Abstract
Background:
Cisplatin (CP) is a chemotherapy drug, with the major side effect of nephrotoxicity. The level of endothelin-1 (ET-1) increases during nephrotoxicity, which is accompanied with vasoconstrictive properties. Bosentan (BOS) is a nonselective ET-1 receptor antagonist, having vasodilatory and anti-hypertension effects. The purpose of this study was to investigate the renoprotective effect of BOS against CP-induced nephrotoxicity in male and female rats.
Materials and Methods:
Male and female rats were divided into six groups; groups 1–3 and 4–6 were male and female rats, respectively. Animals in groups 1 and 4 were considered as negative control and groups 2 and 5 considered as positive control groups received BOS (30 mg/kg/day) alone and CP (2.5 mg/kg/day) alone, respectively, for 1-week. The animals in groups 3 and 6 were treated with both CP and BOS. Finally, serum parameters were measured, and the kidney tissue was subjected to staining to evaluate tissue damage.
Results:
The serum levels of blood urea nitrogen and creatinine, kidney tissue damage score and kidney weight elevated, and body weight significantly decreased in both CP alone and in CP plus BOS-treated groups when compared with the control groups (P < 0.05), while BOS did not ameliorate these parameters neither in males nor in females. No significant differences were observed in serum levels of nitrite and malondialdehyde between the groups, but kidney tissue level of nitrite decreased significantly in CP alone and CP plus BOS-treated groups (P < 0.05).
Conclusion:
Renoprotective effect of BOS, as ET-1 blocker, was not observed against CP-induced nephrotoxicity neither in male nor in female rats. This is while BOS promoted the severity of injuries in females.
Keywords: Bosentan, cisplatin, gender, nephrotoxicity, rat
INTRODUCTION
Cisplatin (CP) is one of most common anticancer drug in clinic, used for treatment of solid tumors.[1,2] However, it is accompanied with side effects such as hearing disturbance,[3] hepatotoxicity,[4] and testicular and renal toxicity.[5] CP accumulates in kidneys,[6] resulting in nephrotoxicity that involves inflammation,[7] oxidative stress, apoptosis, and necrosis in kidney tubules.[8] Nephrotoxicity causes elevation in serum levels of creatinine (Cr) and blood urea nitrogen (BUN).[9,10] The endothelin (ET) axis includes ET-1 which decreases glomerular filtration rate (GFR)[11] and renal blood flow,[12] ET-2, and ET-3 isoforms while ET-1 shows a pivotal role in regulation of vascular tone and has two receptors as ETA and ETB.[13] Both receptors have been identified on vascular smooth muscle cells and mediates vasoconstriction where the ETB receptor has been identified on endothelial cells and mediates vasodilatation.[14] Selective and nonselective antagonists of ET are commonly used in different diseases such as cardiovascular disorders,[15] acute renal failure,[16] and pulmonary hypertension.[17] Bosentan (BOS) was recognized as a dual ET receptor antagonist that is shown to act as a vasodilator and improve heart failure and reduce renal dysfunction in experimental models.[18] Gender difference in renal function related to renin angiotensin system is reported,[19,20] while the function of ET is reported to be gender related also.[21] It has been shown that females and males exhibit different responses against aminoglycosides,[22] and some studies have been investigated the effects of agents such as Vitamin E,[23] L-arginine,[24] and angiotensin II receptor type 1 blocker; losartan[25] against CP-induced nephrotoxicity in both genders. Based on the induction of renal expression of ET by CP[11] and the reports on gender related CP-induced nephrotoxicity,[26,27,28] we hypothesized that ETA antagonist may prevent CP-induced renal toxicity. To test this hypothesis, co-administration of CP and BOS in rats was performed, and the results related to renal function were compared with the control groups.
MATERIALS AND METHODS
Animals
A total of 40 adult male (weighing 205.7 ± 3.5 g) and female (weighing 190.1 ± 2.9 g) Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) were used in this study. The animals were housed under standard conditions with 12-h light/12-h dark cycle and had free access to water and food. Prior to the experiment, the protocols were confirmed to be in accordance with the guidelines of Animal Ethics Committee of Isfahan University of Medical Sciences.
Drugs
BOS, DMSO, and CP were purchased from Osvah Pharmaceutical Co. (OSVE) (Tehran, Iran), Merck (Germany), and EBEWE Pharma Ges.M.B.H (Australia), respectively.
