Abstract
The purpose of this study was to evaluate 64Cu-labeled hexadecyl-1,4,7,10-tetraazacyclododecane-tetraacetic acid-benzoate (64Cu-DOTA-HB) (1) as positron emission tomography (PET) radiotracer for stem cell imaging. Hexadecyl-DOTA-benzoate (DOTA-HB) (2) was efficiently labeled with 64Cu (>99%), and cell labeling efficiency with adipose-derived stem cells (ADSCs) was over 50%. Labeling with 1 did not compromise cell viability. In the PET imaging, intramuscularly transplanted 1-labeled ADSCs were monitored for 18 h in normal rat heart. These results indicate that 1 can be utilized as a promising radiotracer for monitoring of transplanted stem cells.
Keywords: Adipose-derived stem cells, copper-64, hexadecyl benzoate, radiotracer, PET
Longitudinal monitoring of transplanted stem cells is important for assessment of cell therapeutic effect. Molecular imaging technique allows noninvasive monitoring of the fate of transplanted stem cells in living bodies.
The molecular imaging modalities using radionuclide with positron emission tomography (PET) or single photon emission computed tomography provide high sensitivity (10–8 to 10–9 μm/L) and spatial resolution (1–2 mm).1,2
64Cu has relatively long half-life (12.7 h) and its decay property (β–, 0.573 MeV [38.4%]; β+, 0.655 MeV [17.8%]; γ, 0.511 MeV [35.6%]) allows longitudinal PET imaging and tracking for transplanted cells.3,464Cu can react with a wide variety of chelator systems including 1,4,7,10-tetraazacyclododecane-tetraacetic acid (DOTA) or 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) due to its well-established coordination chemistry.5,6 DOTA can bind with many different metal ions; thus, it leads to a decrease in vivo stability. Nevertheless, DOTA is currently used in 64Cu-diacetyl-bis(N4-methylthiosemicarbazone (64Cu-ATSM), approved by the U.S. Food and Drug Administration (FDA) in the United States. So, DOTA chelator is better clinically available and has a simple synthetic process.764Cu can potentially be linked to target including peptide, antibody, or nanoparticle via specified chelator system.
Membrane anchoring methods using lipophilic long alkyl chain or hydrophobic oleyl group is widely used for tight anchoring with lipid bilayer of cell membrane.8−11 Hexadecyl benzoate (HB) has lipophilic property, so it easily and tightly anchors with cell membrane. Moreover, it is free from effects mediated by other receptors that exist in the cell. Ma et al. developed hexadecyl-4-18F-fluorobenzoate (18F-HFB), a long chain fluorinated benzoic acid ester, and labeled with rat mesenchymal stem cell with approximately 25% labeling efficiency. They reported that intravenously injected 18F-HFB-labeled mesenchymal stem cells showed persistent accumulation of radioactivity in the lungs by PET imaging.12 However, half-life of 18F (110 min) is too short to longitudinally monitor the transplanted stem cells in vivo. Here, we developed HB conjugated DOTA bifunctional chelator and labeled it with 64Cu (1), then labeled it with rat adipose-derived stem cells (ADSCs). Next, we evaluated the efficiency of longitudinal monitoring of transplanted 1-labeled ADSCs in rat normal heart.
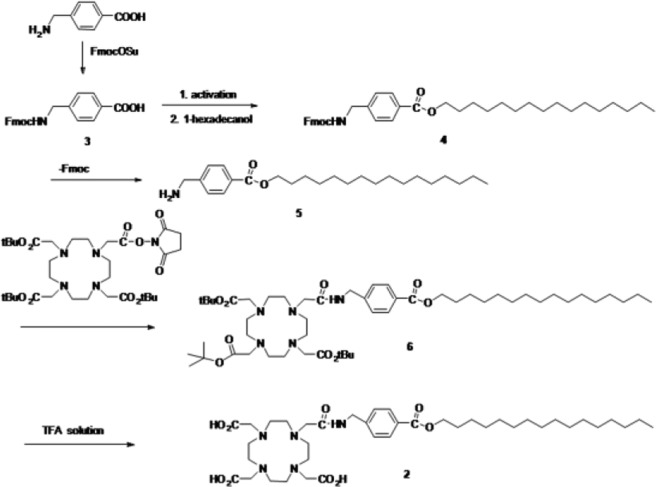
The precursor of 2 was prepared in five steps from p-amino methyl benzoic acid. Hexadecyl ester (5) was prepared in high yield by the condensation of 1-hexadecanol and triphenyl phosphite at 0 °C for 1 h. Compound 2 was prepared by conjugation of hexadecyl ester (5) and DOTA-mono-NHS-tris(tBu ester) using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and then hydrolysis using trifluoroacetic acid (TFA)/DCM/Et3SiH = 8:2:1 solution, giving the desired products with 59% yields (Scheme 1 and Supporting Information Figures 1S–6S).
