve optimal AUC in the presence of herbs that reduce bioavailability of that drug may provide clinicians and patients with an approach to their concomitant use.
Supplementary Material
 A flow chart showing selection of studies for inclusion and a table with further details about the studies are on bmj.com
A flow chart showing selection of studies for inclusion and a table with further details about the studies are on bmj.com
Contributors: The study was conceptualised by EM, VMM, and GG; EM and PW did the literature searches; EM, PW did the data abstraction; VMM, EM, and PW did the analysis; and EM, VMM, MC, GG, and KG wrote and critically revised the manuscript. EM will act as guarantor of this manuscript.
Funding: This study was funded by the Ontario HIV Treatment Network.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med 2001;9: 2-11. [DOI] [PubMed] [Google Scholar]
- 2.Altman DG, Gardner MJ. Means and their differences. In: Altman DG, Bryant TN, Gardner MJ, eds. Statistics with confidence. Bristol: BMJ Publishing, 2000: 28-35.
- 3.Food and Drug Administration. In vivo drug metabolism/drug interaction studies—study design, data analysis, and recommendations for dosing and labeling. 1999. www.fda.gov/cber/gdlns/metabol.pdf (accessed 20 May 2004)
- 4.Johne A, Schmider J, Brockmoller J, Stadelmann AM, Stormer E, Bauer S, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John's wort (Hypericum perforatum). J Clin Psychopharmacol 2002;22: 46-54. [DOI] [PubMed] [Google Scholar]
- 5.Mathijssen RH, Verweij J, de Bruijn P, Loos WJ, Sparreboom A. Effects of St John's wort on irinotecan metabolism. J Natl Cancer Inst 2002;94: 1247-9. [DOI] [PubMed] [Google Scholar]
- 6.Bauer S, Stormer E, Johne A, Kruger H, Budde K, Neumayer HH, et al. Alterations in cyclosporin A pharmacokinetics and metabolism during treatment with St John's wort in renal transplant patients. Br J Clin Pharmacol 2003;55: 203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai I, Stormer E, Bauer S, Kruger H, Budde K, Roots I. Impact of St John's wort treatment on the pharmacokinetics of tacrolimus and mycophenolic acid in renal transplant patients. Nephrol Dial Transplant 2003;18: 819-22. [DOI] [PubMed] [Google Scholar]
- 8.Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, et al. The interaction between St John's wort and an oral contraceptive. Clin Pharmacol Ther 2003;74: 525-35. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Hamman MA, Huang SM, Lesko LJ, Hall SD. Effect of St John's wort on the pharmacokinetics of fexofenadine. Clin Pharmacol Ther 2002;71: 414-20. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effects of St John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther 2001;70: 317-26. [PubMed] [Google Scholar]
- 11.Roby CA, Dryer DA, Burstein AH. St John's wort: effect on CYP2D6 activity using dextromethorphan-dextrorphan ratios. J Clin Psychopharmacol 2001;21: 530-2. [DOI] [PubMed] [Google Scholar]
- 12.Wenk M, Todesco L, Krahenbuhl S. Effect of St John's wort on the activities of CYP1A2, CYP3A4, CYP2D6, n-acetyltransferase 2, and xanthine oxidase in healthy males and females. Br J Clin Pharmacol 2004;57: 495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John's wort (Hypericum perforatum). Clin Pharmacol Ther 1999;66: 338-45. [DOI] [PubMed] [Google Scholar]
- 14.Burstein AH, Horton RL, Dunn T, Alfaro RM, Piscitelli SC, Theodore W. Lack of effect of St John's wort on carbamazepine pharmacokinetics in healthy volunteers. Clin Pharmacol Ther 2000;68: 605-12. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, et al. Different effects of St John's wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther 2001;70: 518-24. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz JS, deVane CL, Boulton DW, Carson SW, Nahas Z, Risch SC. Effect of St John's wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Life Sci 2000;66: PL133-9. [DOI] [PubMed] [Google Scholar]
- 17.Pfrunder A, Schiesser M, Gerber S, Haschke M, Bitzer J, Drewe J. Interaction of St John's wort with low-dose oral contraceptive therapy: a randomized controlled trial. Br J Clin Pharmacol 2003;56: 683-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LS, Zhou G, Zhu B, Wu J, Wang JG, El-Aty Am AA, et al. St John's wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther 2004;75: 191-7. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto T, Kotegawa T, Tsutsumi K, Ohtani Y, Imai H, Nakano S. Effect of St John's wort on the pharmacokinetics of theophylline in healthy volunteers. J Clin Pharmacol 2004;44: 95-101. [DOI] [PubMed] [Google Scholar]
- 20.Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John's wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 2000;68: 598-604. [DOI] [PubMed] [Google Scholar]
- 21.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet 2000;355: 547-8. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, William KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 2004:57: 592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert MF, Park JM, Chen YL, Akhtar S, Larson AM. Effects of St John's wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol 2004;44: 89-94. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang J, et al. Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290: 1500-4. [DOI] [PubMed] [Google Scholar]
- 25.Dresser GK, Schwarz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St John's wort in healthy subjects. Clin Pharmacol Ther 2003;73: 41-50. [DOI] [PubMed] [Google Scholar]
- 26.Hammerness P, Basch E, Ulbricht C, Barrette EP, Foppa I, Basch S, et al. St John's wort: a systematic review of adverse effects and drug interactions for the consultation psychiatrist. Psychosomatics 2003;44: 271-82. [DOI] [PubMed] [Google Scholar]
- 27.Markowitz JS, deVane CL. The emerging recognition of herb-drug interactions with a focus on St John's wort (Hypericum perforatum). Psychopharmacol Bull 2001;35: 53-64. [PubMed] [Google Scholar]
- 28.Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St John's wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol 2002;54: 349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draves AH, Walker SE. Analysis of the hypericin and pseudohypericin content of commercially available St John's wort preparations. Can J Clin Pharmacol 2003;10: 114-8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.