Abstract
Leptin is an endocrine hormone synthesized by adipocytes. It plays a key role in the energy homeostasis in central and peripheral tissues and has additional roles are attributed to it, such as the regulation of reproduction, immune function, bone homeostasis, and angiogenesis. The plasma concentration of leptin significantly increases in obese individuals. In the present review, we give an introduction concerning leptin, its receptors, signaling pathways, and its effect on cardiovascular system, especially on angiogenesis.
Keywords: Angiogenesis, cardiovascular disease, leptin, leptin resistance
INTRODUCTION
Leptin is an adipocyte-derived hormone discovered in 1994 which created dramatic interest in the field of white adipose tissue (WAT) research and related diseases such as hypertension, coronary atherosclerosis, myocardial hypertrophy, diabetes, and dyslipidemia.[1,2] Leptin mirrors the body's fat stores and acts for maintenance of energy homeostasis in central and peripheral tissues.[3] Also, it is involved in the regulation of other physiological processes such as reproduction, bone homeostasis, and immune function.[4]
Leptin acts on target cells by binding to plasma membrane receptors. Six isoforms of ob-R (a–f) have been identified based on different lengths of the intracellular domains.[5] These isoforms are classified into three groups: long form (ob-Rb), short form (ob-Ra, c, d, f) (these forms represent the dominant isoforms in the heart and most of the biological effects of leptin are mediated by ob-Rb isoform), and the third isoform is secretory form (ob-Re).[6,7] The actions of leptin are mediated via ob-R modulation of the janus-activated kinase/signal transducers and activators of transcription (Jak/STAT) as the main signaling pathway in addition to phosphatidylinositol 3-kinase (PI-3K) and mitogen-activated protein kinase (MAPK) signaling pathways.[8]
Endothelium plays a crucial role in modulating both vascular health and tone by producing both vasodilating and vasoconstricting substances.[9] Leptin increases vasodilation and blood perfusion in the adipose tissue through induction of endothelial nitric oxide synthase (eNOS) activity in the endothelial cells and smooth muscle cells. This may be essential to supply of growing adipose tissue in obesity that is associated with high leptin levels.[10,11]
Interactions of leptin with some hormones and neuropeptides in some studies have been demonstrated. Leptin interacts with neuropeptides present in central nervous system (CNS) in different ways. It suppresses neuropeptide Y (NPY) neurons in arcuate nucleus of hypothalamus and inhibits orexigenic peptide, the Aguti regulated peptide (AgRP); it also stimulates the neurons of alpha-melanocyte stimulating hormone (α-MSH) and cocaine-amphetamine regulated transcripts.[12] In addition, leptin interacts with hormone regulators of energy metabolism, including insulin.[13] Current studies show that basal plasma leptin and insulin concentrations parallel each other as leptin resistance (a state of decreased sensitivity to leptin action or, in other words, despite increased leptin level, there is inadequate response) can lead to insulin resistance and diabetes.[14,15] In one study, leptin treatment could reduce plasma insulin concentration in children with congenital leptin deficiency.[16] Also, several studies have demonstrated the inhibitory effect of leptin at supraphysiological concentration on glucose-stimulated insulin secretion in vitro both in rodent and human isolated pancreatic islets.[17,18,19] Meanwhile, at a gene expression level, leptin reduces preproinsulin mRNA in pancreatic beta cells.[20]
Leptin concentration is important for puberty spurt initiation and normal reproductive life through stimulation of gonadotropin releasing hormone (GnRH) and, consequently, follicle stimulating hormone (FSH) and luteinizing hormone (LH).[21] Study on leptin (ob/ob) or leptin receptor (db/db) with knock-out mice reveals low gonadotropin concentration and incomplete development of reproductive organs.[22] Both ovaries and testes express leptin receptors. Thus, in obesity with high leptin levels, sex hormone steriodogenesis is reduced.[23]
ROLE OF LEPTIN IN CARDIOVASCULAR DISEASES