Scheme 1. Synthesis of Precursor of 64Cu-Labeled DOTA-HB, 1.
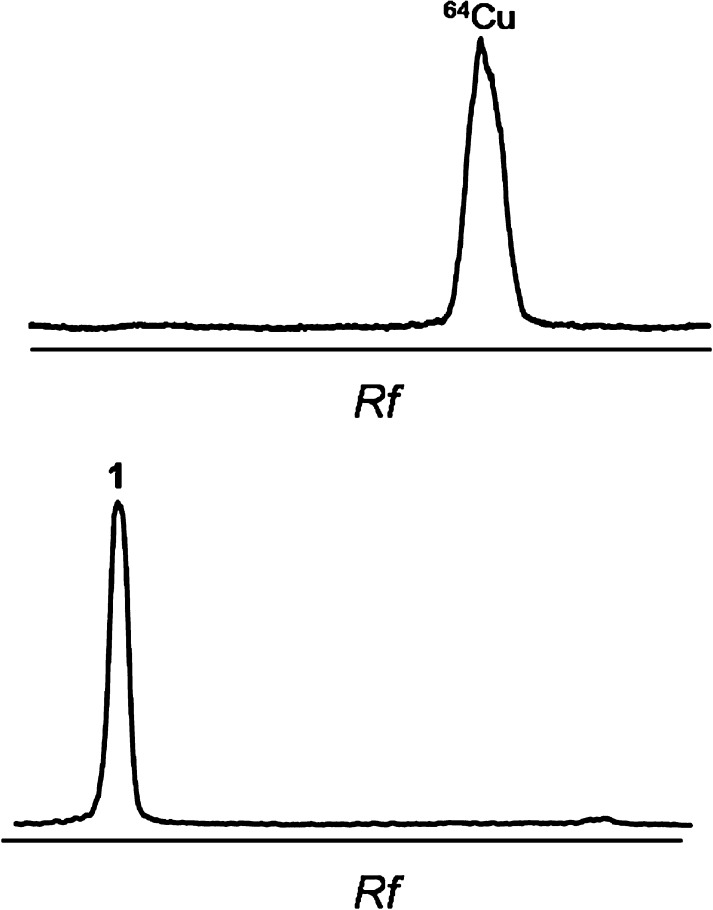
Scheme 2 shows the chemical structure of 64Cu-labeled DOTA-HB, 1. 64Cu and HB were conjugated via DOTA bifunctional chelator. The labeling yield of 2 with 64Cu was 99.7 ± 0.2% (Figure 1).
Scheme 2. Chemical Structure of 64Cu-labeled DOTA-HB, 1.
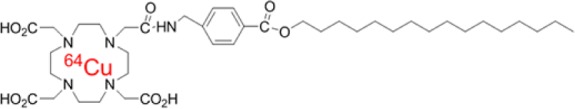
Figure 1.
Radio-TLC of 1. Rf = retention factor.
Isolated ADSCs highly expressed stem cell specific markers including CD44 (78.9%) and CD90 (82.5%), whereas expression levels of endothelial or hematopoietic cell markers including CD31 or CD45 were as low as 1.1% and 0.8%, respectively (Supporting Information Figure 7S).
Cell labeling efficiency was 25.7 ± 1.4%, 53.0 ± 10.2%, and 59.4 ± 1.2% at 0.5, 1, and 2 h, respectively (Figure 2A), which is higher than previously reported results using lipophilic cell labeling agent.12,13 Cell labeling efficiency of 1 was high; thus, only the low dose of 1 is sufficient for labeling a number of stem cells.