Experimental protocol
Wistar rats were randomly assigned to six groups and were treated for 7 days. Groups 1 (male, n = 7) and 4 (female, n = 7) received BOS (30 mg/kg/day ip). Groups 2 (male, n = 7) and 5 (female, n = 7) received CP (2.5 mg/kg/day ip). Groups 3 (male, n = 5) and 6 (female, n = 7) received both BOS and CP. BOS was dissolved in DMSO and was injected 1-h before CP administration every day. At the end of day 7, the rats were anesthetized with chloral hydrate, and blood samples were taken by heart puncture. All animals were sacrificed, and the kidneys were immediately removed and weighed. The left kidney was kept in formalin 10% for pathological investigations. The right kidney was homogenized and centrifuged to measure biochemical parameters.
Measurements
The levels of serum Cr and BUN were measured using RA1000 auto-analyzer (Technicon, Ireland) and Pars Azmoon (Tehran, Iran) kits. The kidney and serum levels of nitrite were determined using an assay kit that involves the Griess reaction. Kidney tissue and serum levels of malondialdehyde (MDA) were measured by the manual method.[29]
Histopathological procedures
The removed kidney was fixed in 10% formalin solution and prepared for histopathological staining. Hematoxylin and eosin stain was applied to determine the kidney injury; including tubular dilation and swelling, and debris in the luminal cell with inflammation. We scored the damage as kidney tissue damage score (KTDS) in the range of 1–4 while zero was considered for normal tissue without damage.
Statistical analysis
The data are presented as mean ± standard error of the mean. ANOVA with least significant difference as post-hoc was applied to compare the groups in terms of quantitative parameters. The Student's t-test was applied to find the statistical difference between genders. The data for KTDS were compared between the groups using the Kruskal–Wallis and Mann–Whitney tests.
RESULTS
Effect of BOS on serum levels of BUN, Cr, kidney weight, and KTDS
The results indicated that the serum levels of BUN and Cr significantly increased in CP alone-treated (positive control group) and CP plus BOS-treated groups when compared with negative control group in both genders (P < 0.05). In addition, BOS did not ameliorate these parameters neither in males nor in females [Figure 1], although kidney weight (KW) and KTDS were significantly different between female and male rats (P < 0.05). The sample images of kidney tissues are demonstrated in Figure 2.
Figure 1.
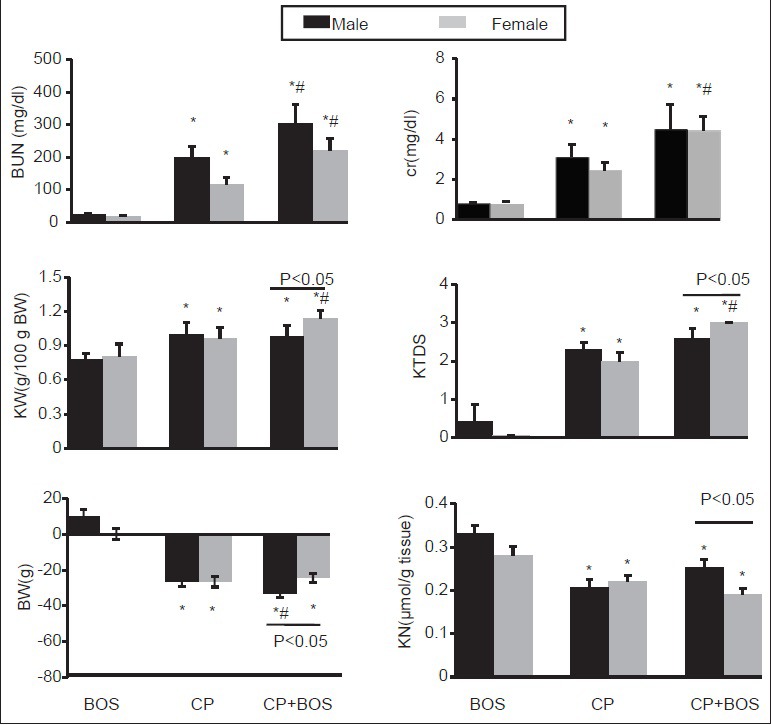
Comparison of the sham (treated with bosentan [BOS] alone), positive control (treated with cisplatin [CP] alone), and case (treated with BOS plus CP) groups in each gender with regard to the measured BUN, creatinine, kidney weight, bodyweight change, kidney tissue damage score, and kidney nitrite. Data are reported as mean ± standard error of mean *indicates significant difference from the sham group and #indicates significant difference from the positive control group, and -indicates significant difference between the case groups in the two genders. BUN: Blood urea nitrogen, Cr: Creatinine, KW: Kidney weight/100 g bodyweight, BW: Bodyweight change, KTDS: Kidney tissue damage score, KN: Kidney nitrite, BOS: Bosentan, and CP: Cisplatin
Figure 2.