Extensive investigation in the general population and animal studies focusing on beneficial or detrimental effects of leptin on cardiovascular function including hypertension, diabetes, atherosclerosis, and coronary heart disease have shown paradoxical evidences. For example, leptin can lead to obesity associated with hypertension through central sympathetic activation,[24] whereas several in vitro studies demonstrated an activation of endothelial nitric oxide in human aortic endothelial cells,[25] besides the endothelium-independent vasodilator effects of leptin in saphenous vein and internal mammary artery vascular ring of coronary artery disease (CAD) patients.[26]
Also, acute hyperleptinemia through an increased expression of peroxisome proliferator activated receptor alpha (PPAR-α) following fatty acid oxidation and down-regulation of lipogenesis is protective in the heart and other tissues.[27] On the contrary, long-term exposure to hyperleptinemia decreases the fatty acid oxidation and increases its cellular uptake, and therefore can lead to fatty acid loading in cardiomyocytes and programmed cell death or lipoapoptosis.[28,29]
On the other hand, a community-based Framingham Heart Study showed cardioprotective influence of leptin on left ventricular (LV) remodeling; also, leptin concentrations were inversely associated with LV mass, LV wall thickness, and left atrial size.[30] In a murine model, leptin at levels of 10 nM in obese humans reduced the infarct size via p38 MAPK pathway.[31] In addition, leptin may protect the heart in an autocrine manner against ischemia/reperfusion (I/R) injury by delaying mitochondrial permeability transition pore (MPTP) opening.[32]
This apparent discrepancy between the protective actions and impaired cardiovascular outcome of leptin in many studies results in a broad spectrum of cardiovascular effects of leptin and dose-dependent and time-course effects of leptin.
Several studies have shown that hyperleptinemia is associated with cardiovascular diseases such as hypertension, diabetes, atherosclerosis, and coronary heart disease.[33]
Clinical and animal studies have shown a strong relationship between obesity and hypertension. Obesity is associated with chronic low-grade inflammatory condition, and serum leptin levels increase in obesity and correlate with the body mass index (BMI).[3,34,35] Leptin can be involved in hypertension [Table 1] through an increase in the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and reactive oxygen species (ROS) in the endothelial cells and induction of endothelial dysfunction by reducing the bioavailability of NO in the endothelial cells.[37,38]
Table 1.
Effect of leptin on cardiovascular diseases
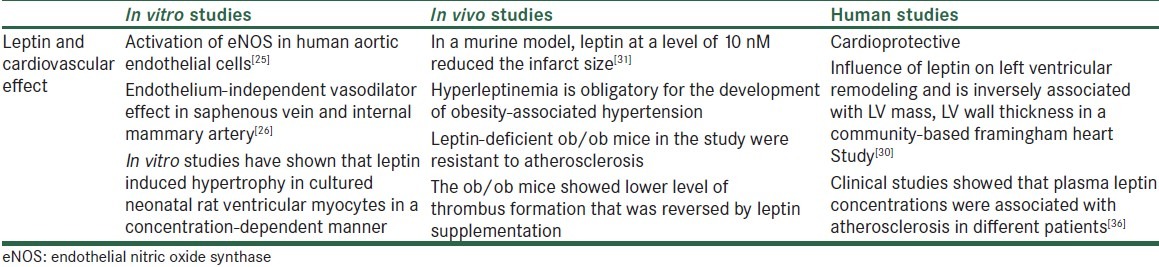
Leptin facilitates thrombosis formation and atherosclerosis in obesity through mechanisms including stimulation of hypertrophy, and proliferation, migration, and calcification of vascular smooth muscle cells (VSMCs). Also, its angiogenic effects on endothelial cell proliferation and remodeling and endothelial dysfunction[39] can result in increased oxidative stress and decrease in NO bioavailability.[40,41] In an in vivo study, leptin-deficient ob/ob mice were resistant to atherosclerosis. Also, in human studies, plasma leptin concentrations were associated with atherosclerosis in different patients.[36,42] Another in vivo study in ob/ob mice showed lower level of thrombus formation that was reversed by leptin supplementation.[43]
Also, via endothelin-1 (ET-1) and ROS generation, leptin can participate in cardiomyocyte hypertrophy, as in a study, the hypertrophic effect of ET-1 was found to be inhibited by antibodies to either leptin or leptin receptors.[44] In addition, in vitro studies have shown that leptin induced hypertrophy in cultured neonatal rat ventricular myocytes in a concentration-dependent manner.[45,46]
LEPTIN AND ANGIOGENESIS
Angiogenesis is defined as a biological mechanism of new blood vessel formation from preexisting ones and plays important roles in many physiological and pathological conditions such as wound healing, menstrual cycles, placenta and mammary gland growth during pregnancy, tumor development, cardiovascular diseases, arthritis, retinopathies, and atherosclerosis.[47,48] Angiogenesis includes coordinated events such as extracellular matrix degradation, migration and proliferation of the endothelial cells and mural cells to assemble the new vessel, and lumen formation and construction of the vessel wall via the mural cell layer which is associated with pericytes and/or smooth muscle cells.[49]