Figure 2.
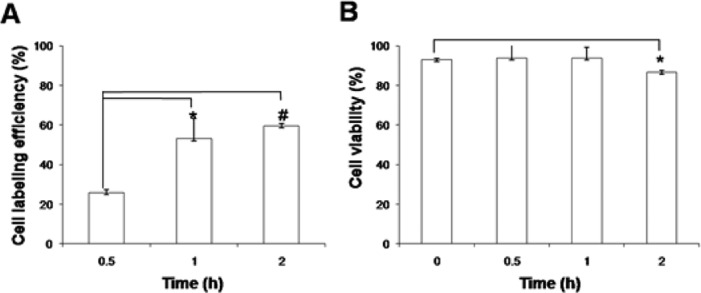
Cell labeling efficiency and cell viability with time. (A) Cell labeling efficiency of 1 with ADSCs for 2 h. (B) Cell viability of 1-labeled ADSCs for 2 h. Error bars denote the standard deviation (n = 3). *P < 0.05; #P < 0.001.
Cell viability of 1-labeled ADSCs was 92.9 ± 0.6, 93.8 ± 6.7, 93.7 ± 5.6, and 86.5 ± 0.9% at 0 min, 30 min, 1 h, and 2 h, respectively (Figure 2B). Adonai et al. reported that 64Cu labeled pyruvaldehyde-bis(N4-methylthiosemicarbazone) (64Cu-PTSM)-labeled C6 glioma cells showed 85 and 80% of cell viability at 35 and 270 min, respectively.3 Labeling with 1 showed slightly higher cell viability compared with 64Cu-PTSM,3 and it did not significantly affect the viability of ADSCs (>85%) up to 2 h (Figure 2B). We labeled with 1 for 1 h to obtain optimal cell viability.
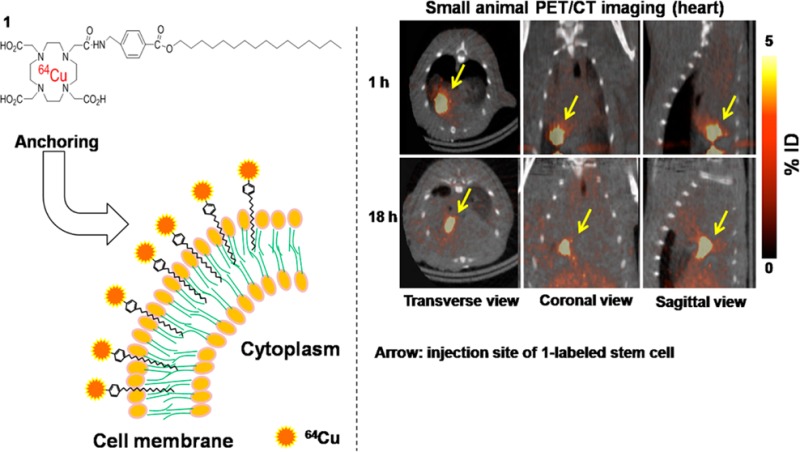
The in vivo distribution of ADSCs labeled with 1 was observed at 1 h postinjection by a small animal PET/computed tomography (CT) scanner. Radioactivity associated with ADSCs was deposited in the lungs by the injection of 1-labeled ADSCs, whereas in the group of 1 injection most radioactivity was accumulated in the liver, then excreted via bladder (Supporting Information Figure 8S). Intravenously injected 1-labeled ADSCs were efficiently trapped in the lungs, but intravenously injected labeling agent 1 did not react with cells in the mouse and was metabolized and excreted through liver and bladder. The high lung uptake of intravenously injected cells reported by many previous studies for investigation of in vivo stability in the cells labeled with radioactive agents is the same as the results in the current study.14−16 To confirm the accumulation of 1-labeled ADSCs in the lungs, digital autoradiography was performed at 1 h after cell injection. In the lungs, high radioactivity of ADSCs was observed; thus, this result showed that 1 was tightly anchored with cell membrane of ADSCs (Supporting Information Figure 9S).