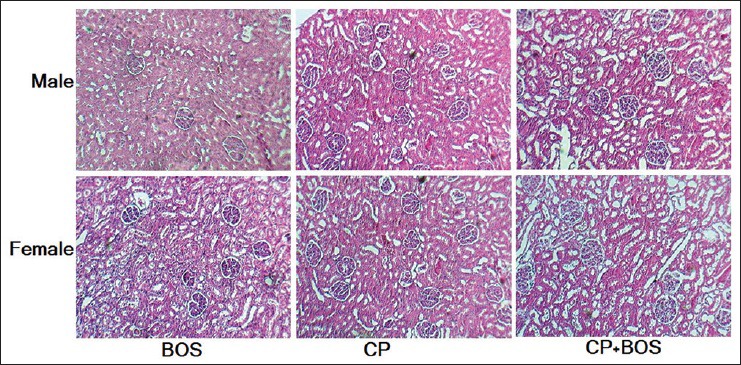
Sample image from kidney tissue of all experimental groups
Effect of BOS on serum and kidney tissue levels of MDA and nitrite.
No significant differences were observed in serum and kidney tissue levels of MDA and the serum level of nitrite between the groups while kidney tissue level of nitrite significantly decreased in CP alone and CP plus BOS-treated groups [Table 1].
Table 1.
Serum and kidney levels of nitrite and malondialdehyde (MDA) and testis weight and uterus weight in six experimental groups

Effect of BOS on testis and uterus weight.
The male groups were not significantly different with regard to the testis weight; however, the uterus weight in CP alone and CP plus BOS-treated groups decreased significantly (P < 0.05) [Table 1].
Effect of BOS on bodyweight
Bodyweight decreased significantly in CP alone and CP plus BOS-treated groups compared with the negative control in both genders (P < 0.05). However, this body weight loss was higher in male animals (P < 0.05) [Figure 1].
DISCUSSION
This study is intended to investigate the role of BOS on nephrotoxicity induced by CP in male and female rats. BOS did not show nephroprotective effect against CP, and kidney injury was greater in female than in male rats. CP alone increased serum levels of BUN and Cr, KTDS, and KW; and decreased bodyweight and kidney nitrite level in both males and females, which indicates induced nephrotoxicity. These findings are in agreement with other studies.[24,25,30,31,32,33,34] CP nephrotoxicity is related to apoptosis,[35] oxidative stress,[36] and accumulation of CP in the proximal tubule.[37] Increasing KW by CP may be related to kidney injury and changes in GFR[38] and consequently aggregation of water and salt in the renal tissue. Helmy et al. reported that co-administration of BOS and CP did not decrease the serum levels of BUN and Cr in male rats treated with CP[39] that is in agreement with our results. In the present study, administration of BOS accompanied with CP failed to decrease KW and KTDS in both genders. Some other studies illustrated that BOS could not protect these factors in renal injury. BOS itself may increase ET-1 level in blood,[40,41] and this may promote CP effects. Furthermore, our results showed that BOS exacerbated CP-induced nephrotoxicity in females. It was reported that administration of compounds such as L-arginine, erythropoietin, and losartan aggravate CP-induced injury in females.[24,25,42] It seems that female sex hormone plays an important role in nephrotoxicity induced by CP. Estrogen itself promote CP-induced nephrotoxicity in ovariectomized female rats.[43] Furthermore, the presence of estrogen reversed renoprotective effects of Vitamins C and E and losartan against CP-induced nephrotoxicity in ovariectomized female rats.[25] Probably in our study presence of gonad hormones influenced BOS effect on CP-induced nephrotoxicity. Furthermore, ETB receptor expression in males is higher than that in female,[44] and accordingly the vasodilatory aspect of ETB receptor.[45] It seems that decrement of injury in males is due to this gender difference of ETB receptor. The results of this study also showed that CP decreased kidney nitrite level. Nitric oxide (NO) is synthesized in the vasculature by three isoforms of NO synthases (NOS); namely, neuronal NOS, endothelial NOS (eNOS), and inducible NOS.[46] Administration of CP decreases expression of eNOS in the outer medulla of the kidney.[47] In addition, the plasma level of ET-1 was increased by CP,[11] and ET-1 impairs the production of NO through inhibition of eNOS synthase.[48] Therefore, we may conclude that nitrite renal level was decreased in both genders probably by elevating ET-1 and reducing eNOS following CP administration. CP decreased uterus weight probably via apoptosis and necrosis.[49,50] We observed that CP decreased bodyweight in both males and females, which is in agreement with the findings of previous studies.[9,25,51,52] Body weight loss is induced by CP due to gastrointestinal problems such as diarrhea and appetite loss.