Several studies have shown that leptin can be a potent angiogenic factor or angiogenesis inducer. Leptin was found to induce angiogenesis in two in vivo angiogenesis assays of cornea pocket and chick chorioallantoic membrane (CAM).[50] Also, another study using two different in vitro models of angiogenesis (endothelial cell–coated microcarrier-induced and monolayer-induced formation of capillary-like tubes in fibrin gels) has demonstrated that leptin-induced proliferation and/or survival and 3D matrix formation of capillary-like tubes was similar with that elicited by vascular endothelial growth factor (VEGF) 165. Whereas VEGF165 is considered as a major proangiogenic factor thus support from leptin as an endothelial growth factor.[51]
Leptin and VEGF induce similar increase in vascular permeability in mice and vascular fenestrations in cornea pocket vessels, as leptin may potentiate VEGF-mediated angiogenesis dose-dependently because of increased endothelial cell VEGF secretion.[52,53] In CAM assay, leptin neovascularization in developing embryo was impaired by inhibition of fibroblast growth factor 2) FGF2 (function, suggesting that FGF2 signaling pathways in endothelial cells are necessary for leptin angiogenesis.[54] Meanwhile, leptin indirectly augments angiogenesis through induction of matrix metalloproteinase-2 (MMP-2) and MMP-9 activity.[53]
In a study, tissue burn wound was created in male rats by electrocautery and then, the burn wounds were treated with leptin recombinant. In those animals that received leptin, there was greater number of total blood vessels in the subcutaneous tissue of the wound and leptin could mitigate cellular response of burn injury by augmenting the production of nutrient blood vessels; this supports the role of leptin in wound healing via angiogenesis process.[55]
On the other hand, through induction of eNOS activity, leptin regulates vasodilatation and vascular permeability in the adipose tissue. Garonna et al. showed that leptin promotes proliferation, directional migration, and differentiation of endothelial cells through increase in cyclooxygenase-2 (COX-2) activity and causes rapid VEGFR2 phosphorylation upstream P38 MAPK/AKT/COX-2 which is needed for leptin-stimulated neoangiogenesis in vivo, and MAPK including P38 MAPK regulates COX-2 expression in endothelial cells treated with physiological and pathological stimuli.[56]
It is demonstrated that both short and long isoform receptors of leptin which exist in endometrial cancer cells can potently enhance endometrial cancer growth and invasiveness through JAK/STAT and AKT pathways. Also, in the same study it was demonstrated that leptin potently induced the invasion of endometrial cancer cells in a matrigel invasion assay.[57] In addition, leptin is a mammary tumor growth promoting factor causing increase in cell number and the expression of VEGF/VEGFR2.[58]
Although there is growing evidence for the angiogenic effects of leptin, on the contrary, some studies have indicated the antiangiogenic properties of leptin. It was found in a study that leptin, as an inflammatory cytokine and antiangiogenic protein, increased 2 months earlier than the appearance of symptoms of preeclampsia in amniotic fluid, and could possibly be considered as a predictive biomarker for preeclampsia.[59] Also, there was inverse correlation between plasma levels of leptin and prostate cancer weight, and leptin appeared as a cellular proliferation and angiogenesis repressor in androgen-insensitive cells RM1 in vivo.[60]
A recent study has reported that despite several strong evidences regarding the angiogenic effects of leptin in vitro and in animal studies, metreleptin administration in pharmacological (0.3 mg/kg) or physiological (0.1 mg/kg) dose in vivo to 15 healthy normoleptinemic volunteers and (50 and 100 ng/ml) in vitro in a three-dimensional human umbilical vein endothelial cell (HUVEC) angiogenesis model did not regulate circulating angiogenic factors in humans[61] [Table 2]. It seems further studies are required to evaluate the mechanisms underlying the broad-spectrum effects of leptin as an angiogenic and/or antiangiogenic factor. The underlying mechanisms by which leptin induces angiogenesis in physiological and pathological conditions have been illustrated in Figure 1.