Moreover, the intramuscularly transplanted 1-labeled ADSCs were longitudinally monitored for 18 h in normal rat heart by PET/CT imaging (Figure 3). Zhang et al. reported that hexadecyl-4-18F-fluorobenzoate (18F-HFB)-labeled human circulating progenitor cells were noninvasively monitored in a rat myocardial infarction model up to 4 h, but long-term monitoring is impossible due to the short half-life of 18F.13 However, in the current study, we used 64Cu, which has a relatively long half-life; thus, 1-labeled ADSCs were monitored in vivo up to 18 h. Adonai et al. reported that a lipophilic agent, 64Cu-PTSM, was rapidly effluxed from C6 rat glioma cells; radioactivity decreased by ∼80% at 24 h.3 Also, Park et al. reported that radioactivity of intravenously injected 64Cu-PTSM-labeled K562 (human erythromyeloblastoid leukemia cell line) cells rapidly decreased in the lungs from 2 to 24 h.17 In the current results, however, the intramuscularly injected 1-labeled ADSCs were clearly visualized at the injection site for 18 h.
Figure 3.
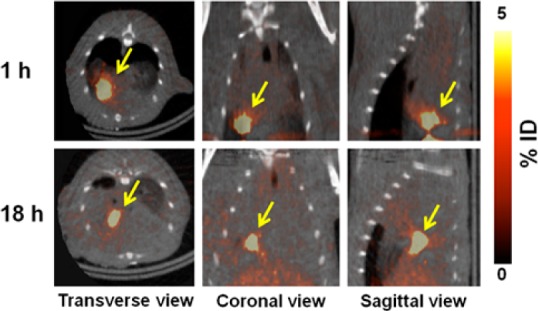
Small animal PET/CT image of intra-myocardially injected 1-labeled ADSCs (1.8–3.7 MBq, 100 μL) in normal heart for 18 h. Arrow indicates the site of 1-labeled ADSC injection.
Although we did not investigate the effect of 1-labeling on the functionality of stem cells, through our previous study a lipophilic labeling agent similar to 1, 124I-HIB, did not affect stem cell differentiation including osteogenic, chondrogenic, adipogenic, and cardiomyogenic lineage.18 Thus, labeling with 1 may not affect stem cell functionality.
In conclusion, we have designed and synthesized 1, a PET imaging agent, and evaluated the efficiency of monitoring transplanted stem cells in normal rat heart. Compound 1 was efficiently labeled with ADSCs, and 1-labeled ADSCs were successfully monitored in vivo by PET imaging. Cell labeling with 1 is a simple and suitable cell labeling agent for monitoring of in vivo distribution and deposition of transplanted stem cells.
Glossary
ABBREVIATIONS
- ADSCs
adipose-derived stem cells
- CT
computed tomography
- DOTA
1,4,7,10-tetraazacyclododecane-tetraacetic acid
- HB
hexadecyl benzoate
- PET
positron emission tomography
- TLC
thin layer chromatography
Supporting Information Available
Detailed experimental procedures including synthesis, ADSCs isolation, cell labeling with 1, small animal PET/CT imaging, and digital whole-body autoradiography. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was funded by the Nuclear R&D (grant code: NRF-2012M2A2A7013480 and 2011-0030162) and Basic Science Research (grant code: NRF-2012R1A1A2044945) Programs of NRF, funded by MEST-Republic of Korea.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang S. J.; Wu J. C. Comparison of imaging techniques for tracking cardiac stem cell therapy. J. Nucl. Med. 2007, 48, 1916–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton P. D.; Kung H. F. Small animal imaging with high resolution single photon emission tomography. Nucl. Med. Biol. 2003, 30, 889–895. [DOI] [PubMed] [Google Scholar]