[51] BOS could not recover body weight loss in both genders; however, the damage was greater in male compared with female rats. It was reported that treatment with BOS could not ameliorate body weight loss in diabetic rats.[53] Accordingly, the patients received BOS exhibited the same clinical problems including skin ulcers, fatigue, upper respiratory tract infection, cough, arthralgia, peripheral edema, diarrhea, bronchitis, sinusitis, and anemia.[54] Therefore, it is possible that side effects of BOS such as gastrointestinal problems; especially diarrhea affect the body weight loss in our study. ET-1 also mediated water and sodium excretion through ETB-receptors.[55] Karet et al. reported that ETB receptor is available in the kidney.[44] Since BOS has a 20-30 folds higher potency for the ETA-receptor than for the ETB-receptor,[40] the greater weight loss in males is probably due to the BOS action on ETB receptors that results in increased diuresis. In summary, BOS could not protect renal tissue against nephrotoxicity in males and females; however, this damage was greater in female rats.
ACKNOWLEDGMENTS
This research was supported by Isfahan University of Medical Sciences.
Footnotes
Source of Support: Supported by Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Hartmann JT, Fels LM, Knop S, Stolt H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide–based combination chemotherapy with or without amifostine in patients with solid tumors. Invest New Drugs. 2000;18:281–9. doi: 10.1023/a:1006490226104. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann JT, Knop S, Fels LM, van Vangerow A, Stolte H, Kanz L, et al. The use of reduced doses of amifostine to ameliorate nephrotoxicity of cisplatin/ifosfamide-based chemotherapy in patients with solid tumors. Anticancer Drugs. 2000;11:1–6. doi: 10.1097/00001813-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Blakley BW, Gupta AK, Myers SF, Schwan S. Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg. 1994;120:541–6. doi: 10.1001/archotol.1994.01880290051009. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998;149:24–31. doi: 10.1006/taap.1997.8325. [DOI] [PubMed] [Google Scholar]
- 5.Nematbakhsh M, Ashrafi F, Pezeshki Z, Fatahi Z, Kianpoor F, Sanei MH, et al. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: Which one is the first observation as side effect of Cisplatin-induced toxicity in animal model? J Nephropathol. 2012;1:190–3. doi: 10.5812/nephropathol.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safirstein R, Miller P, Guttenplan JB. Uptake and metabolism of cisplatin by rat kidney. Kidney Int. 1984;25:753–8. doi: 10.1038/ki.1984.86. [DOI] [PubMed] [Google Scholar]
- 7.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos NA, Catão CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 9.Soltani N, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Effect of oral administration of magnesium on Cisplatin-induced nephrotoxicity in normal and streptozocin-induced diabetic rats. Nephrourol Mon. 2013;5:884–90. doi: 10.5812/numonthly.11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashrafi F, Haghshenas S, Nematbakhsh M, Nasri H, Talebi A, Eshraghi-Jazi F, et al. The role of magnesium supplementation in cisplatin-induced nephrotoxicity in a rat model: No nephroprotectant effect. Int J Prev Med. 2012;3:637–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Ahn D. Expression of endothelin-1 and its receptors in Cisplatin-induced acute renal failure in mice. Korean J Physiol Pharmacol. 2008;12:149–53. doi: 10.4196/kjpp.2008.12.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winston JA, Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985;249:F490–6. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- 13.Bagnato A, Spinella F, Rosanò L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–72. doi: 10.1677/erc.1.01077. [DOI] [PubMed] [Google Scholar]