Table 2.
Effect of leptin on angiogenesis including in vivo, in vivo, and human studies

Figure 1.
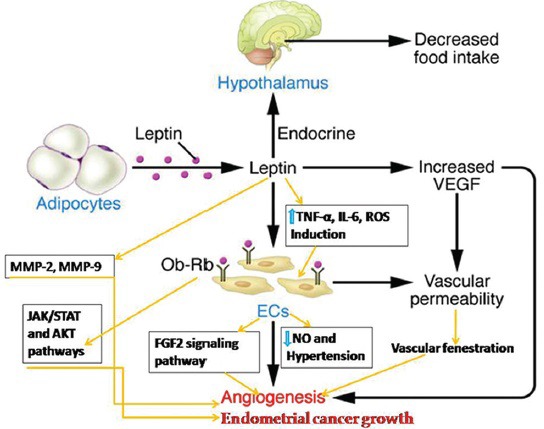
Underlying mechanisms by which leptin induces angiogenesis in physiological and pathological conditions[62]
LEPTIN RESISTANCE
Leptin resistance means reduced ability of leptin to suppress appetite and control weight gain, which can be considered as an important risk factor for the development of obesity.[63] Although the exact mechanisms of leptin resistance are still unclear, however, there are many factors underlying the molecular mechanism leading to this phenomenon.
Leptin resistance can be inherited. In other words, ob gene mutation produces leptin whose ineffective signaling leads to hyperleptinemia and leptin resistance. Also, leptin receptor mutation can be created in diabetic db/db mice and Zucker fatty (fa/fa) rats. Of course, genetic mutation in humans is uncommon in typical obese population.[64,65]
Leptin, like other biological signaling pathways, regulates its own receptor and signaling. Receptor down-regulation can be involved in leptin resistance[66] that is observed in rodent models of diet-induced obesity (DIO).[67] Saturation in transport mechanism of entry into CNS by limited tissue access can lead to leptin resistance.[68]
Intracellular suppressor of cytokine signaling 3 (SOCS3) by inhibiting leptin JAK/STAT signaling, together with protein tyrosine phosphatase 1B (PTP1B) and cytokine-inducible SH2 protein can be candidate components of the intracellular leptin negative feedback loop.[69,70,71] It is indicated that PTP1B, via dephosphorylation of jak2, results in diminished LRb (leptin receptor) signaling, which is involved in endoplasmic reticulum (ER) stress that induces leptin resistance.[63] Stress signals lead to accumulation of unfolded proteins, consequently impairing ER function and causing ER stress. Homocysteine, a product of demethylation of methionine, is increased in obese patients and is positively associated with the serum leptin levels. High levels of plasma homocysteine may cause leptin resistance through ER stress.[72,73]
Several studies have demonstrated that DIO animals are leptin resistant through neural mechanisms such as decreased anorectic response or diminished amplitude of maximal LRb signaling in the hypothalamus in response to leptin treatment by reduction in STAT3 phosphorylation.[74,75] Cellular leptin resistance was prominently detected in the arcuate nucleus of hypothalamus of DIO animals because of increased access of leptin from the circulation to the arcuate nucleus.[76,77] Obesogenic effects of tasty foods, due to their nutrient content besides the rewarding properties of these foods in DIO animals, can be involved in cellular leptin resistance, which is reversed by replacing them with the standard chow.[74,78] Leptin may control food reward in consequence of its interaction with the mesolimbic dopamine system and the core of this system lies in a set of dopamine neurons in the ventral tegmental area (VTA).[79]
Extracellular circulating factors including five serum leptin interacting proteins (SLIPs) in human blood were introduced by Chen et al., which alter leptin bioavailability and bioactivity.[75] SLIP-1 is identified as C-reactive protein (CRP) and SLIP-2 as APOJ or clusterin.[80,81] SLIPs 3–5 have not been characterized. In a study in ob/ob mice, increased human CRP in continuous infusion mitigated the physiological actions of leptin administration on food intake, body weight, blood glucose and leptin metabolism.[80]
CONCLUSIONS