- Adonai N.; Nguyen K. N.; Walsh J.; Iyer M.; Toyokuni T.; Phelps M. E.; McCarthy T.; McCarthy D. W.; Gambhir S. S. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 3030–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. J.; Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Zhang F.; Wang H.; Niu G.; Choi K. Y.; Swierczewska M.; Zhang G.; Gao H.; Wang Z.; Zhu L.; Choi H. S.; Lee S.; Chen X. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials 2013, 34, 1772–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D.; Desai A. V.; Ranganathan D.; Wheeler T. D.; Kenis P. J.; Reichert D. E. Microfluidic radiolabeling of biomolecules with PET radiometals. Nucl. Med. Biol. 2013, 40, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes A.; Prata M. I.; Geraldes C. F.; André J. P. Ga(III) chelates of amphiphilic DOTA-based ligands: synthetic route and in vitro and in vivo studies. Nucl. Med. Biol. 2011, 38, 363–370. [DOI] [PubMed] [Google Scholar]
- Carney C. E.; MacRenaris K. W.; Mastarone D. J.; Kasjanski D. R.; Hung A. H.; Meade T. J. Cell labeling via membrane-anchored lipophilic MR contrast agents. Bioconjugate Chem. 2014, 25, 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Pandya D. N.; Lee W.; Park J. W.; Kim Y. J.; Kwak W.; Ha Y. S.; Chang Y.; An G. I.; Yoo J. Vivid tumor imaging utilizing liposome-carried bimodal radiotracer. ACS Med. Chem. Lett. 2014, 5, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K.; Umezawa K.; Funeriu D. P.; Miyake M.; Miyake J.; Nagamune T. Immobilized culture of nonadherent cells on an oleyl poly(ethylene glycol) ether-modified surface. Biotechniques 2003, 35, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Kawamura R.; Mishima M.; Ryu S.; Arai Y.; Okose M.; Silberberg Y. R.; Rao S. R.; Nakamura C. Controlled cell adhesion using a biocompatible anchor for membrane-conjugated bovine serum albumin/bovine serum albumin mixed layer. Langmuir 2013, 29, 6429–6433. [DOI] [PubMed] [Google Scholar]
- Ma B.; Hankenson K. D.; Dennis J. E.; Caplan A. I.; Goldstein S. A.; Kilbourn M. R. A simple method for stem cell labeling with fluorine 18. Nucl. Med. Biol. 2005, 32, 701–705. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Dasilva J. N.; Hadizad T.; Thorn S.; Kuraitis D.; Renaud J. M.; Ahmadi A.; Kordos M.; Dekemp R. A.; Beanlands R. S.; Suuronen E. J.; Ruel M. 18F-FDG cell labeling may underestimate transplanted cell homing: more accurate, efficient, and stable cell labeling with hexadecyl-4-18F-fluorobenzoate for in vivo tracking of transplanted human progenitor cells by positron emission tomography. Cell Transplant. 2012, 21, 1821–1835. [DOI] [PubMed] [Google Scholar]
- Barbash I. M.; Chouraqui P.; Baron J.; Feinberg M. S.; Etzion S.; Tessone A.; Miller L.; Guetta E.; Zipori D.; Kedes L. H.; Kloner R. A.; Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [DOI] [PubMed] [Google Scholar]
- Allers C.; Sierralta W. D.; Neubauer S.; Rivera F.; Minguell J. J.; Conget P. A. Dynamics of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation 2004, 78, 503–508. [DOI] [PubMed] [Google Scholar]
- Ma B.; Hankenson K. D.; Dennis J. E.; Caplan A. I.; Goldstein S. A.; Kilbourn M. R. A simple method for stem cell labeling with fluorine 18. Nucl. Med. Biol. 2005, 32, 701–705. [DOI] [PubMed] [Google Scholar]
- Park J. J.; Lee T. S.; Son J. J.; Chun K. S.; Song I. H.; Park Y. S.; Kim K. I.; Lee Y. J.; Kang J. H. Comparison of cell-labeling methods with 124I-FIAU and 64Cu-PTSM for cell tracking using chronic myelogenous leukemia cells expressing HSV1-tk and firefly luciferase. Cancer Biother. Radiopharm. 2012, 27, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H.; Woo S. K.; Lee K. C.; An G. I.; Pandya D.; Park N. W.; Nahm S. S.; Eom K. D.; Kim K. I.; Lee T. S.; Kim C. W.; Kang J. H.; Yoo J.; Lee Y. J. Longitudinal monitoring adipose-derived stem cell survival by PET imaging hexadecyl-4-124I-iodobenzoate in rat myocardial infarction model. Biochem. Biophys. Res. Commun. 2014, 456, 13–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.