- 14.Ohlstein EH, Elliott JD, Feuerstein GZ, Ruffolo RR., Jr Endothelin receptors: Receptor classification, novel receptor antagonists, and potential therapeutic targets. Med Res Rev. 1996;16:365–90. doi: 10.1002/(SICI)1098-1128(199607)16:4<365::AID-MED4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2002;3:1–15. doi: 10.3317/jraas.2002.001. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka M, Kuro T, Matsumura Y. Role of endothelin in the pathogenesis of acute renal failure. Drug News Perspect. 2000;13:141–6. doi: 10.1358/dnp.2000.13.3.858437. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman A, Grossman E, Goldstein DS, Gill JR, Jr, Keiser HR. Urinary excretion rate of endothelin-1 in patients with essential hypertension and salt sensitivity. Kidney Int. 1994;45:556–60. doi: 10.1038/ki.1994.72. [DOI] [PubMed] [Google Scholar]
- 18.Roux S, Breu V, Ertel SI, Clozel M. Endothelin antagonism with bosentan: A review of potential applications. J Mol Med (Berl) 1999;77:364–76. doi: 10.1007/s001090050363. [DOI] [PubMed] [Google Scholar]
- 19.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 2012;206:150–6. doi: 10.1111/j.1748-1716.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 20.Safari T, Nematbakhsh M. Angiotensin 1-7 receptor and angiotensin II receptor 2 blockades prevent the increased serum and kidney nitric oxide levels in response to angiotensin II administration: Gender-related difference. Int J Prev Med. 2013;4:311–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285:511–7. [PubMed] [Google Scholar]
- 22.Sweileh WM. Gender differences in aminoglycoside induced nephrotoxicity: A prospective, hospital-based study. Curr Clin Pharmacol. 2009;4:229–32. doi: 10.2174/157488409789375339. [DOI] [PubMed] [Google Scholar]
- 23.Jilanchi S, Nematbakhsh M, Bahadorani M, Talebi A, Eshraghi-Jazi F, Mansouri A, et al. Vitamin E is a nephroprotectant agent in male but not in female in a model of Cisplatin-induced nephrotoxicity. ISRN Nephrol 2013. 2013 doi: 10.5402/2013/280395. 280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 25.Nematbakhsh M, Pezeshki Z, Eshraghi-Jazi F, Ashrafi F, Nasri H, Talebi A, et al. Vitamin E, Vitamin C, or losartan is not nephroprotectant against Cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol 2012. 2012 doi: 10.1155/2012/284896. 284896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nematbakhsh M, Nasri H. Cisplatin nephrotoxicity may be sex related. Kidney Int. 2013;83:1201. doi: 10.1038/ki.2013.37. [DOI] [PubMed] [Google Scholar]
- 28.Nematbakhsh M, Talebi A, Nasri H, Safari T, Dolatkhah S, Ashrafi F. Some evidence for sex-based differences in cisplatin-induced nephrotoxicity in rats. Clin Exp Med Lett. 2012;53:29–32. [Google Scholar]
- 29.Moeini M, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Azarkish F, et al. Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model. Int J Prev Med. 2013;4:648–55. [PMC free article] [PubMed] [Google Scholar]
- 30.Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, et al. Effects of fennel essential oil on Cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol Int. 2013;20:138–45. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad AA, Youssef MI, El-Shennawy LK. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47:1499–506. doi: 10.1016/j.fct.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 32.Baldew GS, McVie JG, van der Valk MA, Los G, de Goeij JJ, Vermeulen NP. Selective reduction of cis-diamminedichloroplatinum (II) nephrotoxicity by ebselen. Cancer Res. 1990;50:7031–6. [PubMed] [Google Scholar]
- 33.Mohamed HE, El-Swefy SE, Mohamed RH, Ghanim AM. Effect of erythropoietin therapy on the progression of cisplatin induced renal injury in rats. Exp Toxicol Pathol. 2013;65:197–203. doi: 10.1016/j.etp.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Joy J, Nair CK. Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J Cancer Res Ther. 2008;4:111–5. doi: 10.4103/0973-1482.43139. [DOI] [PubMed] [Google Scholar]
- 35.Lau AH. Apoptosis induced by cisplatin nephrotoxic injury. Kidney Int. 1999;56:1295–8. doi: 10.1046/j.1523-1755.1999.00687.x. [DOI] [PubMed] [Google Scholar]