Leptin as an adipocyte-derived hormone primarily acts in the hypothalamus and plays a crucial role in the regulation of food intake, body weight, and energy expenditure. Changes in leptin concentration are involved in several pathological conditions including cardiovascular diseases, obesity, immune and reproductive disorders, and recently, it has been identified as a potent angiogenic factor. With regard to the paradoxical and complex effects of leptin on angiogenesis, understanding these results requires further investigations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Butler KR, Buxbaum SG, Sung JH, Campbell BW, Taylor HA. Leptinemia and its association with stroke and coronary heart disease in the Jackson Heart Study. Clin Endocrinol (Oxf) 2010;72:32–7. doi: 10.1111/j.1365-2265.2009.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905–13. doi: 10.1111/j.1440-1681.2011.05619.x. [DOI] [PubMed] [Google Scholar]
- 4.Wauman J, Tavernier J. Leptin receptor signaling: Pathways to leptin resistance. Front Biosci (Landmark Ed) 2011;16:2771–93. doi: 10.2741/3885. [DOI] [PubMed] [Google Scholar]
- 5.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Barouch LA. Leptin signaling and obesity: Cardiovascular consequences. Circ Res. 2007;101:545–59. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 8.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 9.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, et al. Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Tigno XT, Selaru IK, Angeloni SV, Hansen BC. Is microvascular flow rate related to ghrelin, leptin and adiponectin levels? Clin Hemorheol Microcirc. 2003;29:409–16. [PubMed] [Google Scholar]
- 11.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult Microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 12.Petzel M. Action of leptin on bone and its relationship to menopause. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:195–9. doi: 10.5507/bp.2007.034. [DOI] [PubMed] [Google Scholar]
- 13.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 14.Ahrén B, Larsson H, Wilhelmsson C, Näsman B, Olsson T. Regulation of circulating leptin in humans. Endocrine. 1997;7:1–8. doi: 10.1007/BF02778056. [DOI] [PubMed] [Google Scholar]
- 15.Yildiz BO, Haznedaroglu IC. Rethinking leptin and insulin action: Therapeutic Opportunities for diabetes. Int J Biochem Cell Biol. 2006;38:820–30. doi: 10.1016/j.biocel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, et al. Beneficial effects of leptin on obesity, T cell hyporesponsivness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrén B, Havel PJ. Leptin inhibits insulin secretion induced by cellular cAMP in a pancreatic B cell line (INS-1 cells) Am J Physiol. 1999;277:R959–66. doi: 10.1152/ajpregu.1999.277.4.R959. [DOI] [PubMed] [Google Scholar]
- 18.Lupi R, Marchetti P, Maffei M, Del Guerra S, Benzi L, Marselli L, et al. Effects of acute or prolonged exposure to human leptin on isolated human islet function. Biochem Biophys Res Commun. 1999;256:637–41. doi: 10.1006/bbrc.1999.0384. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson E, Stridsberg M, Sandler S. Leptin regulation of islet amyloid polypeptide Secretion from mouse pancreatic islets. Biochem Pharmacol. 1998;56:1339–46. doi: 10.1016/s0006-2952(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 20.Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptindeficient ob/ob mice. Proc Natl Acad Sci U S A. 1999;96:674–9. doi: 10.1073/pnas.96.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. 2013;62:457–78. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Donato J, Jr, Cravo RM, Frazão R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology. 2011;93:9–18. doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldelli R, Dieguez C, Casanueva FF. The role of leptin in reproduction: Experimental and clinical aspects. Ann Med. 2002;34:5–18. doi: 10.1080/078538902317338599. [DOI] [PubMed] [Google Scholar]
- 24.Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens. 2011;29:758–62. doi: 10.1097/HJH.0b013e328344280b. [DOI] [PubMed] [Google Scholar]
- 25.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, et al. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–73. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 26.Momin AU, Melikian N, Shah AM, Grieve DJ, Wheatcroft SB, John L, et al. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: Evidence for tissue specificity of leptin resistance. Eur Heart J. 2006;27:2294–9. doi: 10.1093/eurheartj/ehi831. [DOI] [PubMed] [Google Scholar]
- 27.Wolk R, Somers VK. Leptin and vascular function: Friend or foe? Eur Heart J. 2006;27:2263–5. doi: 10.1093/eurheartj/ehl246. [DOI] [PubMed] [Google Scholar]
- 28.Palanivel R, Eguchi M, Shuralyova I, Coe I, Sweeney G. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism. 2006;55:1067–75. doi: 10.1016/j.metabol.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 29.McGaffin KR, Zou B, McTiernan CF, O’Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–24. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieb W, Sullivan LM, Aragam J, Harris TB, Roubenoff R, Benjamin EJ, et al. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–5. doi: 10.1016/j.amjcard.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149:5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 33.Wannamethee SG, Tchernova J, Whincup P, Lowe GD, Kelly A, Rumley A, et al. Plasma leptin: Associations with metabolic, inflammatory and haemostatic risk factors for cardiovascular disease. Atherosclerosis. 2007;191:418–26. doi: 10.1016/j.atherosclerosis.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Tahergorabi Z, Khazaei M. Obesity and angiogenesis. J Isfahan Med School. 2012;29:1–16. [Google Scholar]
- 35.Tahergorabi Z, Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA Atheroscler. 2013;9:247–53. [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon M, Skaggs BJ, Sahakian L, Grossman J, FitzGerald J, Ragavendra N, et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann Rheum Dis. 2011;70:1619–24. doi: 10.1136/ard.2010.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez A, Fortuño A, Gómez-Ambrosi J, Zalba G, Díez J, Frühbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–31. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 38.Beltowski J, Wójcicka G, Jamroz A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis. 2003;170:21–9. doi: 10.1016/s0021-9150(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 39.Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 40.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–17. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tahergorabi Z, Khazaei M. Changes of serum angiogenic biomarkers and their correlations with serum leptin concentration. Bratisl Lek Listy. 2014;115:330–3. doi: 10.4149/bll_2014_065. [DOI] [PubMed] [Google Scholar]
- 42.Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, et al. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–8. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 43.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–9. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 44.Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41:265–74. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110:1269–75. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 46.Zeidan A, Hunter JC, Javadov S, Karmazyn M. mTOR mediates RhoA-dependent leptin-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2011;352:99–108. doi: 10.1007/s11010-011-0744-2. [DOI] [PubMed] [Google Scholar]
- 47.Salehi E, Amjadi F, Khazaei M. Angiogenesis in Health and Disease: Role of Vascular Endothelial Growth Factor (VEGF) J Isfahan Med School. 2011;29:1–15. [Google Scholar]
- 48.Salehi E, Khazaei M, Rashidi B, Javanmard SH. Effect of rosiglitazone on coronary angiogenesis in diabetic and control rats. J Isfahan Med School. 2011;29:1–8. [Google Scholar]
- 49.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 50.Artwohl M, Roden M, Hölzenbein T, Freudenthaler A, Waldhäusl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577–80. doi: 10.1038/sj.ijo.0801947. [DOI] [PubMed] [Google Scholar]