- 36.Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates Cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–61. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- 37.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: Apoptosis vs. necrosis. Am J Physiol. 1996;270:F700–8. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 38.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334:115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 39.Helmy MM, Helmy MW, Abd Allah DM, Abo Zaid AM, Mohy El-Din MM. Selective ET (A) receptor blockade protects against cisplatin-induced acute renal failure in male rats. Eur J Pharmacol. 2014;730:133–9. doi: 10.1016/j.ejphar.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Clozel M, Breu V, Gray GA, Kalina B, Löffler BM, Burri K, et al. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994;270:228–35. [PubMed] [Google Scholar]
- 41.Weitzberg E, Hemsén A, Rudehill A, Modin A, Wanecek M, Lundberg JM. Bosentan-improved cardiopulmonary vascular performance and increased plasma levels of endothelin-1 in porcine endotoxin shock. Br J Pharmacol. 1996;118:617–26. doi: 10.1111/j.1476-5381.1996.tb15446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Estrogen abolishes protective effect of erythropoietin against Cisplatin-Induced nephrotoxicity in ovariectomized rats. ISRN Oncol 2012. 2012 doi: 10.5402/2012/890310. 890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karet FE, Kuc RE, Davenport AP. Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney Int. 1993;44:36–42. doi: 10.1038/ki.1993.210. [DOI] [PubMed] [Google Scholar]
- 45.Resta TC, Walker BR. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am J Physiol. 1996;270:H888–96. doi: 10.1152/ajpheart.1996.270.3.H888. [DOI] [PubMed] [Google Scholar]
- 46.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. 837a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae EH, Lee J, Ma SK, Kim IJ, Frøkiaer J, Nielsen S, et al. Alpha-Lipoic acid prevents cisplatin-induced acute kidney injury in rats. Nephrol Dial Transplant. 2009;24:2692–700. doi: 10.1093/ndt/gfp176. [DOI] [PubMed] [Google Scholar]
- 48.Ramzy D, Rao V, Tumiati LC, Xu N, Sheshgiri R, Miriuka S, et al. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation. 2006;114:I319–26. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- 49.Nozaki Y, Furubo E, Matsuno T, Fukui R, Kizawa K, Kozaki T, et al. Collaborative work on evaluation of ovarian toxicity 6) Two- or four-week repeated-dose studies and fertility study of cisplatin in female rats. J Toxicol Sci. 2009;34(Suppl 1):SP73–81. doi: 10.2131/jts.34.s73. [DOI] [PubMed] [Google Scholar]
- 50.Harima Y, Harima K, Hasegawa T, Shikata N, Tanaka Y. Histopathological changes in rabbit uterus carcinoma after transcatheter arterial embolization using cisplatin. Cancer Chemother Pharmacol. 1996;38:317–22. doi: 10.1007/s002800050489. [DOI] [PubMed] [Google Scholar]
- 51.Moslemi F, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Nasri H, Ashrafi F, et al. Inhibition of nitric oxide synthase by L-name promotes cisplatin-induced nephrotoxicity in male rats. ISRN Toxicol 2013. 2013 doi: 10.1155/2013/242345. 242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastghalam R, Nematbakhsh M, Bahadorani M, Eshraghi-Jazi F, Talebi A, Moeini M, et al. Angiotensin type-1 receptor blockade may not protect kidney against Cisplatin-induced nephrotoxicity in rats. ISRN Nephrol 2014. 2014 doi: 10.1155/2014/479645. 479645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens EJ, Tomlinson DR. Effects of endothelin receptor antagonism with bosentan on peripheral nerve function in experimental diabetes. Br J Pharmacol. 1995;115:373–9. doi: 10.1111/j.1476-5381.1995.tb15888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seibold JR, Denton CP, Furst DE, Guillevin L, Rubin LJ, Wells A, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum. 2010;62:2101–8. doi: 10.1002/art.27466. [DOI] [PubMed] [Google Scholar]
- 55.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294:F1205–11. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