- 51.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 52.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–5. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: Leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 54.Ribatti D, Nico B, Belloni AS, Vacca A, Roncali L, Nussdorfer GG. Angiogenic activity of leptin in the chick embryo chorioallantoic membrane is in part mediated by endogenous fibroblast growth factor-2. Int J Mol Med. 2001;8:265–8. doi: 10.3892/ijmm.8.3.265. [DOI] [PubMed] [Google Scholar]
- 55.Liapaki I, Anagnostoulis S, Karayiannakis A, Korkolis D, Labropoulou M, Matarasso A, et al. Burn wound angiogenesis is increased by exogenously administered recombinant leptin in rats. Acta Cir Bras. 2008;23:118–24. doi: 10.1590/s0102-86502008000200002. [DOI] [PubMed] [Google Scholar]
- 56.Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. PLoS One. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 59.Wang CN, Chang SD, Peng HH, Lee YS, Chang YL, Cheng PJ, et al. Change in amniotic fluid levels of multiple anti-angiogenic proteins before development of preeclampsia and intrauterine growth restriction. J Clin Endocrinol Metab. 2010;95:1431–41. doi: 10.1210/jc.2009-1954. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro AM, Andrade S, Pinho F, Monteiro JD, Costa M, Lopes C, et al. Prostate cancer cell proliferation and angiogenesis in different obese mice models. Int J Exp Pathol. 2010;91:374–86. doi: 10.1111/j.1365-2613.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aronis KN, Diakopoulos KN, Fiorenza CG, Chamberland JP, Mantzoros CS. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia. 2011;54:2358–67. doi: 10.1007/s00125-011-2201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Y. Angiogenesis modulate adipogenesis and obesity. J Clin Invest. 2007;117:2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, Yoshii M, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610–9. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 65.Considine RV, Considine EL, Williams CJ, Nyce MR, Magosin SA, Bauer TL, et al. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995;95:2986–8. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–56. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 67.Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: Role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol. 2004;181:297–306. doi: 10.1677/joe.0.1810297. [DOI] [PubMed] [Google Scholar]
- 68.Martin SS, Qasim A, Reilly MP. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 70.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 71.Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999;455:170–4. doi: 10.1016/s0014-5793(99)00874-1. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 73.Narin F, Atabek ME, Karakukcu M, Narin N, Kurtoglu S, Gumus H, et al. The association of plasma homocysteine levels with serum leptin and apolipoprotein B levels in childhood obesity. Ann Saudi Med. 2005;25:209–14. doi: 10.5144/0256-4947.2005.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Münzberg H, Björnholm M, Bates SH, Myers MG., Jr Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–52. doi: 10.1007/s00018-004-4432-1. [DOI] [PubMed] [Google Scholar]
- 76.Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, et al. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology. 2004;145:1185–93. doi: 10.1210/en.2003-1382. [DOI] [PubMed] [Google Scholar]
- 77.Krol E, Tups A, Archer ZA, Ross AW, Moar KM, Bell LM, et al. Altered expression of SOCS3 in the hypothalamic arcuate nucleus during seasonal body mass changes in the field vole, Microtus agrestis. J Neuroendocrinol. 2007;19:83–94. doi: 10.1111/j.1365-2826.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- 78.Figlewicz DP. Adiposity signals and food reward: Expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–92. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 79.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 81.Gertler A, Niv-Spector L, Reicher S. Is leptin an important physiological regulator of CRP? Nat Med. 2007;13:18–21. doi: 10.1038/nm0107-18. [DOI] [PubMed] [Google Scholar]


